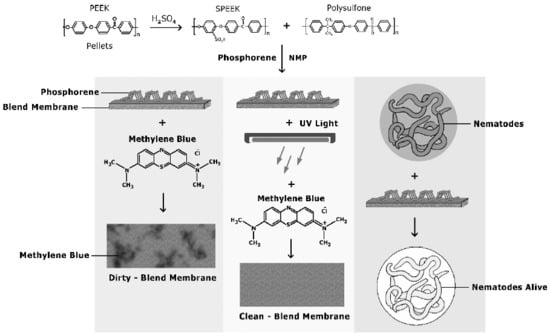
Nanohybrid Membrane Synthesis with Phosphorene Nanoparticles: A Study of the Addition, Stability and Toxicity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sulfonation of PEEK and Determination of Degree of Sulfonation
2.3. Fourier-Transform Infrared Spectroscopy (FTIR)
2.4. Exfoliation of Bulk Black Phosphorus
2.5. Transmission Electron Microscopy (TEM) and HAADF–STEM
2.6. Optical Profilometer
2.7. Electrokinetic Potential Measurement
2.8. Contact Angle Measurement
2.9. Leaching Studies
2.10. Inductively Coupled Plasma-Optical Emission Spectrometry (ICP-OES) Study
2.11. Morphologic Characterization of Membranes Using X-ray Photoelectron Spectroscopy (XPS) and Scanning Electron Microscope (SEM)
2.12. Membrane Synthesis
2.13. Flux Analysis
2.14. Toxicity Testing
3. Results and Discussions
3.1. Degree of Sulfonation and Membrane Fabrication
3.2. Structural Membrane Polymer Evolution
3.3. TEM Analysis
3.4. Morphologic Characterization of Membranes
3.5. Phosphorene Leaching
3.6. Pore Structure Comparison
3.7. Phosphorene Distribution on the Membrane
3.8. Flux Discussion
3.9. Operational Performance of Phosphorene Membranes
3.10. Toxicity of Phosphorene
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Warsinger, D.M.; Chakraborty, S.; Tow, E.W.; Plumlee, M.H.; Bellona, C.; Loutatidou, S.; Karimi, L.; Mikelonis, A.M.; Achilli, A.; Ghassemi, A. A review of polymeric membranes and processes for potable water reuse. Prog. Polym. Sci. 2018, 81, 209–237. [Google Scholar] [CrossRef] [PubMed]
- Arumugham, T.; Amimodu, R.G.; Kaleekkal, N.J.; Rana, D. Nano cuo/g-c3n4 sheets-based ultrafiltration membrane with enhanced interfacial affinity, antifouling and protein separation performances for water treatment application. J. Environ. Sci. 2019, 82, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.-T.; Kook, S.; Lee, C.; Field, R.W.; Kim, I.S. Critical flux-based membrane fouling control of forward osmosis: Behavior, sustainability, and reversibility. J. Membr. Sci. 2019, 570, 380–393. [Google Scholar] [CrossRef] [Green Version]
- Xu, P.; Drewes, J.E.; Kim, T.-U.; Bellona, C.; Amy, G. Effect of membrane fouling on transport of organic contaminants in NF/RO membrane applications. J. Membr. Sci. 2006, 279, 165–175. [Google Scholar] [CrossRef]
- Sun, M.; Zucker, I.; Davenport, D.M.; Zhou, X.; Qu, J.; Elimelech, M. Reactive, self-cleaning ultrafiltration membrane functionalized with iron oxychloride nanocatalysts. Environ. Sci. Technol. 2018, 52, 8674–8683. [Google Scholar] [CrossRef] [PubMed]
- Eke, J.; Elder, K.; Escobar, I.C. Self-cleaning nanocomposite membranes with phosphorene-based pore fillers for water treatment. Membranes 2018, 8, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.; Hou, J.; Uliana, A.; Zhang, Y.; Tian, M.; Van der Bruggen, B. The rapid emergence of two-dimensional nanomaterials for high-performance separation membranes. J. Mater. Chem. A 2018, 6, 3773–3792. [Google Scholar] [CrossRef]
- Mas-Balleste, R.; Gomez-Navarro, C.; Gomez-Herrero, J.; Zamora, F. 2D materials: To graphene and beyond. Nanoscale 2011, 3, 20–30. [Google Scholar] [CrossRef]
- Li, L.; Yu, Y.; Ye, G.J.; Ge, Q.; Ou, X.; Wu, H.; Feng, D.; Chen, X.H.; Zhang, Y. Black phosphorus field-effect transistors. Nat. Nanotechnol. 2014, 9, 372. [Google Scholar] [CrossRef] [Green Version]
