Polymethyl Methacrylate-Based Bone Cements Containing Carbon Nanotubes and Graphene Oxide: An Overview of Physical, Mechanical, and Biological Properties
Abstract
:1. Introduction
Historical Background of PMMA- based BCs
2. Composition of PMMA-based BCs
2.1. Handling Properties of PMMA-Based BCs
2.2. Thermal Properties of PMMA-Based BCs
2.3. Mechanical Properties of PMMA-Based BCs
2.4. In Vivo Assessments of PMMA-Based BCs
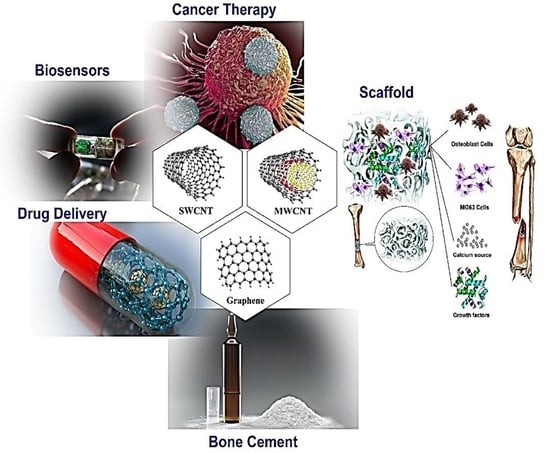
3. Use of Carbon Compounds in PMMA-Based BCs
4. CNTs in PMMA-Based BCs
4.1. Mechanical and Setting Properties of CNTs in PMMA-Based BCs
4.2. Biological Properties of CNTs in PMMA-Based BCs
5. Graphene (G) and GO in PMMA-Based BCs
5.1. Mechanical and Setting Properties of Graphene (G) and GO in PMMA-Based BCs
5.2. Biological Properties of Graphene (G) and GO in PMMA-Based BCs
6. Strengthening Mechanisms and Future Outlook of the PMMA-Carbon-Based BCs
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABCs | Acrylic bone cements | GO | Graphene oxide |
| BCs | Bone cements | HA | Hydroxyapatite |
| BPO | Benzoyl peroxide | HOb | Human osteoblast |
| CBNs | Carbon-based nanomaterials | MMA | Methyl methacrylate |
| CNTs | Carbon nanotubes | Mon | Monticellite |
| CS | Chitosan | MPS | [3-(Methacryloyloxy)propyl]trimethoxysilane |
| Ca-P | Calcium phosphate | MWCNTs | Multi-walled carbon nanotubes |
| DMA | Dynamic mechanical analysis | PCL | Polycaprolactone |
| DmpT | N,N-dimethyl-p-toluidine | PMMA | Polymethyl methacrylate |
| ECM | Extra-cellular matrix | rBMSC | Rat bone marrow mesenchymal stem cell |
| FA | Fluorapatite | RIC | Radical initiator concentration |
| f-CNT | Functionalized carbon nanotube | SIF | Stress intensity factor |
| FTIR | Fourier transform infrared spectroscopy | SWCNTs | Single-walled carbon nanotubes |
| FBT | Fractured bone treatments | TJR | Total joint replacement |
| G | Graphene | TNI | Thermal necrosis index |
References
- Deb, S. Acrylic Bone Cements for Joint Replacement, in Biomedical Composites; Elsevier Woodhead Publishing: Amsterdam, The Netherlands, 2010; pp. 210–233. [Google Scholar]
- Dunne, N.; Ormsby, R.W. MWCNT Used in Orthopaedic Bone Cements; Naraghi, M., Ed.; InTech Open Access Publisher: London, UK, 2011; pp. 337–342. [Google Scholar]
- Dunne, N.; Clements, J.; Wang, J. Acrylic cements for bone fixation in joint replacement. In Joint Replacement Technology; Elsevier Woodhead Publishing: Amsterdam, The Netherlands, 2014; pp. 212–256. [Google Scholar]
- Kleinschmitt, O. Plexiglas zur deckung von schdellcken. Chirurgie 1941, 13, 273. [Google Scholar]
- Judet, J.; Judet, R. The use of an artificial femoral head for arthroplasty of the hip joint. J. Bone Jt. Surg. 1950, 32, 166–173. [Google Scholar] [CrossRef]
- Charnley, J. Anchorage of the femoral head prosthesis to the shaft of the femur. J. Bone Jt. Surg. 1960, 42, 28–30. [Google Scholar] [CrossRef] [Green Version]
- Charnley, J. Clinical experiences with self-curing acrylic cement. In Acrylic Cement in Orthopaedic Surgery; E & S Livingstone: Edinburgh, Scotland, 1970; pp. 33–35. [Google Scholar]
- Buchholz, H.; Elson, R.; Engelbrecht, E.; Lodenkamper, H.; Rottger, J.; Siegel, A. Management of deep infection of total hip replacement. J. Bone Jt. Surg. 1981, 63, 342–353. [Google Scholar] [CrossRef]
- Dunne, N.; Ormsby, R.; Mitchell, C.A. Carbon nanotubes in acrylic bone cement. In Biologically Responsive Biomaterials for Tissue Engineering; Springer: New York, NY, USA, 2013; pp. 173–199. [Google Scholar]
- Daniëlle, N.; Kluin, O.S.; Thompson, J.; van der Mei, H.C.; Busscher, H.J. Gentamicin release from commercially-available gentamicin-loaded PMMA bone cements in a prosthesis-related interfacial gap model and their antibacterial efficacy. BMC Musculoskelet. Disord. 2010, 11, 258. [Google Scholar]
- Dunne, N. Mechanical properties of bone cements. In Orthopaedic Bone Cements; Woodhead Publishing: Cambridge, UK, 2008; pp. 233–264. [Google Scholar]
- Ali, U.; Karim, K.J.B.A.; Buang, N.A. A review of the properties and applications of poly (methyl methacrylate)(PMMA). Polym. Rev. 2015, 55, 678–705. [Google Scholar] [CrossRef]
- Dunne, N.; Orr, J. Curing characteristics of acrylic bone cement. J. Mater. Sci. Mater. Med. 2002, 13, 17–22. [Google Scholar] [CrossRef]
- Huang, K.Y.; Yan, J.J.; Lin, R.M. Histopathologic findings of retrieved specimens of vertebroplasty with polymethylmethacrylate cement: Case control study. Spine 2005, 30, E585–E588. [Google Scholar] [CrossRef]
- Kuehn, K.D. Bone Cements: Up-to-date Comparison of Physical and Chemical Properties of Commercial Materials; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Jimenez, E.P. Bone Cements Reinforced with Carbon Based Nanomaterials. Ph.D Thesis, Universidad Pontificia Comillas, Comillas, Spain, 2017. [Google Scholar]
- Starke, G.; Birnie, C.; van den Blink, P. Numerical modelling of cement polymerisation and thermal bone necrosis. In Third International Symposium on Computer Methods in Biomechanics and Biomedical Engineering; Gordon and Breach: London, UK, 1997. [Google Scholar]
- DiPisa, J.A.; Sih, G.S.; Berman, A.T. The temperature problem at the bone-acrylic cement interface of the total hip replacement. Clin. Orthop. Relat. Res. 1976, 95–98. [Google Scholar] [CrossRef]
- Standard, I.S. International Standard ISO 5833. In Implants for Surgery—Acrylic Resin Cements; International Standards Organization: Geneva, Switzerland, 2002. [Google Scholar]
- ASTM F451-99a. Standard Specification for Acrylic Bone Cement; ASTM International: West Conshohocken, PA, USA, 2007. [Google Scholar]
- Gruenert, A.; Ritter, G. Alterations of the physical properties of so-called bone cements after admixing of foreign incredients (author’s transl). Arch. Fur Orthop. Und Unf. Chir. 1974, 78, 336. [Google Scholar]
