Association of Hydrophobic Carboxyl-Terminal Dendrimers with Lymph Node-Resident Lymphocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Carboxyl-Terminal Dendrimers
2.3. Characterization
2.4. Animals
2.5. Biodistribution
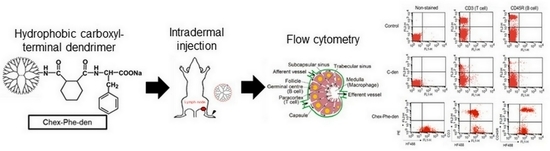
2.6. Flow Cytometry
2.7. Fluorescence Imaging
3. Results
3.1. Association of Hydrophobic Carboxyl-terminal Dendrimers with Immune Cells
3.2. Biodistribution of the Anionic Dendrimers via Intradermal Administration
3.3. Fluorescence Imaging of the Lymph Nodes in Mice Injected with Chex-Phe-den
3.4. Association of Chex-Phe-den with Lymph Node-Resident Lymphocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mellman, I.; Coukos, G.; Dranoff, G. Cancer immunotherapy comes of age. Nature 2011, 480, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Cochran, A.J.; Huang, R.-R.; Lee, J.; Itakura, E.; Leong, S.P.L.; Essner, R. Tumour-induced immune modulation of sentinel lymph nodes. Nat. Rev. Immunol. 2006, 6, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, R.; Sakakibara, M.; Nagashima, T.; Sangai, T.; Arai, M.; Fujimori, T.; Takano, S.; Shida, T.; Nakatani, Y.; Miyazaki, M. Accumulation of regulatory T cells in sentinel lymph nodes is a prognostic predictor in patients with node-negative breast cancer. Eur. J. Cancer 2009, 45, 2123–2131. [Google Scholar] [CrossRef] [PubMed]
- Xia, A.; Zhang, Y.; Xu, J.; Yin, T.; Lu, X.-J. T cell dysfunction in cancer immunity and immunotherapy. Front. Immunol. 2019, 10, 1719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmid, D.; Park, C.G.; Hartl, C.A.; Subedi, N.; Cartwright, A.N.; Puerto, R.B.; Zheng, Y.; Maiarana, J.; Freeman, G.J.; Wucherpfennig, K.W.; et al. T cell-targeting nanoparticles focus delivery of immunotherapy to improve antitumor immunity. Nat. Commun. 2017, 8, 1747. [Google Scholar] [CrossRef] [PubMed]
- Mignani, S.; Rodrigues, J.; Tomas, H.; Zablocka, M.; Shi, X.; Caminade, A.M.; Majoral, J.P. Dendrimers in combination with natural products and analogues as anticancer agents. Chem. Soc. Rev. 2018, 47, 514–532. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, M.; Dehghan, G.; Abedi-Gaballu, F.; Kashanian, S.; Baradaran, B.; Ezzati Nazhad Dolatabadi, J.; Losic, D. Surface functionalized dendrimers as controlled-release delivery nanosystems for tumor targeting. Eur. J. Pharm. Sci. 2018, 122, 311–330. [Google Scholar] [CrossRef] [PubMed]
- Sherje, A.P.; Jadhav, M.; Dravyakar, B.R.; Kadam, D. Dendrimers: A versatile nanocarrier for drug delivery and targeting. Int. J. Pharm. 2018, 548, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Niki, Y.; Ogawa, M.; Makiura, R.; Magata, Y.; Kojima, C. Optimization of dendrimer structure for sentinel lymph node imaging: Effects of generation and terminal group. Nanomed. NBM 2015, 11, 2119–2127. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, Y.; Nagashima, S.; Nakajima, K.; Ohira, T.; Sato, T.; Izawa, T.; Yamate, J.; Higashikawa, K.; Kuge, Y.; Ogawa, M.; et al. Carboxyl-, sulfonyl-, and phosphate-terminal dendrimers as a nanoplatform with lymph node targeting. Int. J. Pharm. 2020, 576, 119021. [Google Scholar] [CrossRef]
- Kaminskas, L.M.; Kota, J.; McLeod, V.M.; Kelly, B.D.; Karellas, P.; Porter, C.J. PEGylation of polylysine dendrimers improves absorption and lymphatic targeting following SC administration in rats. J. Control. Release 2009, 140, 108–116. [Google Scholar] [CrossRef]