- Sadki, S.; Drissi, L.B. Tunable optical and excitonic properties of phosphorene via oxidation. J. Phys. Condens. Matter 2018, 30, 255703. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Lin, L.; Zhang, R.; Yang, C.; Yang, J. Highly efficient photocatalytic water splitting over edge-modified phosphorene nanoribbons. J. Am. Chem. Soc. 2017, 139, 15429–15436. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Sa, B.; Xu, C.; Zhan, H.; Anpo, M.; Sun, Z. Enhanced photocatalytic performance of black phosphorene by isoelectronic co-dopants. Inorg. Chem. Front. 2019, 6, 2369–2378. [Google Scholar] [CrossRef]
- Pei, J.; Gai, X.; Yang, J.; Wang, X.; Yu, Z.; Choi, D.-Y.; Luther-Davies, B.; Lu, Y. Producing air-stable monolayers of phosphorene and their defect engineering. Nat. Commun. 2016, 7, 10450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Neal, A.T.; Zhu, Z.; Luo, Z.; Xu, X.; Tománek, D.; Ye, P.D. Phosphorene: An unexplored 2D semiconductor with a high hole mobility. ACS Nano 2014, 8, 4033–4041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reich, E.S. Phosphorene excites materials scientists. Nature 2014, 506, 19. [Google Scholar] [CrossRef]
- Fonsaca, J.E.; Domingues, S.H.; Orth, E.S.; Zarbin, A.J. Air stable black phosphorous in polyaniline-based nanocomposite. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Peruzzini, M.; Bini, R.; Bolognesi, M.; Caporali, M.; Ceppatelli, M.; Cicogna, F.; Coiai, S.; Heun, S.; Ienco, A.; Benito, I.I. A perspective on recent advances in Phosphorene functionalization and its applications in devices. Eur. J. Inorg. Chem. 2019, 2019, 1476–1494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, H.; Lu, N.; Dai, J.; Wu, X.; Zeng, X.C. Phosphorene nanoribbons, phosphorus nanotubes, and van der Waals multilayers. J. Phys. Chem. C 2014, 118, 14051–14059. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.Z.; Kwong, C.W.; Davey, K.; Qiao, S.Z. 2D phosphorene as a water splitting photocatalyst: Fundamentals to applications. Energy Environ. Sci. 2016, 9, 709–728. [Google Scholar] [CrossRef]
- Lin, Y.-J.; Cao, W.-Z.; Ou Yang, T.; Feng, C.-H.; Chang, C.-T. Deciphering the effect of citric acid on arsenic adsorption with phosphorene in aqueous solution. Sustain. Environ. Res. 2019, 29, 22. [Google Scholar] [CrossRef] [Green Version]
- Walia, S.; Balendhran, S.; Ahmed, T.; Singh, M.; El-Badawi, C.; Brennan, M.D.; Weerathunge, P.; Karim, M.N.; Rahman, F.; Rassell, A. Ambient protection of few-layer black phosphorus via sequestration of reactive oxygen species. Adv. Mater. 2017, 29, 1700152. [Google Scholar] [CrossRef] [PubMed]
- Ryder, C.R.; Wood, J.D.; Wells, S.A.; Yang, Y.; Jariwala, D.; Marks, T.J.; Schatz, G.C.; Hersam, M.C. Covalent functionalization and passivation of exfoliated black phosphorus via aryl diazonium chemistry. Nat. Chem. 2016, 8, 597. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Zhou, Y.; Zhou, X.; Zhang, T.; Wang, C.; Yuen, R.K.; Hu, W.; Hu, Y. Air-Stable Polyphosphazene-Functionalized Few-Layer Black Phosphorene for Flame Retardancy of Epoxy Resins. Small 2019, 15, 1805175. [Google Scholar] [CrossRef] [PubMed]
- Zoubeik, M.; Ismail, M.; Salama, A.; Henni, A. New developments in membrane technologies used in the treatment of produced water: A review. Arab. J. Sci. Eng. 2018, 43, 2093–2118. [Google Scholar] [CrossRef]
- Polte, J. Fundamental growth principles of colloidal metal nanoparticles–a new perspective. CrystEngComm 2015, 17, 6809–6830. [Google Scholar] [CrossRef] [Green Version]