- De Wijn, J.; Slooff, T.; Driessens, F. Characterization of bone cements. Acta Orthop. Scand. 1975, 46, 38–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuhn, K. Bone Cements Berlin; Springer: Berlin/Heidelberg, Germany, 2000; Volume 21, pp. 813–819. [Google Scholar]
- Jasty, M.; Davies, J.P.; O’Connor, D.O.; Burke, D.W.; Harrigan, T.P.; Harris, W.H. Porosity of various preparations of acrylic bone cements. Clin. Orthop. 1990, 259, 9. [Google Scholar] [CrossRef]
- Lewis, G.; Mladsi, S. Effect of sterilization method on properties of Palacos® R acrylic bone cement. Biomaterials 1998, 19, 117–124. [Google Scholar] [CrossRef]
- Dunne, N.; Orr, J.; Mushipe, M.; Eveleigh, R. The relationship between porosity and fatigue characteristics of bone cements. Biomaterials 2003, 24, 239–245. [Google Scholar] [CrossRef]
- Lidgren, L.; Bodelind, B.; Möller, J. Bone cement improved by vacuum mixing and chilling. Acta Orthop. Scand. 1987, 58, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.P.; O’Connor, D.O.; Greer, J.A.; Harris, W.H. Comparison of the mechanical properties of Simplex P, Zimmer Regular, and LVC bone cements. J. Biomed. Mater. Res. 1987, 21, 719–730. [Google Scholar] [CrossRef]
- Wixson, R.L.; Lautenschalager, E.P.; Novak, M.A. Vacuum mixing of acrylic bone cement. J. Arthroplast. 1987, 2, 141–149. [Google Scholar] [CrossRef]
- Dunne, N.; Orr, J. Influence of mixing techniques on the physical properties of acrylic bone cement. Biomaterials 2001, 22, 1819–1826. [Google Scholar] [CrossRef]
- Freitag, T.A.; Cannon, S.L. Fracture characteristics of acrylic bone cements. II. Fatigue. J. Biomed. Mater. Res. 1977, 11, 609–624. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.; Provan, J.; Krygier, J.; Chan, K.; Miller, J. Fatigue of acrylic bone cement–effect of frequency and environment. J. Biomed. Mater. Res. 1989, 23, 819–831. [Google Scholar] [CrossRef]
- Webb, J.; Spencer, R. The role of polymethylmethacrylate bone cement in modern orthopaedic surgery. J. Bone Jt. Surg. 2007, 89, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.; Oshida, Y.; Hancock, E.; Kowolik, M.; Barco, T.; Zunt, S. Hydroxyapatite/PMMA composites as bone cements. Bio-Med. Mater. Eng. 2004, 14, 87–105. [Google Scholar]
- Lee, R.R.; Ogiso, M.; Watanabe, A.; Ishihara, K. Examination of hydroxyapatite filled 4-META/MMA-TBB adhesive bone cement in in vitro and in vivo environment. J. Biomed. Mater. Res. 1997, 38, 11–16. [Google Scholar] [CrossRef]
- Mousa, W.F.; Kobayashi, M.; Shinzato, S.; Kamimura, M.; Neo, M.; Yoshihara, S.; Nakamura, T. Biological and mechanical properties of PMMA-based bioactive bone cements. Biomaterials 2000, 21, 2137–2146. [Google Scholar] [CrossRef]
- Shinzato, S.; Kobayashi, M.; Mousa, W.F.; Kamimura, M.; Neo, M.; Kitamura, Y.; Kokubo, T.; Nakamura, T. Bioactive polymethyl methacrylate-based bone cement: Comparison of glass beads, apatite-and wollastonite-containing glass–ceramic, and hydroxyapatite fillers on mechanical and biological properties. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2000, 51, 258–272. [Google Scholar] [CrossRef]
- Pawar, E. A Review Article on Acrylic PMMA. IOSR J. Mech. Civ. Eng. 2016, 13, 1–4. [Google Scholar]
- Cha, C.; Shin, S.R.; Annabi, N.; Dokmeci, M.R.; Khademhosseini, A. Carbon-based nanomaterials: Multifunctional materials for biomedical engineering. ACS Nano 2013, 7, 2891–2897. [Google Scholar] [CrossRef]
- Curl, R.F.; Smalley, R.E.; Kroto, H.W.; O’Brien, S.; Heath, J.R. How the news that we were not the first to conceive of soccer ball C_ (60) got to us. J. Mol. Graph. Model. 2001, 19, 185–186. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Shi, Z.; Lian, Y.; Liao, F.H.; Zhou, X.; Gu, Z.; Zhang, Y.; Iijima, S.; Li, H.; Yue, K.T.; Zhang, S.L. Large scale synthesis of single-wall carbon nanotubes by arc-discharge method. J. Phys. Chem. Solids 2000, 61, 1031–1036. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, H.; Iijima, S. Single-wall carbon nanotubes synthesized by laser ablation in a nitrogen atmosphere. Appl. Phys. Lett. 1998, 73, 3827–3829. [Google Scholar] [CrossRef]
- Sinnott, S.B.; Andrews, R.; Qian, D.; Rao, A.M.; Mao, Z.; Dickey, E.; Derbyshire, F. Model of carbon nanotube growth through chemical vapor deposition. Chem. Phys. Lett. 1999, 315, 25–30. [Google Scholar] [CrossRef]
- Andrews, R.; Jacques, D.; Qian, D.; Rantell, T. Multiwall carbon nanotubes: Synthesis and application. Acc. Chem. Res. 2002, 35, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- Santangelo, S.; Messina, G.; Donato, M.G.; Lanza, M.; Milone, C.; Pistone, A. Low-frequency Raman study of hollow multiwalled nanotubes grown by Fe-catalyzed chemical vapor deposition. J. Appl. Phys. 2006, 100, 104311. [Google Scholar] [CrossRef]
- Thostenson, E.T.; Ren, Z.; Chou, T.W. Advances in the science and technology of carbon nanotubes and their composites: A review. Compos. Sci. Technol. 2001, 61, 1899–1912. [Google Scholar] [CrossRef] [Green Version]
- Ajayan, P. Carbon nanotubes: Novel architecture in nanometer space. Prog. Cryst. Growth Charact. Mater. 1997, 34, 37–51. [Google Scholar] [CrossRef]
- Iijima, S. Carbon nanotubes: Past, present, and future. Phys. B Condens. Matter 2002, 323, 1–5. [Google Scholar] [CrossRef]
- Baughman, R.H.; Zakhidov, A.A.; de Heer, W.A. Carbon nanotubes—The route toward applications. Science 2002, 297, 787–792. [Google Scholar] [CrossRef] [Green Version]
- Colbert, D.T. Single-wall nanotubes: A new option for conductive plastics and engineering polymers. Plast. Addit. Compd. 2003, 5, 8–25. [Google Scholar]
- Shadjou, N.; Hasanzadeh, M. Graphene and its nanostructure derivatives for use in bone tissue engineering: Recent advances. J. Biomed. Mater. Res. Part A 2016, 104, 1250–1275. [Google Scholar] [CrossRef] [PubMed]
