Synthetic Approaches for Poly(Phenylene) Block Copolymers via Nickel Coupling Reaction for Fuel Cell Applications
Abstract
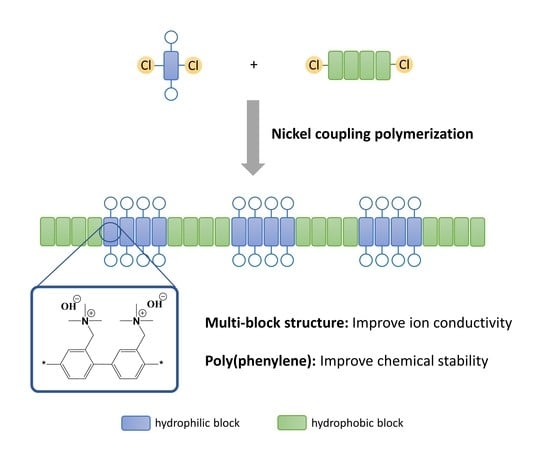
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Syntheses of Random Poly(Phenylene)s
2.3. Syntheses of Model Poly(Phenylene) Multi-Block Copolymer with NiX2 and Ni(COD)2
2.4. Syntheses of the Amine-Containing Multi-Block Copolymer with Ni(COD)2
2.5. Membrane Preparation and Quaternization
2.6. Characterization
3. Results and Discussion
3.1. Synthesis of Random Poly(Phenylene)s
3.2. Synthesis of Poly(Phenylene) from 3,5-DCBP Monomer and Chlorine-Terminated Hydrophobic Oligomer
3.3. Synthesis of Amine-Containing Poly(Phenylene) Block Copolymer with Ni(COD)2
3.4. Membrane Preparation, Quaternization, and Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Carrette, L.; Friedrich, K.A.; Stimming, U. Fuel Cells—Fundamentals and Applications. Fuel Cells 2001, 1, 5–39. [Google Scholar] [CrossRef]
- Jacobson, M.Z.; Colella, W.G.; Golden, D.M. Cleaning the Air and Improving Health with Hydrogen Fuel-Cell Vehicles. Science 2005, 308, 1901–1905. [Google Scholar] [CrossRef] [Green Version]
- Hickner, M.A.; Pivovar, B.S. The Chemical and Structural Nature of Proton Exchange Membrane Fuel Cell Properties. Fuel Cells 2005, 5, 213–229. [Google Scholar] [CrossRef]
- Smitha, B.; Sridhar, S.; Khan, A.A. Solid polymer electrolyte membranes for fuel cell applications—A review. J. Membr. Sci. 2005, 259, 10–26. [Google Scholar] [CrossRef]
- Wang, B. Recent development of non-platinum catalysts for oxygen reduction reaction. J. Power Sources 2005, 152, 1–15. [Google Scholar] [CrossRef]
- Gottesfeld, S.; Dekel, D.R.; Page, M.; Bae, C.; Yan, Y.; Zelenay, P.; Kim, Y.S. Anion exchange membrane fuel cells: Current status and remaining challenges. J. Power Sources 2018, 375, 170–184. [Google Scholar] [CrossRef]
- Varcoe, J.R.; Atanassov, P.; Dekel, D.R.; Herring, A.M.; Hickner, M.A.; Kohl, P.A.; Kucernak, A.R.; Mustain, W.E.; Nijmeijer, K.; Scott, K.; et al. Anion-exchange membranes in electrochemical energy systems. Energy Environ. Sci. 2014, 7, 3135–3191. [Google Scholar] [CrossRef] [Green Version]
- Jeon, T.-Y.; Kim, S.K.; Pinna, N.; Sharma, A.; Park, J.; Lee, S.Y.; Lee, H.C.; Kang, S.-W.; Lee, H.-K.; Lee, H.H. Selective Dissolution of Surface Nickel Close to Platinum in PtNi Nanocatalyst toward Oxygen Reduction Reaction. Chem. Mater. 2016, 28, 1879–1887. [Google Scholar] [CrossRef]
- Weiber, E.A.; Meis, D.; Jannasch, P. Anion conducting multiblock poly(arylene ether sulfone)s containing hydrophilic segments densely functionalized with quaternary ammonium groups. Polym. Chem. 2015, 6, 1986–1996. [Google Scholar] [CrossRef] [Green Version]
- You, W.; Noonan, K.J.T.; Coates, G.W. Alkaline-stable anion exchange membranes: A review of synthetic approaches. Prog. Polym. Sci. 2020, 100, 101177. [Google Scholar] [CrossRef]