- Esfahani, M.R.; Aktij, S.A.; Dabaghian, Z.; Firouzjaei, M.D.; Rahimpour, A.; Eke, J.; Escobar, I.C.; Abolhassani, M.; Greenlee, L.F.; Esfahani, A.R. Nanocomposite membranes for water separation and purification: Fabrication, modification, and applications. Sep. Purif. Technol. 2018, 213, 465–499. [Google Scholar] [CrossRef]
- Israelachvili, J.N. Intermolecular and Surface Forces; Academic Press: Waltham, MA, USA, 2015. [Google Scholar]
- Rabkin, E.; Gottstein, G.; Shvindlerman, L. Why do crystalline nanoparticles agglomerate with low misorientations? Scr. Mater. 2011, 65, 1101–1104. [Google Scholar] [CrossRef]
- Petosa, A.R.; Jaisi, D.P.; Quevedo, I.R.; Elimelech, M.; Tufenkji, N. Aggregation and deposition of engineered nanomaterials in aquatic environments: Role of physicochemical interactions. Environ. Sci. Technol. 2010, 44, 6532–6549. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Ralston, J.; Sedev, R.; Beattie, D.A. Functionalized gold nanoparticles: Synthesis, structure and colloid stability. J. Colloid Interface Sci. 2009, 331, 251–262. [Google Scholar] [CrossRef]
- Viota, J.; De Vicente, J.; Duran, J.; Delgado, A. Stabilization of magnetorheological suspensions by polyacrylic acid polymers. J. Colloid Interface Sci. 2005, 284, 527–541. [Google Scholar] [CrossRef]
- Lyklema, J. Fundamentals of Interface and Colloid Science: Particulate Colloids; Elsevier: San Diego, CA, USA, 2005. [Google Scholar]
- Latiff, N.M.; Teo, W.Z.; Sofer, Z.; Fisher, A.C.; Pumera, M. The cytotoxicity of layered black phosphorus. Chem. A Eur. J. 2015, 21, 13991–13995. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Zhang, X.; Zhang, S.; Lei, L.; Ma, W.; Li, D.; Wang, W.; Zhao, Q.; Xing, B. Bacterial toxicity of exfoliated black phosphorus nanosheets. Ecotoxicol. Environ. Saf. 2018, 161, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.R.; Yong, K.W.; Choi, J.Y.; Nilghaz, A.; Lin, Y.; Xu, J.; Lu, X. Black phosphorus and its biomedical applications. Theranostics 2018, 8, 1005. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Wang, J.-Y.; Bai, X.; Xu, F.; Liu, H.; Yang, J.; Jing, Y.; Liu, L.; Xue, X.; Dai, H. Black phosphorus quantum dot induced oxidative stress and toxicity in living cells and mice. ACS Appl. Mater. Interfaces 2017, 9, 20399–20409. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, Y.; Huynh, T.; Yang, Y.; Yang, X.; Zhou, R. Directional Extraction and Penetration of Phosphorene Nanosheets to Cell Membranes. Nanoscale 2020, 4, 2810–2819. [Google Scholar] [CrossRef]
- Choi, J.; Tsyusko, O.V.; Unrine, J.M.; Chatterjee, N.; Ahn, J.-M.; Yang, X.; Thornton, B.L.; Ryde, I.T.; Starnes, D.; Meyer, J.N. A micro-sized model for the in vivo study of nanoparticle toxicity: What has Caenorhabditis elegans taught us? Environ. Chem. 2014, 11, 227–246. [Google Scholar] [CrossRef] [Green Version]
- Starnes, D.; Unrine, J.; Chen, C.; Lichtenberg, S.; Starnes, C.; Svendsen, C.; Kille, P.; Morgan, J.; Baddar, Z.E.; Spear, A. Toxicogenomic responses of Caenorhabditis elegans to pristine and transformed zinc oxide nanoparticles. Environ. Pollut. 2019, 247, 917–926. [Google Scholar] [CrossRef]
- Sang, D.K.; Wang, H.; Guo, Z.; Xie, N.; Zhang, H. Recent Developments in Stability and Passivation Techniques of Phosphorene toward Next-Generation Device Applications. Adv. Funct. Mater. 2019, 29, 1903419. [Google Scholar] [CrossRef]
