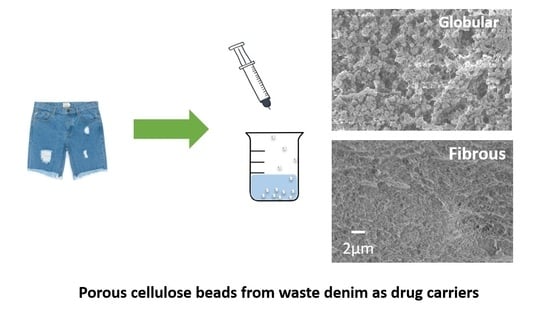
Cellulose Beads Derived from Waste Textiles for Drug Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Methodology
2.2.1. Preparation of Cellulose Beads from Denim
2.2.2. Characterisations of Cellulose–IL Solutions and Cellulose Beads
2.2.3. Drug Loading and In-Vitro Release
Drug Loading
In Vitro Drug Release
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Koszewska, M. Circular Economy—Challenges for the Textile and Clothing Industry. Autex Res. J. 2018, 18, 337–347. [Google Scholar] [CrossRef] [Green Version]
- Sull, D.N.; Turconi, S. Fast fashion lessons. Bus. Strat. Rev. 2008, 19, 4–11. [Google Scholar] [CrossRef]
- Kerr, J.; Landry, J. Pulse the Fashion Industry. The Sustainbility Portal 2017. Available online: http://www.sustainabilityportal.net/blog/pulseofthefashionindustry (accessed on 3 September 2019).
- Sandin, G.; Peters, G.M. Environmental impact of textile reuse and recycling—A review. J. Clean. Prod. 2018, 184, 353–365. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Chemin- 2005, 36, 3358–3393. [Google Scholar] [CrossRef]
- Wang, S.; Lu, A.; Zhang, L. Recent advances in regenerated cellulose materials. Prog. Polym. Sci. 2016, 53, 169–206. [Google Scholar] [CrossRef]
- Abeer, M.M.; Amin, M.C.I.M.; Martin, C. A review of bacterial cellulose-based drug delivery systems: Their biochemistry, current approaches and future prospects. J. Pharm. Pharmacol. 2014, 66, 1047–1061. [Google Scholar] [CrossRef]
- Qiu, X.; Hu, S. “Smart” Materials Based on Cellulose: A Review of the Preparations, Properties, and Applications. Materials 2013, 6, 738–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, B.; Wang, X.; Byrne, N. Development of cellulose based aerogel utilizing waste denim—A Morphology study. Carbohydr. Polym. 2019, 205, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Budtova, T.; Ratke, L.; Gurikov, P.; Baudron, V.; Preibisch, I.; Niemeyer, P.; Smirnova, I.; Milow, B. Review on the Production of Polysaccharide Aerogel Particles. Materials 2018, 11, 2144. [Google Scholar] [CrossRef] [Green Version]
- Luo, X.; Zhang, L. Creation of regenerated cellulose microspheres with diameter ranging from micron to millimeter for chromatography applications. J. Chromatogr. A 2010, 1217, 5922–5929. [Google Scholar] [CrossRef] [PubMed]
- Gericke, M.; Trygg, J.; Fardim, P. Functional cellulose beads: Preparation, characterization, and applications. Chem. Rev. 2013, 113, 4812–4836. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Meng, H.; Ouyang, Y.; Chang, J. Nanoporous Magnetic Cellulose–Chitosan Composite Microspheres: Preparation, Characterization, and Application for Cu(II) Adsorption. Ind. Eng. Chem. Res. 2014, 53, 2106–2113. [Google Scholar] [CrossRef]
- Roy, I.; Gupta, M.N. Lactose hydrolysis by Lactozym™ immobilized on cellulose beads in batch and fluidized bed modes. Process Biochem. 2003, 39, 325–332. [Google Scholar] [CrossRef]
- Lu, H.; Luo, H.; Leventis, N. Aerogel Handbook; Springer: New York, NY, USA, 2011. [Google Scholar]
- Budtova, T. Cellulose II aerogels: A review. Cellulose 2019, 26, 81–121. [Google Scholar] [CrossRef]
- García-González, C.A.; Budtova, T.; Durães, L.; Erkey, C.; Del Gaudio, P.; Gurikov, P.; Koebel, M.; Liebner, F.; Neagu, M.; Smirnova, I. An Opinion Paper on Aerogels for Biomedical and Environmental Applications. Molecules 2019, 24, 1815. [Google Scholar] [CrossRef] [Green Version]