- Kaminskas, L.M.; Porter, C.J. Targeting the lymphatics using dendritic polymers (dendrimers). Adv. Drug Deliv. Rev. 2011, 63, 890–900. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Kawamoto, S.; Choyke, P.L.; Sato, N.; Knopp, M.V.; Star, R.A.; Waldmann, T.A.; Tagaya, Y.; Brechbiel, M.W. Comparison of dendrimer-based macromolecular contrast agents for dynamic micro-magnetic resonance lymphangiography. Magn. Reson. Med. 2003, 50, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Kawamoto, S.; Bernardo, M.; Brechbiel, M.W.; Knopp, M.V.; Choyke, P.L. Delivery of gadolinium-labeled nanoparticles to the sentinel lymph node: Comparison of the sentinel node visualization and estimations of intra-nodal gadolinium concentration by the magnetic resonance imaging. J. Control. Release 2006, 111, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Moyano, D.F.; Goldsmith, M.; Solfiell, D.J.; Landesman-Milo, D.; Miranda, O.R.; Peer, D.; Rotello, V.M. Nanoparticle hydrophobicity dictates immune response. J. Am. Chem. Soc. 2012, 134, 3965–3967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shima, F.; Akagi, T.; Akashi, M. Effect of hydrophobic side chains in the induction of immune responses by nanoparticle adjuvants consisting of amphiphilic poly(γ-glutamic acid). Bioconjugate Chem. 2015, 26, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, M.; Fukushima, D.; Kojima, C. Dual pH-sensitive and UCST-type thermosensitive dendrimers: Phenylalanine-modified polyamidoamine dendrimers with carboxyl termini. RSC Adv. 2018, 8, 28147–28151. [Google Scholar] [CrossRef] [Green Version]
- Ghose, A.K.; Crippen, G.M. Atomic physicochemical parameters for three-dimensional-structure-directed quantitative structure-activity relationships. 2. Modeling dispersive and hydrophobic interactions. J. Chem. Inf. Comput. Sci. 1987, 27, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Takayama, K.; Hirose, H.; Tanaka, G.; Pujals, S.; Katayama, S.; Nakase, I.; Futaki, S. Effect of the attachment of a penetration accelerating sequence and the influence of hydrophobicity on octaarginine-mediated intracellular delivery. Mol. Pharm. 2012, 9, 1222–1230. [Google Scholar] [CrossRef] [PubMed]





| Dendrimer | Bound Ratio | ζ-Potential (mV) | LogP | Double Positive Cells | |||
|---|---|---|---|---|---|---|---|
| Suc | Chex | Phe | T Cells | B Cells | |||
| C-den | ~100% | − | − | −21.0 ± 0.9 | −1.29 | 0.74% ± 0.06 | 1.05% ± 0.10 |
| C-Phe-den | ~100% | − | ~100% | −24.2 ± 1.6 | −0.27 | 1.36% ± 0.08 | 3.89% ± 0.09 |
| Chex-den | − | 100% | − | −22.6 ± 2.2 | 0.18 | 1.31% ± 0.06 | 2.20% ± 0.26 |
| Chex-Phe-den | − | 100% | 91% | −19.1 ± 0.2 | 1.20 | 20.03% ± 0.57 (4.87% ± 0.35) * | 23.81% ± 0.91 (12.09% ± 0.41) * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishimoto, Y.; Nishio, M.; Nagashima, S.; Nakajima, K.; Ohira, T.; Nakai, S.; Nakase, I.; Higashikawa, K.; Kuge, Y.; Matsumoto, A.; et al. Association of Hydrophobic Carboxyl-Terminal Dendrimers with Lymph Node-Resident Lymphocytes. Polymers 2020, 12, 1474. https://doi.org/10.3390/polym12071474
Nishimoto Y, Nishio M, Nagashima S, Nakajima K, Ohira T, Nakai S, Nakase I, Higashikawa K, Kuge Y, Matsumoto A, et al. Association of Hydrophobic Carboxyl-Terminal Dendrimers with Lymph Node-Resident Lymphocytes. Polymers. 2020; 12(7):1474. https://doi.org/10.3390/polym12071474
Chicago/Turabian StyleNishimoto, Yutaka, Misaki Nishio, Shu Nagashima, Kohei Nakajima, Takayuki Ohira, Shinya Nakai, Ikuhiko Nakase, Kei Higashikawa, Yuji Kuge, Akikazu Matsumoto, and et al. 2020. "Association of Hydrophobic Carboxyl-Terminal Dendrimers with Lymph Node-Resident Lymphocytes" Polymers 12, no. 7: 1474. https://doi.org/10.3390/polym12071474
APA StyleNishimoto, Y., Nishio, M., Nagashima, S., Nakajima, K., Ohira, T., Nakai, S., Nakase, I., Higashikawa, K., Kuge, Y., Matsumoto, A., Ogawa, M., & Kojima, C. (2020). Association of Hydrophobic Carboxyl-Terminal Dendrimers with Lymph Node-Resident Lymphocytes. Polymers, 12(7), 1474. https://doi.org/10.3390/polym12071474