- Dinh, K.N.; Zhang, Y.; Zhu, J.; Sun, W. Phosphorene-based Electrocatalysts. Chem. A Eur. J. 2020. [Google Scholar] [CrossRef]
- Qu, Z.; Wu, K.; Meng, W.; Nan, B.; Hu, Z.; Xu, C.-a.; Tan, Z.; Zhang, Q.; Meng, H.; Shi, J. Surface Coordination of Black Phosphorene for Excellent Stability, Flame Retardancy and Thermal Conductivity in Epoxy Resin. Chem. Eng. J. 2020, 125416. [Google Scholar] [CrossRef]
- Li, H.; Lian, P.; Lu, Q.; Chen, J.; Hou, R.; Mei, Y. Excellent air and water stability of two-dimensional black phosphorene/MXene heterostructure. Mater. Res. Express 2019, 6, 065504. [Google Scholar] [CrossRef]
- Paik, Y.; Chae, S.A.; Han, O.H.; Hwang, S.Y.; Ha, H.Y. Influence of water and degree of sulfonation on the structure and dynamics of SPEEK studied by solid-state 13C and 1H NMR. Polymer 2009, 50, 2664–2673. [Google Scholar] [CrossRef]
- Jin, X.; Bishop, M.T.; Ellis, T.S.; Karasz, F.E. A sulphonated poly (aryl ether ketone). Br. Polym. J. 1985, 17, 4–10. [Google Scholar] [CrossRef]
- Ji, Y.; Tay, Z.Y.; Li, S.F.Y. Highly selective sulfonated poly (ether ether ketone)/titanium oxide composite membranes for vanadium redox flow batteries. J. Membr. Sci. 2017, 539, 197–205. [Google Scholar] [CrossRef]
- Zaidi, S.; Mikhailenko, S.; Robertson, G.P.; Guiver, M.D.; Kaliaguine, S. Proton Conducting Composite Membranes from Polyether Ether Ketone and Heteropolyacids for Fuel Cell Application. J. Membr. Sci. 2000, 173, 17–34. [Google Scholar] [CrossRef] [Green Version]
- Dole, M.N.; Patel, P.A.; Sawant, S.D.; Shedpure, P.S. Advance applications of Fourier transform infrared spectroscopy. Int. J. Pharm. Sci. Rev. Res. 2011, 7, 159–166. [Google Scholar]
- Amand, L.-E.; Tullin, C.J. The Theory behind FTIR Analysis; Department Of Energy Conversion, Chalmers University of Technology: Gothenburg, Sweden, 1999. [Google Scholar]
- Berthomieu, C.; Hienerwadel, R. Fourier transform infrared (FTIR) spectroscopy. Photosynth. Res. 2009, 101, 157–170. [Google Scholar] [CrossRef]
- Davis, R.; Mauer, L. Fourier transform infrared (FT-IR) spectroscopy: A rapid tool for detection and analysis of foodborne pathogenic bacteria. Curr. Res. Technol. Educ. Top. Appl. Microbiol. Microb. Biotechnol. 2010, 2, 1582–1594. [Google Scholar]
- Guo, Z.; Zhang, H.; Lu, S.; Wang, Z.; Tang, S.; Shao, J.; Sun, Z.; Xie, H.; Wang, H.; Yu, X.F. From black phosphorus to phosphorene: Basic solvent exfoliation, evolution of Raman scattering, and applications to ultrafast photonics. Adv. Funct. Mater. 2015, 25, 6996–7002. [Google Scholar] [CrossRef]
- Vrijenhoek, E.M.; Hong, S.; Elimelech, M. Influence of membrane surface properties on initial rate of colloidal fouling of reverse osmosis and nanofiltration membranes. J. Membr. Sci. 2001, 188, 115–128. [Google Scholar] [CrossRef]
- Kwak, S.-Y.; Yeom, M.-O.; Roh, I.J.; Kim, D.Y.; Kim, J.-J. Correlations of chemical structure, atomic force microscopy (AFM) morphology, and reverse osmosis (RO) characteristics in aromatic polyester high-flux RO membranes. J. Membr. Sci. 1997, 132, 183–191. [Google Scholar] [CrossRef]
- Koyuncu, I.; Brant, J.; Lüttge, A.; Wiesner, M.R. A comparison of vertical scanning interferometry (VSI) and atomic force microscopy (AFM) for characterizing membrane surface topography. J. Membr. Sci. 2006, 278, 410–417. [Google Scholar] [CrossRef]