- Sherazi, T.A.; Yong Sohn, J.; Moo Lee, Y.; Guiver, M.D. Polyethylene-based radiation grafted anion-exchange membranes for alkaline fuel cells. J. Membr. Sci. 2013, 441, 148–157. [Google Scholar] [CrossRef] [Green Version]
- Mohanty, A.D.; Ryu, C.Y.; Kim, Y.S.; Bae, C. Stable Elastomeric Anion Exchange Membranes Based on Quaternary Ammonium-Tethered Polystyrene-b-poly(ethylene-co-butylene)-b-polystyrene Triblock Copolymers. Macromolecules 2015, 48, 7085–7095. [Google Scholar] [CrossRef]
- Dang, H.-S.; Jannasch, P. High-Performing Hydroxide Exchange Membranes with Flexible Tetra-Piperidinium Side Chains Linked by Alkyl Spacers. ACS Appl. Energy Mater. 2018, 1, 2222–2231. [Google Scholar] [CrossRef]
- Li, N.; Guiver, M.D.; Binder, W.H. Towards High Conductivity in Anion-Exchange Membranes for Alkaline Fuel Cells. ChemSusChem 2013, 6, 1376–1383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.; Moh, L.C.H.; Swager, T.M. Anion Exchange Membranes: Enhancement by Addition of Unfunctionalized Triptycene Poly(Ether Sulfone)s. ACS Appl. Mater. Interfaces 2017, 9, 42409–42414. [Google Scholar] [CrossRef]
- Yang, Z.; Guo, R.; Malpass-Evans, R.; Carta, M.; McKeown, N.B.; Guiver, M.D.; Wu, L.; Xu, T. Highly Conductive Anion-Exchange Membranes from Microporous Tröger’s Base Polymers. Angew. Chem. Int. Ed. 2016, 55, 11499–11502. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.L.; Lin, C.X.; Hu, E.N.; Soyekwo, F.; Zhang, Q.G.; Zhu, A.M.; Liu, Q.L. Imidazolium-functionalized anion exchange membranes using poly(ether sulfone)s as macrocrosslinkers for fuel cells. RSC Adv. 2017, 7, 27342–27353. [Google Scholar] [CrossRef] [Green Version]
- Park, J.-S.; Park, S.-H.; Yim, S.-D.; Yoon, Y.-G.; Lee, W.-Y.; Kim, C.-S. Performance of solid alkaline fuel cells employing anion-exchange membranes. J. Power Sources 2008, 178, 620–626. [Google Scholar] [CrossRef]
- Kim, E.; Lee, S.; Woo, S.; Park, S.-H.; Yim, S.-D.; Shin, D.; Bae, B. Synthesis and characterization of anion exchange multi-block copolymer membranes with a fluorine moiety as alkaline membrane fuel cells. J. Power Sources 2017, 359, 568–576. [Google Scholar] [CrossRef]
- Shin, D.; Nugraha, A.F.; Wijaya, F.; Lee, S.; Kim, E.; Choi, J.; Kim, H.-J.; Bae, B. Synthetic approaches for advanced multi-block anion exchange membranes. RSC Adv. 2019, 9, 21106–21115. [Google Scholar] [CrossRef] [Green Version]
- Fujimoto, C.; Kim, D.-S.; Hibbs, M.; Wrobleski, D.; Kim, Y.S. Backbone stability of quaternized polyaromatics for alkaline membrane fuel cells. J. Membr. Sci. 2012, 423–424, 438–449. [Google Scholar] [CrossRef]
- Choe, Y.-K.; Fujimoto, C.; Lee, K.-S.; Dalton, L.T.; Ayers, K.; Henson, N.J.; Kim, Y.S. Alkaline Stability of Benzyl Trimethyl Ammonium Functionalized Polyaromatics: A Computational and Experimental Study. Chem. Mater. 2014, 26, 5675–5682. [Google Scholar] [CrossRef]