- De Cicco, F.; Russo, P.; Reverchon, E.; García-González, C.A.; Aquino, R.P.; Del Gaudio, P. Prilling and supercritical drying: A successful duo to produce core-shell polysaccharide aerogel beads for wound healing. Carbohydr. Polym. 2016, 147, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Sescousse, R.; Gavillon, R.; Budtova, T. Wet and dry highly porous cellulose beads from cellulose–NaOH–water solutions: Influence of the preparation conditions on beads shape and encapsulation of inorganic particles. J. Mater. Sci. 2010, 46, 759–765. [Google Scholar] [CrossRef]
- Trygg, J.; Fardim, P.; Gericke, M.; Mäkilä, E.M.; Salonen, J.J. Physicochemical design of the morphology and ultrastructure of cellulose beads. Carbohydr. Polym. 2013, 93, 291–299. [Google Scholar] [CrossRef]
- Druel, L.; Niemeyer, P.; Milow, B.; Budtova, T. Rheology of cellulose-[DBNH][CO2Et] solutions and shaping into aerogel beads. Green Chem. 2018, 20, 3993–4002. [Google Scholar] [CrossRef]
- Ishimura, D.; Morimoto, Y.; Saito, H. Influences of chemical modifications on the mechanical strength of cellulose beads. Cellulose 1998, 5, 135–151. [Google Scholar] [CrossRef]
- Biganska, O.; Navard, P. Morphology of cellulose objects regenerated from cellulose–N-methylmorpholine N-oxide–water solutions. Cellulose 2008, 16, 179–188. [Google Scholar] [CrossRef] [Green Version]
- Trygg, J.; Yildir, E.; Kolakovic, R.; Sandler, N.; Fardim, P. Anionic cellulose beads for drug encapsulation and release. Cellulose 2014, 21, 1945–1955. [Google Scholar] [CrossRef]
- Yildir, E.; Kolakovic, R.; Genina, N.; Trygg, J.; Gericke, M.; Hanski, L.; Ehlers, H.; Rantanen, J.; Tenho, M.; Vuorela, P.; et al. Tailored beads made of dissolved cellulose—Investigation of their drug release properties. Int. J. Pharm. 2013, 456, 417–423. [Google Scholar] [CrossRef]
- Pircher, N.; Carbajal, L.; Schimper, C.; Bacher, M.; Rennhofer, H.; Nedelec, J.-M.; Lichtenegger, H.; Rosenau, T.; Liebner, F. Impact of selected solvent systems on the pore and solid structure of cellulose aerogels. Cellulose 2016, 23, 1949–1966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sescousse, R.; Gavillon, R.; Budtova, T. Aerocellulose from cellulose–ionic liquid solutions: Preparation, properties and comparison with cellulose–NaOH and cellulose–NMMO routes. Carbohydr. Polym. 2011, 83, 1766–1774. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, S.; Matsumoto, Y.; Kuga, S. Cellulose gel and aerogel from LiCl/DMSO solution. Cellulose 2012, 19, 393–399. [Google Scholar] [CrossRef]
- Innerlohinger, J.; Weber, H.K.; Kraft, G. Aerocellulose: Aerogels and Aerogel-like Materials made from Cellulose. In Macromolecular Symposia; Wiley Online Library: Weinheim, Germany, 2006; Volume 244, pp. 126–135. [Google Scholar]
- Ma, Y.; Zeng, B.; Wang, X.; Byrne, N. Circular textiles: Closed loop fibre to fibre wet spun process for recycling cotton from denim. ACS Sustain. Chem. Eng. 2019, 14, 11937–11943. [Google Scholar] [CrossRef]
- Sing, K. The use of nitrogen adsorption for the characterisation of porous materials. Colloids Surfaces A Physicochem. Eng. Asp. 2001, 187, 3–9. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Miyazaki, S.; Kubo, W.; Attwood, D. Oral sustained delivery of theophylline using in-situ gelation of sodium alginate. J. Control. Release 2000, 67, 275–280. [Google Scholar] [CrossRef]