- Boussu, K.; Van der Bruggen, B.; Volodin, A.; Snauwaert, J.; Van Haesendonck, C.; Vandecasteele, C. Roughness and hydrophobicity studies of nanofiltration membranes using different modes of AFM. J. Colloid Interface Sci. 2005, 286, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S. DLS and zeta potential–what they are and what they are not? J. Control Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Uskoković, V.; Castiglione, Z.; Cubas, P.; Zhu, L.; Li, W.; Habelitz, S. Zeta-potential and Particle Size Analysis of Human Amelogenins. J. Dent. Res. 2010, 89, 149–153. [Google Scholar] [CrossRef] [Green Version]
- Nägele, E.W. The transient zeta potential of hydrating cement. Chem. Eng. Sci. 1989, 44, 1637–1645. [Google Scholar] [CrossRef]
- Van der Bruggen, B.; Kim, J.H.; DiGiano, F.A.; Geens, J.; Vandecasteele, C. Influence of MF pretreatment on NF performance for aqueous solutions containing particles and an organic foulant. Sep. Purif. Technol. 2004, 36, 203–213. [Google Scholar] [CrossRef]
- Yuan, Y.; Lee, T.R. Contact angle and wetting properties. In Surface Science Techniques; Springer: Berlin/Heidelberg, Germany, 2013; pp. 3–34. [Google Scholar]
- Mannan, H.A.; Mukhtar, H.; Murugesan, T.; Nasir, R.; Mohshim, D.F.; Mushtaq, A. Recent applications of polymer blends in gas separation membranes. Chem. Eng. Technol. 2013, 36, 1838–1846. [Google Scholar] [CrossRef]
- Nunes, S.P.; Peinemann, K.V. Ultrafiltration membranes from PVDF/PMMA blends. J. Membr. Sci. 1992, 73, 25–35. [Google Scholar] [CrossRef]
- Bowen, W.R.; Doneva, T.A.; Yin, H. The effect of sulfonated poly (ether ether ketone) additives on membrane formation and performance. Desalination 2002, 145, 39–45. [Google Scholar] [CrossRef]
- Dong, X.; Shannon, H.D.; Amirsoleimani, A.; Brion, G.M.; Escobar, I.C. Thiol-Affinity Immobilization of Casein-Coated Silver Nanoparticles on Polymeric Membranes for Biofouling Control. Polymers 2019, 11, 2057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sprick, C.; Chede, S.; Oyanedel-Craver, V.; Escobar, I.C. Bio-inspired immobilization of casein-coated silver nanoparticles on cellulose acetate membranes for biofouling control. J. Environ. Chem. Eng. 2018, 6, 2480–2491. [Google Scholar] [CrossRef]
- Zereshki, S.; Figoli, A.; Madaeni, S.; Simone, S.; Esmailinezhad, M.; Drioli, E. Pervaporation separation of MeOH/MTBE mixtures with modified PEEK membrane: Effect of operating conditions. J. Membr. Sci. 2011, 371, 1–9. [Google Scholar] [CrossRef]
- Chung, T.S.; Teoh, S.K.; Hu, X. Formation of ultrathin high-performance polyethersulfone hollow-fiber membranes. J. Membr. Sci. 1997, 133, 161–175. [Google Scholar] [CrossRef]
- Wu, H.-L.; Ma, C.-C.M.; Li, C.-H.; Lee, T.-M.; Chen, C.-Y.; Chiang, C.-L.; Wu, C. Sulfonated poly (ether ether ketone)/poly (amide imide) polymer blends for proton conducting membrane. J. Membr. Sci. 2006, 280, 501–508. [Google Scholar] [CrossRef]
- Wu, H.L.; Ma, C.C.M.; Li, C.H.; Chen, C.Y. Swelling behavior and solubility parameter of sulfonated poly (ether ether ketone). J. Polym. Sci. Part B Polym. Phys. 2006, 44, 3128–3134. [Google Scholar] [CrossRef]
- Rajagopalan, G.; Immordino, K.M.; Gillespie, J.W., Jr.; McKnight, S.H. Diffusion and reaction of epoxy and amine in polysulfone studied using fourier transform infrared spectroscopy: Experimental results. Polymer 2000, 41, 2591–2602. [Google Scholar] [CrossRef]
- Dooher, T.; Dixon, D. Multiwalled carbon nanotube/polysulfone composites: Using the Hildebrand solubility parameter to predict dispersion. Polym. Compos. 2011, 32, 1895–1903. [Google Scholar] [CrossRef]