- Fang, J.; Shen, P.K. Quaternized poly(phthalazinon ether sulfone ketone) membrane for anion exchange membrane fuel cells. J. Membr. Sci. 2006, 285, 317–322. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y. Quaternized polyethersulfone Cardo anion exchange membranes for direct methanol alkaline fuel cells. J. Membr. Sci. 2005, 262, 1–4. [Google Scholar] [CrossRef]
- Willdorf-Cohen, S.; Mondal, A.N.; Dekel, D.R.; Diesendruck, C.E. Chemical stability of poly(phenylene oxide)-based ionomers in an anion exchange-membrane fuel cell environment. J. Mater. Chem. A 2018, 6, 22234–22239. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; Li, L.; Lin, C.X.; Gao, X.L.; Zhao, C.H.; Zhang, Q.G.; Zhu, A.M.; Liu, Q.L. Hyperbranched poly(arylene ether ketone) anion exchange membranes for fuel cells. J. Membr. Sci. 2018, 560, 77–86. [Google Scholar] [CrossRef]
- Hibbs, M.R.; Hickner, M.A.; Alam, T.M.; McIntyre, S.K.; Fujimoto, C.H.; Cornelius, C.J. Transport Properties of Hydroxide and Proton Conducting Membranes. Chem. Mater. 2008, 20, 2566–2573. [Google Scholar] [CrossRef]
- Lee, S.; Yuk, J.; Nugraha, A.F.; Shul, Y.-G.; Park, S.-H.; Shin, D.; Bae, B. Partially Fluorinated Multiblock Poly(arylene ether sulfone) Membranes for Fuel Cell Applications. Macromol. Mater. Eng. 2018, 303, 1700650. [Google Scholar] [CrossRef]
- Elabd, Y.A. Ion transport in hydroxide conducting block copolymers. Mol. Syst. Des. Eng. 2019, 4, 519–530. [Google Scholar] [CrossRef]
- Zhu, M.; Zhang, X.; Wang, Y.; Wu, Y.; Wang, H.; Zhang, M.; Chen, Q.; Shen, Z.; Li, N. Novel anion exchange membranes based on quaternized diblock copolystyrene containing a fluorinated hydrophobic block. J. Membr. Sci. 2018, 554, 264–273. [Google Scholar] [CrossRef]
- Yokota, N.; Ono, H.; Miyake, J.; Nishino, E.; Asazawa, K.; Watanabe, M.; Miyatake, K. Anion Conductive Aromatic Block Copolymers Containing Diphenyl Ether or Sulfide Groups for Application to Alkaline Fuel Cells. ACS Appl. Mater. Interfaces 2014, 6, 17044–17052. [Google Scholar] [CrossRef] [PubMed]
- Arges, C.G.; Ramani, V. Two-dimensional NMR spectroscopy reveals cation-triggered backbone degradation in polysulfone-based anion exchange membranes. Proc. Natl. Acad. Sci. USA 2013, 110, 2490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohanty, A.D.; Tignor, S.E.; Krause, J.A.; Choe, Y.-K.; Bae, C. Systematic Alkaline Stability Study of Polymer Backbones for Anion Exchange Membrane Applications. Macromolecules 2016, 49, 3361–3372. [Google Scholar] [CrossRef]
- Hibbs, M.R.; Fujimoto, C.H.; Cornelius, C.J. Synthesis and Characterization of Poly(phenylene)-Based Anion Exchange Membranes for Alkaline Fuel Cells. Macromolecules 2009, 42, 8316–8321. [Google Scholar] [CrossRef]
- Olsson, J.S.; Pham, T.H.; Jannasch, P. Poly(arylene piperidinium) Hydroxide Ion Exchange Membranes: Synthesis, Alkaline Stability, and Conductivity. Adv. Funct. Mater. 2018, 28, 1702758. [Google Scholar] [CrossRef]