- Eivazzadeh-Keihan, R.; Maleki, A.; de la Guardia, M.; Bani, M.S.; Chenab, K.K.; Pashazadeh-Panahi, P.; Baradaran, B.; Mokhtarzadeh, A.; Hamblin, M.R. Carbon based nanomaterials for tissue engineering of bone: Building new bone on small black scaffolds: A review. J. Adv. Res. 2019, 18, 185–201. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, M.; Karbasi, S.; Bakhtiari, S.S.E. Evaluation of physical, mechanical, and biodegradation of chitosan/graphene oxide composite as bone substitutes. Polym.-Plast. Technol. Mater. 2020, 59, 430–440. [Google Scholar] [CrossRef]
- Depan, D.; Pesacreta, T.; Misra, R. The synergistic effect of a hybrid graphene oxide–chitosan system and biomimetic mineralization on osteoblast functions. Biomater. Sci. 2014, 2, 264–274. [Google Scholar] [CrossRef]
- Pistone, A.; Ferlazzo, A.; Lanza, M.; Milone, C.; Iannazzo, D.; Piperno, A.; Piperopoulos, E.; Galvagno, S. Morphological modification of MWCNT functionalized with HNO3/H2SO4 mixtures. J. Nanosci. Nanotechnol. 2012, 12, 5054–5060. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Robinson, J.T.; Tabakman, S.M.; Yang, K.; Dai, H. Carbon materials for drug delivery & cancer therapy. Mater. Today 2011, 14, 316–323. [Google Scholar]
- Demoustier, S.; Minoux, E.; le Baillif, M.; Charles, M.; Ziaei, A. Review of two microwave applications of carbon nanotubes: Nano-antennas and nano-switches. Comptes Rendus Phys. 2008, 9, 53–66. [Google Scholar] [CrossRef]
- Nayak, T.R.; Andersen, H.; Makam, V.S.; Khaw, C.; Bae, S.; Xu, X.; Ee, P.L.R.; Ahn, J.; Hong, B.; Pastorin, G.; et al. Graphene for controlled and accelerated osteogenic differentiation of human mesenchymal stem cells. ACS Nano 2011, 5, 4670–4678. [Google Scholar] [CrossRef] [Green Version]
- Crowder, S.W.; Prasai, D.; Rath, R.; Balikov, D.A.; Bae, H.; Bolotin, K.; Sung, H. Three-dimensional graphene foams promote osteogenic differentiation of human mesenchymal stem cells. Nanoscale 2013, 5, 4171–4176. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.C.; Lim, C.H.Y.X.; Shi, H.; Tang, L.A.L.; Wang, Y.; Lim, C.T.; Loh, K.P. Origin of enhanced stem cell growth and differentiation on graphene and graphene oxide. ACS Nano 2011, 5, 7334–7341. [Google Scholar] [CrossRef]
- Yoon, H.H.; Bhang, S.H.; Kim, T.; Yu, T.; Hyeon, T.; Kim, B. Dual Roles of Graphene oxide in chondrogenic differentiation of adult stem cells: Cell-adhesion substrate and growth factor-delivery carrier. Adv. Funct. Mater. 2014, 24, 6455–6464. [Google Scholar] [CrossRef]
- Kim, J.; Choi, K.S.; Kim, Y.; Lim, K.T.; Seonwoo, H.; Park, Y.; Kim, D.; Choung, P.H.; Cho, C.; Kim, S.Y.; et al. Bioactive effects of graphene oxide cell culture substratum on structure and function of human adipose-derived stem cells. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2013, 101, 3520–3530. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Park, S.; Bhang, S.H.; Yoon, J.; Jo, I.; Jeong, G.; Hong, B.; Kim, B. Graphene enhances the cardiomyogenic differentiation of human embryonic stem cells. Biochem. Biophys. Res. Commun. 2014, 452, 174–180. [Google Scholar] [CrossRef]
- Park, J.; Park, S.; Ryu, S.; Bhang, S.H.; Kim, J.; Yoon, J.; Park, Y.H.; Cho, S.; Lee, S.; Hong, B.; et al. Graphene‒regulated cardiomyogenic differentiation process of mesenchymal stem cells by enhancing the expression of extracellular matrix proteins and cell signaling molecules. Adv. Healthc. Mater. 2014, 3, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhang, Q.; Gao, S.; Song, Q.; Huang, R.; Wang, L.; Liu, L.; Dai, J.; Tang, M.; Cheng, G. Three-dimensional graphene foam as a biocompatible and conductive scaffold for neural stem cells. Sci. Rep. 2013, 3, 1604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghuge, A.; Shirode, A.R.; Kadam, V.J. Graphene: A comprehensive review. Curr. Drug Targets 2017, 18, 724–733. [Google Scholar] [CrossRef]
- Maiti, D.; Tong, X.; Mou, X.; Yang, K. Carbon-based nanomaterials for biomedical applications: A recent study. Front. Pharmacol. 2019, 9, 1401. [Google Scholar] [CrossRef] [PubMed]
- Mundra, R.V.; Wu, X.; Sauer, J.; Dordick, J.S.; Kane, R.S. Nanotubes in biological applications. Curr. Opin. Biotechnol. 2014, 28, 25–32. [Google Scholar] [CrossRef]
- Bakhtiari, S.S.E.; Karbasi, S.; Tabrizi, S.A.H.; Ebrahimi-Kahrizsangi, R. Chitosan/MWCNTs composite as bone substitute: Physical, mechanical, bioactivity, and biodegradation evaluation. Polym. Compos. 2019, 40, E1622–E1632. [Google Scholar] [CrossRef]
- Lewis, G. Properties of acrylic bone cement: State of the art review. J. Biomed. Mater. Res. 1997, 38, 155–182. [Google Scholar] [CrossRef]
- Sinnett-Jones, P.; Browne, M.; Moffat, A.; Jeffers, J.; Saffari, N.; Buffière, J.Y.; Sinclair, I. Crack initiation processes in acrylic bone cement. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2009, 89, 1088–1097. [Google Scholar] [CrossRef]
- Dalton, A.B.; Collins, S.; Munoz, E.; Razal, J.M.; Ebron, V.H.; Ferraris, J.P.; Coleman, J.N.; Kim, B.G.; Baughman, R.H. Super-tough carbon-nanotube fibres. Nature 2003, 423, 703. [Google Scholar] [CrossRef]
- Pienkowski, D.A.; Andrews, R.J. Polymethylmethacrylate Augmented with Carbon Nanotubes. Google Patents US 6872403B2, 29 March 2003. [Google Scholar]
- Marrs, B.; Andrews, R.; Rantell, T.; Pienkowski, D. Augmentation of acrylic bone cement with multiwall carbon nanotubes. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2006, 77, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Qian, D.; Andrews, R.; Jacques, D.; Kichambare, P.; Lian, G.; Dickey, E.C. Low-temperature synthesis of large-area CN x nanotube arrays. J. Nanosci. Nanotechnol. 2003, 3, 93–97. [Google Scholar] [CrossRef]
- Marrs, B. Carbon Nanotube Augmentation of a Bone Cement Polymer. Ph.D. Thesis, University of Kentucky, Lexington, KY, USA, 2007. [Google Scholar]
- Marrs, B.; Andrews, R.; Pienkowski, D. Multiwall carbon nanotubes enhance the fatigue performance of physiologically maintained methyl methacrylate–styrene copolymer. Carbon 2007, 45, 2098–2104. [Google Scholar] [CrossRef]