- Klose, D.; Siepmann, J.; Elkharraz, K.; Krenzlin, S.; Siepmann, J. How porosity and size affect the drug release mechanisms from PLGA-based microparticles. Int. J. Pharm. 2006, 314, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Ciobanu, B.C.; Cadinoiu, A.N.; Popa, M.; Desbrieres, J.; Peptu, C.A. Chitosan/poly (vinyl alcohol) hydrogels for entrapment of drug loaded liposomes. Cellul. Chem. Technol. 2014, 48, 485–494. [Google Scholar]
- Van De Witte, P.; Dijkstra, P.; Berg, J.V.D.; Feijen, J. Phase separation processes in polymer solutions in relation to membrane formation. J. Membr. Sci. 1996, 117, 1–31. [Google Scholar] [CrossRef] [Green Version]
- Gavillon, R.; Budtova, T. Aerocellulose: New Highly Porous Cellulose Prepared from Cellulose−NaOH Aqueous Solutions. Biomacromolecules 2008, 9, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.M.; Hu, Z.; Jiang, J. Cellulose regeneration from a cellulose/ionic liquid mixture: The role of anti-solvents. RSC Adv. 2013, 3, 12794–12801. [Google Scholar] [CrossRef]
- Cai, J.; Kimura, S.; Wada, M.; Kuga, S.; Zhang, L. Cellulose Aerogels from Aqueous Alkali Hydroxide–Urea Solution. ChemSusChem 2008, 1, 149–154. [Google Scholar] [CrossRef]
- García-González, C.A.; Alnaief, M.; Smirnova, I. Polysaccharide-based aerogels—Promising biodegradable carriers for drug delivery systems. Carbohydr. Polym. 2011, 86, 1425–1438. [Google Scholar] [CrossRef]




| Bead Name | Dissolving Solvent | Coagulant |
|---|---|---|
| BmimClwater | BmimCl | Water |
| BmimAcwater | BmimAc | Water |
| BmimAcethanol | BmimAc | Ethanol |
| Drug Name | Synonym | Solubility in Water (mg/mL) | Solution Concentration for Drug Loading (mg/mL) | Wavelength in UV-Vis (nm) |
|---|---|---|---|---|
| Lidocaine hydrochloride monohydrate (LiHCl) | 2-(Diethylamino)-N-(2,6-dimethylphenyl)acetamide hydrochloride hydrate | 147 | 20 | 218 |
| Theophylline (Thp) | Dimethylxanthine | 8 | 4 | 271 |
| Bead Name | Specific Surface Area (m2/g) | Average Pore Size (nm) | Pore Volume (cm3/g) | Diameter of the Wet Beads (mm) | Diameter of Dry Beads (mg) | Weight of Dry Beads (mg) |
|---|---|---|---|---|---|---|
| BmimClwater | 382 | 34.3 | 2.4 | 2.00 ± 0.08 | 0.75 ± 0.04 | 0.35 ± 0.05 |
| BmimAcwater | 306 | 13.9 | 1.8 | 2.02 ± 0.08 | 0.80 ± 0.06 | 0.44 ± 0.04 |
| BmimAcethanol | 348 | 34.3 | 3.7 | 2.00 ± 0.09 | 0.65 ± 0.04 | 0.26 ± 0.01 |
| BmimClwater | BmimAcwater | BmimAcethanol | |
|---|---|---|---|
| Swelling capacity | 59.3% | 48.3% | 76.4% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, B.; Wang, X.; Byrne, N. Cellulose Beads Derived from Waste Textiles for Drug Delivery. Polymers 2020, 12, 1621. https://doi.org/10.3390/polym12071621
Zeng B, Wang X, Byrne N. Cellulose Beads Derived from Waste Textiles for Drug Delivery. Polymers. 2020; 12(7):1621. https://doi.org/10.3390/polym12071621
Chicago/Turabian StyleZeng, Beini, Xungai Wang, and Nolene Byrne. 2020. "Cellulose Beads Derived from Waste Textiles for Drug Delivery" Polymers 12, no. 7: 1621. https://doi.org/10.3390/polym12071621
APA StyleZeng, B., Wang, X., & Byrne, N. (2020). Cellulose Beads Derived from Waste Textiles for Drug Delivery. Polymers, 12(7), 1621. https://doi.org/10.3390/polym12071621