- Yamamoto, T.; Hayashi, Y.; Yamamoto, A. A Novel Type of Polycondensation Utilizing Transition Metal-Catalyzed C–C Coupling. I. Preparation of Thermostable Polyphenylene Type Polymers. Bull. Chem. Soc. Jpn. 1978, 51, 2091–2097. [Google Scholar] [CrossRef] [Green Version]
- Rehahn, M.; Schlüter, A.-D.; Wegner, G.; Feast, W.J. Soluble poly(para-phenylene)s. 1. Extension of the Yamamoto synthesis to dibromobenzenes substituted with flexible side chains. Polymer 1989, 30, 1054–1059. [Google Scholar] [CrossRef]
- Kallitsis, J.K.; Naarmann, H. Synthesis of some disubstituted poly(p-terphenylenes). Synth. Met. 1991, 44, 247–257. [Google Scholar] [CrossRef]
- Nattmann, L.; Saeb, R.; Nöthling, N.; Cornella, J. An air-stable binary Ni(0)–olefin catalyst. Nat. Catal. 2020, 3, 6–13. [Google Scholar] [CrossRef]
- Bae, B.; Miyatake, K.; Watanabe, M. Sulfonated Poly(arylene ether sulfone ketone) Multiblock Copolymers with Highly Sulfonated Block. Synthesis and Properties. Macromolecules 2010, 43, 2684–2691. [Google Scholar] [CrossRef]
- Yamamoto, T.; Wakabayashi, S.; Osakada, K. Mechanism of C-C coupling reactions of aromatic halides, promoted by Ni(COD)2 in the presence of 2,2′-bipyridine and PPh3, to give biaryls. J. Organomet. Chem. 1992, 428, 223–237. [Google Scholar] [CrossRef]
- Tanaka, H.; Sumida, S.-I.; Kobayashi, N.; Komatsu, N.; Torii, S. Use of aluminium as an electron pool. Reductive coupling of iodobenzenes promoted by electron transfer in an Ni/Pb/A1 multi redox system. Inorg. Chim. Acta 1994, 222, 323–325. [Google Scholar] [CrossRef]
- Colon, I.; Kelsey, D.R. Coupling of aryl chlorides by nickel and reducing metals. J. Org. Chem. 1986, 51, 2627–2637. [Google Scholar] [CrossRef]
- Bae, J.M.; Honma, I.; Murata, M.; Yamamoto, T.; Rikukawa, M.; Ogata, N. Properties of selected sulfonated polymers as proton-conducting electrolytes for polymer electrolyte fuel cells. Solid State Ionics 2002, 147, 189–194. [Google Scholar] [CrossRef]
- Goto, K.; Rozhanskii, I.; Yamakawa, Y.; Otsuki, T.; Naito, Y. Development of Aromatic Polymer Electrolyte Membrane with High Conductivity and Durability for Fuel Cell. Polym. J. 2009, 41, 95–104. [Google Scholar] [CrossRef] [Green Version]
- Hagberg, E.C.; Olson, D.A.; Sheares, V.V. Advances in Ni(0)-Catalyzed Coupling for the Synthesis of Polythiophenes and Polyphenylenes. Macromolecules 2004, 37, 4748–4754. [Google Scholar] [CrossRef]
- Micovic, V.; Mihailovic, M. The Reduction of Acid Amides with Lithium Aluminum Hydride. J. Org. Chem. 1953, 18, 1190–1200. [Google Scholar] [CrossRef]
- White, T.D.; Berglund, K.D.; Groh, J.M.; Johnson, M.D.; Miller, R.D.; Yates, M.H. Development of a Continuous Schotten–Baumann Route to an Acyl Sulfonamide. Org. Process Res. Dev. 2012, 16, 939–957. [Google Scholar] [CrossRef]
- Miyake, J.; Taki, R.; Mochizuki, T.; Shimizu, R.; Akiyama, R.; Uchida, M.; Miyatake, K. Design of flexible polyphenylene proton-conducting membrane for next-generation fuel cells. Sci. Adv. 2017, 3, eaao0476. [Google Scholar] [CrossRef] [Green Version]