- Nien, Y.H.; Huang, C.L. The mechanical study of acrylic bone cement reinforced with carbon nanotube. Mater. Sci. Eng. B 2010, 169, 134–137. [Google Scholar] [CrossRef]
- Ormsby, R.; McNally, T.; Mitchell, C.; Dunne, N. Incorporation of multiwalled carbon nanotubes to acrylic based bone cements: Effects on mechanical and thermal properties. J. Mech. Behav. Biomed. Mater. 2010, 3, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Ormsby, R.; McNally, T.; Mitchell, C.; Dunne, N. Influence of multiwall carbon nanotube functionality and loading on mechanical properties of PMMA/MWCNT bone cements. J. Mater. Sci. Mater. Med. 2010, 21, 2287–2292. [Google Scholar] [CrossRef]
- Ormsby, R.; McNally, T.; O’Hare, P.; Burke, G.; Mitchell, C.; Dunne, N. Fatigue and biocompatibility properties of a poly (methyl methacrylate) bone cement with multi-walled carbon nanotubes. Acta Biomater. 2012, 8, 1201–1212. [Google Scholar] [CrossRef]
- Ormsby, R.; Ally, T.M.; Mitchell, C.; Halley, P.; Martin, D.; Nicholson, T.; Dunne, N. Effect of MWCNT addition on the thermal and rheological properties of polymethyl methacrylate bone cement. Carbon 2011, 49, 2893–2904. [Google Scholar] [CrossRef]
- Ormsby, R.W.; Modreanu, M.; Mitchell, C.A.; Dunne, N.J. Carboxyl functionalised MWCNT/polymethyl methacrylate bone cement for orthopaedic applications. J. Biomater. Appl. 2014, 29, 209–221. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Y.; Cui, L. An efficient functionalization method for the multiwalled carbon nanotubes and their applications in PMMA bone cement. Sens. Transducers 2013, 21, 36. [Google Scholar]
- Saffar, K.; Najafi, A.R.; Moeinzadeh, M.H.; Sudak, L.J. A finite element study of crack behavior for carbon nanotube reinforced bone cement. World J. Mech. 2013, 3, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Xu, Y.Z. Incorporation of MWCNTs to PMMA bone cements: Effects on fatigue properties. Adv. Mater. Res. 2014. [Google Scholar] [CrossRef]
- Arun, S.; Sreekanth, P.R.; Kanagaraj, S. Mechanical characterisation of PMMA/SWNTs bone cement using nanoindenter. Mater. Technol. 2014, 29, B4–B9. [Google Scholar] [CrossRef]
- Pahlevanzadeh, F.; Bakhsheshi-Rad, H.; Ismail, A.; Aziz, M.; Chen, X. Development of PMMA-Mon-CNT bone cement with superior mechanical properties and favorable biological properties for use in bone-defect treatment. Mater. Lett. 2019, 240, 9–12. [Google Scholar] [CrossRef]
- Soleymani Eil Bakhtiari, S.; Karbasi, S.; Tabrizi, S.A.H.; Ebrahimi-Kahrizsangi, R.; Salehi, H. Evaluation of the effects of chitosan/multiwalled carbon nanotubes composite on physical, mechanical and biological properties of polymethyl methacrylate-based bone cements. Mater. Technol. 2019, 35, 267–280. [Google Scholar] [CrossRef]
- Wang, C.; Yu, B.; Fan, Y.; Ormsby, R.W.; McCarthy, H.O.; Dunne, N.; Li, X. Incorporation of multi-walled carbon nanotubes to PMMA bone cement improves cytocompatibility and osseointegration. Mater. Sci. Eng. C 2019, 103, 109823. [Google Scholar] [CrossRef]
- Gonçalves, G.; Portolés, M.T.; Ramírez-Santillán, C.; Vallet-Regí, M.; Serro, A.P.; Grácio, J.; Marques, P.A. Evaluation of the in vitro biocompatibility of PMMA/high-load HA/carbon nanostructures bone cement formulations. J. Mater. Sci. Mater. Med. 2013, 24, 2787–2796. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Cao, B.; Zhu, J.; Shen, J.; Li, J.; Guo, S.; Wang, Y. Rheological, thermal, and mechanical properties of phosphorus-containing wholly aromatic thermotropic liquid crystalline polymer-filled poly (butylene terephthalate) composites. Polym. Compos. 2012, 33, 1432–1436. [Google Scholar] [CrossRef]
- Depan, D.; Girase, B.; Shah, J.; Misra, R. Structure–process–property relationship of the polar graphene oxide-mediated cellular response and stimulated growth of osteoblasts on hybrid chitosan network structure nanocomposite scaffolds. Acta Biomater. 2011, 7, 3432–3445. [Google Scholar] [CrossRef]
- Paz, E.; Forriol, F.; del Real, J.; Dunne, N. Graphene oxide versus graphene for optimisation of PMMA bone cement for orthopaedic applications. Mater. Sci. Eng. C 2017, 77, 1003–1011. [Google Scholar] [CrossRef]
- Paz, E.; Ballesteros, Y.; Forriol, F.; Dunne, N.; del Real, J. Graphene and graphene oxide functionalisation with silanes for advanced dispersion and reinforcement of PMMA-based bone cements. Mater. Sci. Eng. C 2019, 104, 109946. [Google Scholar] [CrossRef]
- Khan, A.A.; Mirza, E.H.; Mohamed, B.A.; Alharthi, N.H.; Abdo, H.S.; Javed, R.; Alhur, R.S.; Vallittu, P.K. Physical, mechanical, chemical and thermal properties of nanoscale graphene oxide-poly methylmethacrylate composites. J. Compos. Mater. 2018, 52, 2803–2813. [Google Scholar] [CrossRef]
- Khan, A.A.; Mirza, E.H.; Mohamed, B.A.; El-Sharawy, M.A.; Al-Asmari, M.H.; Al-Khureif, A.A.; Dar, M.A.; Vallittu, P.K. Static and dynamic mechanical properties of graphene oxide-based bone cementing agents. J. Compos. Mater. 2019, 53, 2297–2304. [Google Scholar] [CrossRef]
- Gonçalves, G.; Cruz, S.M.; Grácio, J.; Marques, P.A.; Ramírez-Santillán, C.; Vallet-Regí, M.; Portolés, M.T. New bioactive PMMA-hydroxyapatite based bone cement reinforced with graphene oxide. Graphene 2012, 2012, 10–13. [Google Scholar]
- Pahlevanzadeh, F.; Bakhsheshi-Rad, H.R.; Hamzah, E. In-vitro biocompatibility, bioactivity, and mechanical strength of PMMA-PCL polymer containing fluorapatite and graphene oxide bone cements. J. Mech. Behav. Biomed. Mater. 2018, 82, 257–267. [Google Scholar] [CrossRef]