- Barton, A.F.J.C.R. Solubility parameters. Chem. Rev. 1975, 75, 731–753. [Google Scholar] [CrossRef]
- Weber, C.I. Methods for Measuring the Acute Toxicity of Effluents and Receiving Waters to Freshwater and Marine Organisms; Environmental Monitoring Systems Laboratory, Office of Research and Development, US Environmental Protection Agency: Washington, DC, USA, 1991. [Google Scholar]
- Tsyusko, O.V.; Unrine, J.M.; Spurgeon, D.; Blalock, E.; Starnes, D.; Tseng, M.; Joice, G.; Bertsch, P.M. Toxicogenomic responses of the model organism Caenorhabditis elegans to gold nanoparticles. Environ. Sci. Technol. 2012, 46, 4115–4124. [Google Scholar] [CrossRef]
- Starnes, D.L.; Unrine, J.M.; Starnes, C.P.; Collin, B.E.; Oostveen, E.K.; Ma, R.; Lowry, G.V.; Bertsch, P.M.; Tsyusko, O.V. Impact of sulfidation on the bioavailability and toxicity of silver nanoparticles to Caenorhabditis elegans. Environ. Pollut. 2015, 196, 239–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, P.L.; Dusenbery, D.B. Using the nematode Caenorhabditis elegans to predict mammalian acute lethality to metallic salts. Toxicol. Ind. Health 1988, 4, 469–478. [Google Scholar] [CrossRef]
- Huang, R.; Shao, P.; Burns, C.; Feng, X. Sulfonation of poly (ether ether ketone) (PEEK): Kinetic study and characterization. J. Appl. Polym. Sci. 2001, 82, 2651–2660. [Google Scholar] [CrossRef]
- Puro, L.; Mänttäri, M.; Pihlajamäki, A.; Nyström, M. Characterization of modified nanofiltration membranes by octanoic acid permeation and FTIR analysis. Chem. Eng. Res. Des. 2006, 84, 87–96. [Google Scholar] [CrossRef]
- Malik, R.S.; Verma, P.; Choudhary, V.J.E.A. A study of new anhydrous, conducting membranes based on composites of aprotic ionic liquid and cross-linked SPEEK for fuel cell application. Electrochim. Acta 2015, 152, 352–359. [Google Scholar] [CrossRef]
- Destainville, A.; Champion, E.; Bernache-Assollant, D.; Laborde, E. Synthesis, characterization and thermal behavior of apatitic tricalcium phosphate. Mater. Chem. Phys. 2003, 80, 269–277. [Google Scholar] [CrossRef]
- Berg, J.M.; Romoser, A.; Banerjee, N.; Zebda, R.; Sayes, C.M.J.N. The relationship between pH and zeta potential of~ 30 nm metal oxide nanoparticle suspensions relevant to in vitro toxicological evaluations. Nanotoxicology 2009, 3, 276–283. [Google Scholar] [CrossRef]
- Kumar, R.; Seetharamu, S.; Kamaraj, M.J.W. Quantitative evaluation of 3D surface roughness parameters during cavitation exposure of 16Cr–5Ni hydro turbine steel. Wear 2014, 320, 16–24. [Google Scholar] [CrossRef]
- Zhu, W.; Yogeesh, M.N.; Yang, S.; Aldave, S.H.; Kim, J.-S.; Sonde, S.; Tao, L.; Lu, N.; Akinwande, D. Flexible black phosphorus ambipolar transistors, circuits and AM demodulator. Nano Lett. 2015, 15, 1883–1890. [Google Scholar] [CrossRef]
- Dunphy Guzman, K.A.; Finnegan, M.P.; Banfield, J.F. Influence of surface potential on aggregation and transport of titania nanoparticles. Environ. Sci. Technol. 2006, 40, 7688–7693. [Google Scholar] [CrossRef] [PubMed]
- Pettibone, J.M.; Cwiertny, D.M.; Scherer, M.; Grassian, V.H. Adsorption of organic acids on TiO2 nanoparticles: Effects of pH, nanoparticle size, and nanoparticle aggregation. Langmuir 2008, 24, 6659–6667. [Google Scholar] [CrossRef] [PubMed]