- Kimura, T.; Matsumoto, A.; Inukai, J.; Miyatake, K. Highly Anion Conductive Polymers: How Do Hexafluoroisopropylidene Groups Affect Membrane Properties and Alkaline Fuel Cell Performance? ACS Appl. Energy Mater. 2020, 3, 469–477. [Google Scholar] [CrossRef]
- Yuk, J.; Lee, S.; Nugraha, A.F.; Lee, H.; Park, S.-H.; Yim, S.-D.; Bae, B. Synthesis and characterization of multi-block poly(arylene ether sulfone) membranes with highly sulfonated blocks for use in polymer electrolyte membrane fuel cells. J. Membr. Sci. 2016, 518, 50–59. [Google Scholar] [CrossRef]
















| Sample | Reactants (mmol) | Catalyst (mmol) | Zn (mmol) | BPY (mmol) | PPh3 (mmol) | Mn a (kDa) | Mw a (kDa) | PDI |
|---|---|---|---|---|---|---|---|---|
| NiBr2 | 6.4 | 0.5 | 27.5 | - | 3.2 | 1.3 | 2.0 | 1.6 |
| NiCl2 | 6.4 | 0.5 | 27.5 | - | 3.2 | 1.5 | 2.3 | 1.6 |
| Ni(COD)2 | 2.9 | 7.2 | - | 7.2 | - | 1.4 | 2.4 | 1.7 |
| Sample | Reactants (mmol) | Catalyst (mmol) | Zn (mmol) | BPY (mmol) | PPh3 (mmol) | Mn a (kDa) | Mw a (kDa) | PDI |
|---|---|---|---|---|---|---|---|---|
| NiBr2 | 0.65 | 0.05 | 2.75 | - | 0.32 | 3.5 | 6.2 | 1.8 |
| NiCl2 | 0.65 | 0.05 | 2.75 | - | 0.32 | 3.7 | 6.7 | 1.8 |
| Ni(COD)2 | 0.65 | 1.63 | - | 1.63 | - | 2.1 | 4.6 | 2.2 |
| Sample | Reactants (mmol) | Catalyst (mmol) | Zn (mmol) | BPY (mmol) | PPh3 (mmol) | XY a | Mn b (kDa) | Mw b (kDa) | PDI |
|---|---|---|---|---|---|---|---|---|---|
| NiCl2 | 2.9 | 0.2 | 12.2 | - | 1.4 | X5Y5 | 5.6 | 12.3 | 2.2 |
| NiBr2 | 2.9 | 0.2 | 12.2 | - | 1.4 | X5Y5 | 6.7 | 14.3 | 2.1 |
| Ni(COD)2 | 2.9 | 7.3 | - | 7.3 | - | X5Y5 | 23.4 | 131.0 | 5.6 |
| Reactants/BPY/Ni(COD)2 Ratio | Mn a (kDa) | Mw a (kDa) | PDI |
|---|---|---|---|
| 1:2.5:2.5 | 20.5 | 80.1 | 3.9 |
| 1:1.25:1.25 | 2.9 | 38.7 | 13.1 |
| 1:2.5:1.25 | 25.9 | 93.9 | 3.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nugraha, A.F.; Kim, S.; Wijaya, F.; Bae, B.; Shin, D. Synthetic Approaches for Poly(Phenylene) Block Copolymers via Nickel Coupling Reaction for Fuel Cell Applications. Polymers 2020, 12, 1614. https://doi.org/10.3390/polym12071614
Nugraha AF, Kim S, Wijaya F, Bae B, Shin D. Synthetic Approaches for Poly(Phenylene) Block Copolymers via Nickel Coupling Reaction for Fuel Cell Applications. Polymers. 2020; 12(7):1614. https://doi.org/10.3390/polym12071614
Chicago/Turabian StyleNugraha, Adam F., Songmi Kim, Farid Wijaya, Byungchan Bae, and Dongwon Shin. 2020. "Synthetic Approaches for Poly(Phenylene) Block Copolymers via Nickel Coupling Reaction for Fuel Cell Applications" Polymers 12, no. 7: 1614. https://doi.org/10.3390/polym12071614
APA StyleNugraha, A. F., Kim, S., Wijaya, F., Bae, B., & Shin, D. (2020). Synthetic Approaches for Poly(Phenylene) Block Copolymers via Nickel Coupling Reaction for Fuel Cell Applications. Polymers, 12(7), 1614. https://doi.org/10.3390/polym12071614