- Tavakoli, M.; Bakhtiari, S.S.E.; Karbasi, S. Incorporation of chitosan/graphene oxide nanocomposite in to the PMMA bone cement: Physical, mechanical and biological evaluation. Int. J. Biol. Macromol. 2020, 149, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Valencia Zapata, M.E.; Hernandez, M.; Herminsul, J.; Tovar, C.D.G.; Llano, C.H.V.; Escobar, J.A.D.; Vázquez-Lasa, B.; Román, J.S.; Rojo, L. Novel bioactive and antibacterial acrylic bone cement nanocomposites modified with graphene oxide and chitosan. Int. J. Mol. Sci. 2019, 20, 2938. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, G.; Cruz, S.M.; Ramalho, A.; Grácio, J.; Marques, P.A. Graphene oxide versus functionalized carbon nanotubes as a reinforcing agent in a PMMA/HA bone cement. Nanoscale 2012, 4, 2937–2945. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Kapusetti, G.; Bhong, S.Y.; Roy, P.; Singh, S.K.; Singh, S.; Balavigneswaran, C.K.; Mahato, K.K.; Ray, B.; Maiti, B.; et al. Osteoconductive amine-functionalized graphene–poly (methyl methacrylate) bone cement composite with controlled exothermic polymerization. Bioconjugate Chem. 2017, 28, 2254–2265. [Google Scholar] [CrossRef] [PubMed]
- Paz, E.; Ballesteros, Y.; Abenojar, J.; del Real, J.; Dunne, N. Graphene Oxide and Graphene Reinforced PMMA Bone Cements: Evaluation of Thermal Properties and Biocompatibility. Materials 2019, 12, 3146. [Google Scholar] [CrossRef] [Green Version]
- Munir, K.S.; Wen, C.; Li, Y. Carbon nanotubes and graphene as nanoreinforcements in metallic biomaterials: A review. Adv. Biosyst. 2019, 3, 1800212. [Google Scholar] [CrossRef]
- Ge, C.; Du, J.; Zhao, L.; Wang, L.; Liu, Y.; Li, D.; Yang, Y.; Zhou, R.; Zhao, Y.; Chai, Z.; et al. Binding of blood proteins to carbon nanotubes reduces cytotoxicity. Proc. Natl. Acad. Sci. USA 2011, 108, 16968–16973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Ali, S.F.; Dervishi, E.; Xu, Y.; Li, Z.; Casciano, D.; Biris, A.S. Cytotoxicity effects of graphene and single-wall carbon nanotubes in neural phaeochromocytoma-derived PC12 cells. ACS Nano 2010, 4, 3181–3186. [Google Scholar] [CrossRef] [PubMed]
- Porter, A.E.; Gass, M.; Muller, K.; Skepper, J.N.; Midgley, P.A.; Welland, M. Direct imaging of single-walled carbon nanotubes in cells. Nat. Nanotechnol. 2007, 2, 713–717. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Toxicity of graphene and graphene oxide nanowalls against bacteria. ACS Nano 2010, 4, 5731–5736. [Google Scholar] [CrossRef]
- Gurunathan, S.; Han, J.W.; Dayem, A.A.; Eppakayala, V.; Kim, J.H. Oxidative stress-mediated antibacterial activity of graphene oxide and reduced graphene oxide in Pseudomonas aeruginosa. Int. J. Nanomed. 2012, 7, 5901. [Google Scholar] [CrossRef] [Green Version]
- Hegab, H.M.; ElMekawy, A.; Zou, L.; Mulcahy, D.; Saint, C.P.; Ginic-Markovic, M. The controversial antibacterial activity of graphene-based materials. Carbon 2016, 105, 362–376. [Google Scholar] [CrossRef]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: Membrane and oxidative stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef]
- Liu, L.; Liu, J.; Wang, Y.; Yan, X.; Sun, D.D. Facile synthesis of monodispersed silver nanoparticles on graphene oxide sheets with enhanced antibacterial activity. New J. Chem. 2011, 35, 1418–1423. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Umasuthan, N.; Mohan, R.; Lee, J.; Kim, S.J. Antibacterial activity of graphene oxide nanosheets. Sci. Adv. Mater. 2012, 4, 1111–1117. [Google Scholar] [CrossRef]
- Hu, W.; Peng, C.; Luo, W.; Lv, M.; Li, X.; Li, D.; Huang, Q.; Fan, C. Graphene-based antibacterial paper. ACS Nano 2010, 4, 4317–4323. [Google Scholar] [CrossRef]
- Liu, S.; Hu, M.; Zeng, T.H.; Wu, R.; Jiang, R.; Wei, J.; Wang, L.; Kong, J.; Chen, Y. Lateral dimension-dependent antibacterial activity of graphene oxide sheets. Langmuir 2012, 28, 12364–12372. [Google Scholar] [CrossRef]
- Tu, Y.; Lv, M.; Xiu, P.; Huynh, T.; Zhang, M.; Castelli, M.; Liu, Z.; Huang, Q.; Fan, C.; Fang, H. Destructive extraction of phospholipids from Escherichia coli membranes by graphene nanosheets. Nat. Nanotechnol. 2013, 8, 594. [Google Scholar] [CrossRef] [PubMed]
- Olivi, M.; Zanni, E.; de Bellis, G.; Talora, C.; Sarto, M.S.; Palleschi, C.; Flahaut, E.; Monthioux, M.; Rapino, S.; Uccelletti, D. Inhibition of microbial growth by carbon nanotube networks. Nanoscale 2013, 5, 9023–9029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, S.; Pinault, M.; Pfefferle, L.D.; Elimelech, M. Single-walled carbon nanotubes exhibit strong antimicrobial activity. Langmuir 2007, 23, 8670–8673. [Google Scholar] [CrossRef]
- Kang, S.; Herzberg, M.; Rodrigues, D.F.; Elimelech, M. Antibacterial effects of carbon nanotubes: Size does matter. Langmuir 2008, 24, 6409–6413. [Google Scholar] [CrossRef]
- Aslan, S.; Loebick, C.Z.; Kang, S.; Elimelech, M.; Pfefferle, L.D.; van Tassel, P.R. Antimicrobial biomaterials based on carbon nanotubes dispersed in poly (lactic-co-glycolic acid). Nanoscale 2010, 2, 1789–1794. [Google Scholar] [CrossRef] [PubMed]
- Arias, L.R.; Yang, L. Inactivation of bacterial pathogens by carbon nanotubes in suspensions. Langmuir 2009, 25, 3003–3012. [Google Scholar] [CrossRef] [PubMed]
- Topoleski, L.; Ducheyne, P.; Cuckler, J. Microstructural pathway of fracture in poly (methyl methacrylate) bone cement. Biomaterials 1993, 14, 1165–1172. [Google Scholar] [CrossRef]
- Andrews, R.; Weisenberger, M. Carbon nanotube polymer composites. Curr. Opin. Solid State Mater. Sci. 2004, 8, 31–37. [Google Scholar] [CrossRef]
- Gojny, F.H.; Nastalczyk, J.; Roslaniec, Z.; Schulte, K. Surface modified multi-walled carbon nanotubes in CNT/epoxy-composites. Chem. Phys. Lett. 2003, 370, 820–824. [Google Scholar] [CrossRef]
- Bortz, D.R.; Heras, E.G.; Martin-Gullon, I. Impressive fatigue life and fracture toughness improvements in graphene oxide/epoxy composites. Macromolecules 2012, 45, 238–245. [Google Scholar] [CrossRef]
- Bakhsheshirad, H.R.; Hamzah, E.; Kasiriasgarani, M.; Saud, S.N.; Yaghoubidoust, F.; Akbari, E. Structure, corrosion behavior, and antibacterial properties of nano-silica/graphene oxide coating on biodegradable magnesium alloy for biomedical applications. Vacuum 2016, 131, 106–110. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Ismail, A.F.; Aziz, M.; Akbari, M.; Hadisi, Z.; Khoshnava, S.M.; Pagan, E.; Chen, X. Co-incorporation of graphene oxide/silver nanoparticle into poly-L-lactic acid fibrous: A route toward the development of cytocompatible and antibacterial coating layer on magnesium implants. Mater. Sci. Eng. C 2020, 111, 110812. [Google Scholar] [CrossRef] [PubMed]