- Van de Witte, P.; Dijkstra, P.; Van den Berg, J.; Feijen, J. Phase separation processes in polymer solutions in relation to membrane formation. J. Membr. Sci. 1996, 117, 1–31. [Google Scholar] [CrossRef] [Green Version]
- Dong, X.; Al-Jumaily, A.; Escobar, I.C. Investigation of the Use of a Bio-Derived Solvent for Non-Solvent-Induced Phase Separation (NIPS) Fabrication of Polysulfone Membranes. Membranes 2018, 8, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pagliero, M.; Bottino, A.; Comite, A.; Costa, C. Novel hydrophobic PVDF membranes prepared by nonsolvent induced phase separation for membrane distillation. J. Membr. Sci. 2020, 596, 117575. [Google Scholar] [CrossRef]
- Kaya, D.; Keçeci, K. Track-Etched Nanoporous Polymer Membranes as Sensors: A Review. J. Electrochem. Soc. 2020, 167, 037543. [Google Scholar] [CrossRef]
- Yu, L.; Kanezashi, M.; Nagasawa, H.; Tsuru, T. Phase inversion/sintering-induced porous ceramic microsheet membranes for high-quality separation of oily wastewater. J. Membr. Sci. 2020, 595, 117477. [Google Scholar] [CrossRef]
- Kimmerle, K.; Strathmann, H. Analysis of the structure-determining process of phase inversion membranes. Desalination 1990, 79, 283–302. [Google Scholar] [CrossRef]
- Frommer, M.A.; Lancet, D. The mechanism of membrane formation: Membrane structures and their relation to preparation conditions. In Reverse Osmosis Membrane Research; Springer: Boston, MA, USA, 1972; pp. 85–110. [Google Scholar]
- Fujii, Y.; Iwatani, H.; Kigoshi, S. Morphological structures of the membranes prepared from polymer solutions. Polym. J. 1992, 24, 737. [Google Scholar] [CrossRef]
- Sah, B.K.; Das, K.; Kundu, S. pH-dependent structure, pattern and hysteresis behaviour of lipid (DMPA)-protein (BSA) monolayer complex. Colloids Surf. A Physicochem. Eng. Asp. 2019, 579, 123663. [Google Scholar] [CrossRef]















| Number | Wavelength Number (cm−1) | Functional Group |
|---|---|---|
| 1 | 1230 | –O=S=O– |
| 2 | 1487 | C=C |
| 3 | 1586 | C=C |
| 4 | 3450 | OH |
| SPEEK:PSf Membranes | Phosphorene Membranes | |||
|---|---|---|---|---|
| Flux (LMH) | Visible | UV | Visible | UV |
| PWF initial | 126 | 67 | 56 | 107 |
| PWF final | 92 | 37 | 74 | 82 |
| MB initial | 72 | 42 | 71 | 68.1 |
| MB final | 43 | 29 | 42 | 31 |
| Recovered | 40 | 17 | 25 | 70 |
| Normalized membrane surface coverage (%) | 100 | 95 | 76 | 30 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eke, J.; Mills, P.A.; Page, J.R.; Wright, G.P.; Tsyusko, O.V.; Escobar, I.C. Nanohybrid Membrane Synthesis with Phosphorene Nanoparticles: A Study of the Addition, Stability and Toxicity. Polymers 2020, 12, 1555. https://doi.org/10.3390/polym12071555
Eke J, Mills PA, Page JR, Wright GP, Tsyusko OV, Escobar IC. Nanohybrid Membrane Synthesis with Phosphorene Nanoparticles: A Study of the Addition, Stability and Toxicity. Polymers. 2020; 12(7):1555. https://doi.org/10.3390/polym12071555
Chicago/Turabian StyleEke, Joyner, Philip Alexander Mills, Jacob Ryan Page, Garrison P. Wright, Olga V. Tsyusko, and Isabel C. Escobar. 2020. "Nanohybrid Membrane Synthesis with Phosphorene Nanoparticles: A Study of the Addition, Stability and Toxicity" Polymers 12, no. 7: 1555. https://doi.org/10.3390/polym12071555
APA StyleEke, J., Mills, P. A., Page, J. R., Wright, G. P., Tsyusko, O. V., & Escobar, I. C. (2020). Nanohybrid Membrane Synthesis with Phosphorene Nanoparticles: A Study of the Addition, Stability and Toxicity. Polymers, 12(7), 1555. https://doi.org/10.3390/polym12071555