- Saberi, A.; Bakhsheshi-Rad, H.R.; Karamian, E.; Kasiri-Asgarani, M.; Ghomi, H. Magnesium- graphene nano-platelet composites: Corrosion behavior, mechanical and biological properties. J. Alloy. Compd. 2020, 821, 153379. [Google Scholar] [CrossRef]
- Saud, S.N.; Raheleh, H.S.; Bakhsheshirad, H.R.; Yaghoubidoust, F.; Iqbal, N.; Hamzah, E.; Ooi, C.H.R. Corrosion and bioactivity performance of graphene oxide coating on Ti-Nb shape memory alloys in simulated body fluid. Mater. Sci. Eng. C 2016, 68, 687–694. [Google Scholar] [CrossRef]
- Pahlevanzadeh, F.; Bakhsheshi-Rad, H.R.; Ismail, A.F.; Aziz, M. Apatite-forming ability, cytocompatibility and mechanical properties enhancement of PMMA-based bone cements by incorporating of baghdadite nanoparticles. Int. J. Appl. Ceram. Technol. 2019, 16, 2006–2019. [Google Scholar] [CrossRef]
- Yildirim, M.; Kesimer, M.; Hasirci, N.; Kiliç, N.; Hasanreisoğlu, U. Adsorption of human salivary mucin MG1 onto glow-discharge plasma treated acrylic resin surfaces. J. Oral Rehabil. 2006, 33, 775–783. [Google Scholar] [CrossRef]
- Endogan, T.; Kiziltay, A.; Kose, G.T.; Comunoglu, N.; Beyzadeoglu, T.; Hasirci, N. Acrylic bone cements: Effects of the poly (methyl methacrylate) powder size and chitosan addition on their properties. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Katti, K.; Verma, D.; Katti, D. Materials for joint replacement. In Joint Replacement Technology; Elsevier Woodhead Publishing: Amsterdam, The Netherlands, 2008; pp. 81–104. [Google Scholar]
- ŞERBETÇİ, K.; Korkusuz, F.; Hasirci, N. Mechanical and thermal properties of hydroxyapatite-impregnated bone cement. Turk. J. Med Sci. 2000, 30, 543–549. [Google Scholar]
- Berto, F.; Elices, M.; Lazzarin, P.; Zappalorto, M. Fracture behaviour of notched round bars made of PMMA subjected to torsion at room temperature. Eng. Fract. Mechancs 2012, 90, 143–160. [Google Scholar] [CrossRef]
- Zhou, X.P.; Fu, L.; Ju, W.; Berto, F. An experimental study of the mechanical and fracturing behavior in PMMA specimen containing multiple 3D embedded flaws under uniaxial compression. Theor. Appl. Fract. Mech. 2019, 101, 207–216. [Google Scholar] [CrossRef]
- Campagnolo, A.; Berto, F. Tensile fracture analysis of blunt notched PMMA specimens by means of the strain energy density. Eng. Solid Mech. 2015, 3, 35–42. [Google Scholar] [CrossRef]
- Cendón, D.A.; Berto, F.; Lazzarin, P.; Elices Calafat, M. The cohesive crack model applied to notched PMMA specimens obeying a non linear behaviour under torsion loading. Key Eng. Mater. 2014, 577, 49–52. [Google Scholar] [CrossRef]
- Razavi, S.M.J.; Hokstad, H.L.; Berto, F. Mixed mode I/II/III fracture assessment of PMMA using a new test fixture. MATEC Web Conf. 2019, 300, 11003. [Google Scholar] [CrossRef]
- Berto, F.; Cendon, D.A.; Lazzarin, P.; Elices, M. Fracture behaviour of notched round bars made of PMMA subjected to torsion at -60 C. Eng. Fract. Mech. 2013, 102, 271–287. [Google Scholar] [CrossRef] [Green Version]
- Ayatollahi, M.R.; Moghaddam, M.R.; Razavi, S.M.J.; Berto, F. Geometry effects on fracture trajectory of PMMA samples under pure mode-I loading. Eng. Fract. Mech. 2016, 163, 449–461. [Google Scholar] [CrossRef]
- Politakos, N.; Barbarin, I.; Cordero-Lanzac, T.; Gonzalez, A.; Zangi, R.; Tomovska, R. Reduced graphene oxide/polymer monolithic materials for selective CO2 capture. Polymers 2020, 12, 936. [Google Scholar] [CrossRef] [Green Version]
- Araya-Hermosilla, E.A.; Carlotti, M.; Picchioni, F.; Mattoli, V.; Pucci, A. Electrically-conductive polyketone nanocomposites based on reduced graphene oxide. Polymers 2020, 12, 923. [Google Scholar] [CrossRef]
- Liu, F.; Liu, C.; Zheng, B.; He, J.; Liu, J.; Chen, C.; Lee, I.S.; Wang, X.; Liu, Y. Synergistic effects on incorporation of β-tricalcium phosphate and graphene oxide nanoparticles to silk fibroin/soy protein isolate scaffolds for bone tissue engineering. Polymers 2020, 12, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, W.J.; Cha, S.H. Improvement of mechanical and self-healing properties for polymethacrylate derivatives containing maleimide modified graphene oxide. Polymers 2020, 12, 603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabater i Serra, R.; Molina-Mateo, J.; Torregrosa-Cabanilles, C.; Andrio-Balado, A.; Dueñas, J.M.M.; Serrano-Aroca, Á. Bio-nanocomposite hydrogel based on zinc alginate/graphene oxide: Morphology, structural conformation, thermal behavior/degradation, and dielectric properties. Polymers 2020, 12, 702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cobos, M.; De-La-Pinta, I.; Quindós, G.; Fernández, M.J.; Fernández, M.D. Synthesis, Physical, mechanical and antibacterial properties of nanocomposites based on poly(vinyl alcohol)/ graphene oxide–silver nanoparticles. Polymers 2020, 12, 723. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Song, B.; Zhang, S.; Zhang, F.; Liu, C.; Zhang, N.; Yao, H.; Shi, Y. Using 3-isocyanatopropy ltrimethoxysilane to decorate graphene oxide with nano-titanium dioxide for enhancing the anti-corrosion properties of epoxy coating. Polymers 2020, 12, 837. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.S.; Yu, J.; Shim, S.E.; Yang, C.M. Synergistic effects of hybrid carbonaceous fillers of carbon fibers and reduced graphene oxides on enhanced heat-dissipation capability of polymer composites. Polymers 2020, 12, 909. [Google Scholar] [CrossRef]
- Broda, J.; Fabia, J.; Bączek, M.; Ślusarczyk, C. Supramolecular structure of polypropylene fibers extruded with addition of functionalized reduced graphene oxide. Polymers 2020, 12, 910. [Google Scholar] [CrossRef]
- Kukulski, T.; Wacławek, S.; Silvestri, D.; Krawczyk, K.; Padil, V.V.T.; Fryczkowski, R.; Janicki, J.; Černík, M. A Polymeric composite material (rGO/PANI) for acid blue 129 adsorption. Polymers 2020, 12, 1051. [Google Scholar] [CrossRef]
- Hasan, K.M.F.; Horváth, P.G.; Alpár, T. Potential natural fiber polymeric nanobiocomposites: A review. Polymers 2020, 12, 1072. [Google Scholar] [CrossRef]
- Ma, G.; Qi, J.; Cui, Q.; Bao, X.; Gao, D.; Xing, C. Graphene oxide composite for selective recognition, capturing, photothermal killing of bacteria over mammalian cells. Polymers 2020, 12, 1116. [Google Scholar] [CrossRef] [PubMed]
- Aldoasri, M.A.; Alsaud, K.B.B.; Othman, A.; Al-Hindawi, M.; Faisal, N.H.; Ahmed, R.; Michael, F.M.; Krishnan, M.R.; Alsharaeh, E. Microwave irradiation synthesis and characterization of reduced-(graphene oxide-(polystyrene-polymethyl methacrylate))/silver nanoparticle- nanocom posites and their anti-microbial activity. Polymers 2020, 12, 1155. [Google Scholar] [CrossRef] [PubMed]
- Sahu, G.; Das, M.; Yadav, M.; Sahoo, B.P.; Tripathy, J. Dielectric relaxation behavior of silver nanoparticles and graphene oxide embedded poly(vinyl alcohol) nanocomposite film: An effect of ionic liquid and temperature. Polymers 2020, 12, 374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campaña, A.L.; Sotelo, D.C.; Oliva, H.A.; Aranguren, A.; Ornelas-Soto, N.; Cruz, J.C.; Osma, J.F. Fabrication and characterization of a low-cost microfluidic system for the manufacture of alginate–lacasse microcapsules. Polymers 2020, 12, 1158. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.H.; Kammer, H.W. Low frequency dielectric relaxation and conductance of solid polymer electrolytes with PEO and Blends of PEO and PMMA. Polymers 2020, 12, 1009. [Google Scholar] [CrossRef]
- Murali, A.; Sampath, S.; Appukutti Achuthan, B.; Sakar, M.; Chandrasekaran, S.; Suthanthira Vanitha, N.; Joseph Bensingh, R.; Abdul Kader, M.; Jaisankar, S.N. Copper (0) mediated single electron transfer-living radical polymerization of methyl methacrylate: Functionalized graphene as a convenient tool for radical initiator. Polymers 2020, 12, 874. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Najafinezhad, A.; Hamzah, E.; Ismail, A.F.; Berto, F.; Chen, X. Clinoenstatite/tantalum coating for enhancement of biocompatibility and corrosion protection of Mg alloy. J. Funct. Biomater. 2020, 11, 26. [Google Scholar] [CrossRef] [Green Version]
- Pahlevanzadeh, F.; Emadi, R.; Valiani, A.; Kharaziha, M.; Poursamar, S.A.; Bakhsheshi-Rad, H.R.; Ismail, A.F.; RamaKrishna, S.; Berto, F. Three-dimensional printing constructs based on the chitosan for tissue regeneration: State of the art, developing directions and prospect trends. Materials 2020, 13, 2663. [Google Scholar] [CrossRef]
- Parham, S.; Kharazi, A.Z.; Bakhsheshi-Rad, H.R.; Ghayour, H.; Ismail, A.F.; Nur, H.; Berto, F. Electrospun nano-fibers for biomedical and tissue engineering applications: A comprehensive review. Materials 2020, 13, 2153. [Google Scholar] [CrossRef]
- Attar, K.; Demey, H.; Bouazza, D.; Sastre, A.M. Sorption and desorption studies of Pb(II) and Ni(II) from aqueous solutions by a new composite based on alginate and magadiite materials. Polymers 2019, 11, 340. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; Yuan, Q.; Fu, Y.; Liu, W.; Su, Y.-H.; Liu, H.; Wu, C.-Y.; Zhao, L.; Zhang, Q.; Lin, D.-R.; et al. Extraction optimization and effects of extraction methods on the chemical structures and antioxidant activities of polysaccharides from snow chrysanthemum (coreopsis tinctoria). Polymers 2019, 11, 215. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Hai, T.; Feng, Z.; Yu, D.-G.; Yang, Y.; Annie Bligh, S. The relationships between the working fluids, process characteristics and products from the modified coaxial electrospinning of zein. Polymers 2019, 11, 1287. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Sample | Compressive Strength (MPa) | Compressive Modulus (GPa) | Bending Strength (MPa) | Bending Modulus (GPa) | Fracture Toughness (KIC) (MPa.m1/2) | Ref. |
|---|---|---|---|---|---|---|
| PMMA/MWCNTs (with 2 wt % MWCNTs) | - | - | 90.6 ± 3.2 | 3.52 | 1.23 ± 0.22 | [77] |
| PMMA-PMMA/CNTs (17/3 g/g)(in PMMA/CNTs ratio was 100/0.2 g/g) | 130.16 ± 3.83 | - | - | - | - | [79] |
| PMMA/functionalized MWCNTs (with 0.1 wt % MWCNTs) | 62.24 ± 3.60 | 3.21 | 68.48 ± 9.39 | 3.26 | 1.49 ± 0.12 | [80] |
| PMMA/MWCNTs (0.1 wt % carboxyl functionalized MWCNTs added to MMA pre-polymerization) | 86.5 ± 6.6 | 4.8 | 68.8 ± 5.6 | 3.5 | 1.5 | [81] |
| PMMA/MWCNTs-COOH (with 0.1 wt % MWCNTs) | 104.47 ± 6.05 | 3.67 | 102.88 ± 19.26 | 6.12 | 3.07± 0.39 | [84] |
| PMMA/MWCNTs (with 0.6 wt % MWCNTs) Ultrasonic disintegration | 92.9 ± 2.2 | - | 74.1 ± 2.2 | - | - | [85] |
| PMMA/SWCNTs (with 0.15 wt % SWCNTs) | - | 4.58 | - | - | - | [88] |
| PMMA-CS/MWCNTs (with 25 wt % CS/MWCNTs containing 2.5 wt % CS and 0.5 wt % MWCNTs) | 127.33 ± 7.41 | 1.67 | 107.80 ± 2.30 | 5.7 | - | [90] |
| PMMA/GO (with 0.1 wt % of GO) | 120.7 ± 16.1 | - | 66.4 ± 6.50 | 3.29 | 2.17 ± 0.11 | [95] |
| PMMA/ GO (with 0.1 wt % GO) | 81 | - | 57 | - | 1.53 ± 0.07 | [96] |
| PMMA/GO (with 0.048 wt % of GO) | - | - | 87.0 ± 7.2 | - | - | [97] |
| PMMA/GO (with 0.05 wt % of GO corresponding to MMA monomer) | 90.94 | 4.4 | - | - | - | [98] |
| PMMA-PCL-FA-GO | 138 ± 7 | 0.5 | - | - | - | [100] |
| PMMA-CS/GO (with 25 wt % CS/GO containing 2 and 0.3 wt % CS and GO respectively) | 93.0 ± 4.0 | 1.21 | 79.9 ± 1.8 | - | - | [101] |
| PMMA/CS/GO (with 15 wt % of CS and 0.3% of GO) | 77 | - | - | - | - | [102] |
| Sample | T Max (°C) | Setting Time (sec) | Ref. |
|---|---|---|---|
| PMMA/ functionalized MWCNTs (with 0.1 wt % MWCNTs) | 68.53 ± 6 | 1712 ± 7 | [80] |
| PMMA/MWCNTs (with 1 wt % MWCNTs) | ≈57 | ≈1100 | [83] |
| PMMA/ MWCNTs-COOH (with 0.1 wt % MWCNTs) | 70.50 ± 6.87 | 694.2 ± 16.74 | [84] |
| PMMA-CS/MWCNTs (with 25 wt % CS/MWCNTs containing 2.5 wt % CS and 0.5 wt % MWCNTs) | 58.10 ± 2.77 | 970.2 ± 48 | [90] |
| PMMA/GO (with 0.1 wt % of GO) | 79 | 1080 | [95] |
| PMMA-CS/GO (with 25 wt % CS/GO containing 2 and 0.3 wt % CS and GO respectively) | 62.3 ± 1.9 | 768 ± 20.4 | [101] |
| PMMA/AG | 38 ± 1 | 1320 ± 120 | [104] |
| Sample | Cellular Assay | Cell Type | Target Tissue | Application | Ref. |
|---|---|---|---|---|---|
| PMMA/MWCNTs | MG-63 osteoblastic cells successfully adhered to and proliferated on the surfaces of all MWCNTs–PMMA cement | Osteoblast-like MG-63 cells | Bone | In vitro | [82] |
| PMMA-CS/MWCNTs (with 25 wt % CS/MWCNTs containing 2.5 wt % CS and 0.5 wt % MWCNTs) | The activity of the osteocyte cells leads to the formation of the ECM. | Human osteosarcoma cell line MG-63 | Bone | In vitro | [90] |
| PMMA/MWCNTs | Promoted cell adhesion, induced osteogenic differentiation, Promoted osseointegration, | Bone marrow-derived mesenchymal stem cells (rBMSCs) | Bone | In vitro | [91] |
| PMMA/ HA (67 wt %)/GO (0.5 wt/wt %) | Induce calcium phosphate high cell viability, low apoptosis, and extensive spread on disc surfaces | L929 fibroblasts and human Saos-2 osteoblasts | Bone | In vitro | [92] |
| PMMA/HA (67 wt %)/functionalized CNTs (0.1 wt/wt %) | Induce calcium phosphate high cell viability, low apoptosis, and extensive spread on disc surfaces | L929 fibroblasts and human Saos-2 osteoblasts | Bone | In vitro | [92] |
| PMMA- HA/GO | Adhere and then grow on all these surfaces by high cell viability | L929 fibroblasts and human Saos-2 osteoblasts | Bone | In vitro | [99] |
| PMMA-PCL-FA-GO | The viability of MG-63 osteoblast cells enhanced after the use of GO and FA in the PMMA-PCL polymer BC | MG-63 osteoblast cells | Bone | In vitro | [100] |
| PMMA-CS/GO (with 25 wt % CS/GO containing 2 and 0.3 wt % CS and GO respectively) | The improvement of cell viability, growth, and cell adhesion | Human osteosarcoma cell line MG-63 | Bone | In vitro | [101] |
| PMMA/AG | The BC-AG has offered very conducive microenvironment to the surrounding cells for proper growth and proliferation to rapid mineral secretion | - | Bone | In vivo | [104] |
| PMMA/G (0.1 wt % G) | Did not invoke a cytotoxic response, thereby demonstrating an adequate level of biocompatibility | Osteoblast precursor cell line (MC3-T3) | Bone | In vitro | [105] |
| PMMA/GO (0.1 wt % GO) | Did not invoke a cytotoxic response, thereby demonstrating an adequate level of biocompatibility | Osteoblast precursor cell line (MC3-T3) | Bone | In vitro | [105] |
| CNTs and GO Based Materials Features | Antibacterial Evaluation | |||||||
|---|---|---|---|---|---|---|---|---|
| Type | Material | Fabrication Method | Concentration (µg/mL) | Bacterial Strains | Incubation Time (h) | Method | Inhibition | Ref. |
| Nanowalls | GO | Hummers and Offeman | - | E. coli | 24 | PC | 59% | [110] |
| S. aureus | 74% | |||||||
| Nanosheets | GO | Hummers and Offeman | 175 | P. aeruginosa | 2 | PC | 100% | [111] |
| Nanopowder | GO | Hummers and Offeman | 40 | E. coli | 2 | PC | 69.30% | [113] |
| Nanosheets | GO | Hummers and Offeman | 100 | E. coli | 2 | PC | 17% | [114] |
| Nanosheets | GO | Hummers and Offeman | 100 | E.coli | 3 | PC | 100% | [115] |
| 125 | S.iniae | |||||||
| Nanosheets | GO | Hummers and Offeman | 85 | E. coli | 2 | PC | 98.50% | [116] |
| Nanosheets | GO | Hummers and Offeman | 40 | E. coli | 2 | PC | 97.70% | [117] |
| Nanosheets | GO | Modified Hummers’procedure | 100 | E. coli | 2.5 | TEM | - | [118] |
| SWCNTs | CNTs | Electric arc discharge | 100 | P. aeruginosa and S. aureus | 24 | PC | 50–60% and 70% | [119] |
| DWCNTs | CNTs | Catalytic Chemical Vapour Deposition | ||||||
| MWCNTs | CNTs | Catalyst-assisted chemical vapor deposition | ||||||
| SWCNTs | CNTs | CO disproportionation | 5 | E. coli | 1 | PC | 86.80% | [120] |
| SWCNTs | CNTs | CO incorporated MCM-41 | 5 | E. coli | 1 | PC | 80.10% | [121] |
| MWCNTs | CNTs | Chemical Vapour Deposition method | 5 | E. coli | 1 | PC | 24.40% | |
| SWCNTs | CNTs | CO decomposition | 1/70 CNT/polymer(PLGA) | S. epidermidis | 0.5 | PC | 98% | [122] |
| MWCNTs | CNTs | NanoLab productions | 500 | S.typhimurium, B. subtilis, S. aureus | 1 | PC | minor | [123] |
| SWCNTs | CNTs | NanoLab productions | 200–250 | −7 log | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soleymani Eil Bakhtiari, S.; Bakhsheshi-Rad, H.R.; Karbasi, S.; Tavakoli, M.; Razzaghi, M.; Ismail, A.F.; RamaKrishna, S.; Berto, F. Polymethyl Methacrylate-Based Bone Cements Containing Carbon Nanotubes and Graphene Oxide: An Overview of Physical, Mechanical, and Biological Properties. Polymers 2020, 12, 1469. https://doi.org/10.3390/polym12071469
Soleymani Eil Bakhtiari S, Bakhsheshi-Rad HR, Karbasi S, Tavakoli M, Razzaghi M, Ismail AF, RamaKrishna S, Berto F. Polymethyl Methacrylate-Based Bone Cements Containing Carbon Nanotubes and Graphene Oxide: An Overview of Physical, Mechanical, and Biological Properties. Polymers. 2020; 12(7):1469. https://doi.org/10.3390/polym12071469
Chicago/Turabian StyleSoleymani Eil Bakhtiari, Sanaz, Hamid Reza Bakhsheshi-Rad, Saeed Karbasi, Mohamadreza Tavakoli, Mahmood Razzaghi, Ahmad Fauzi Ismail, Seeram RamaKrishna, and Filippo Berto. 2020. "Polymethyl Methacrylate-Based Bone Cements Containing Carbon Nanotubes and Graphene Oxide: An Overview of Physical, Mechanical, and Biological Properties" Polymers 12, no. 7: 1469. https://doi.org/10.3390/polym12071469
APA StyleSoleymani Eil Bakhtiari, S., Bakhsheshi-Rad, H. R., Karbasi, S., Tavakoli, M., Razzaghi, M., Ismail, A. F., RamaKrishna, S., & Berto, F. (2020). Polymethyl Methacrylate-Based Bone Cements Containing Carbon Nanotubes and Graphene Oxide: An Overview of Physical, Mechanical, and Biological Properties. Polymers, 12(7), 1469. https://doi.org/10.3390/polym12071469