Investigation of Biocidal Effect of Microfiltration Membranes Impregnated with Silver Nanoparticles by Sputtering Technique
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microfiltration Membrane
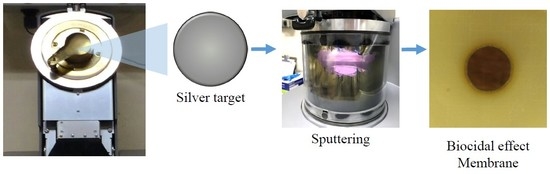
2.3. Silver Nanoparticles Deposition
2.4. MF-AgNps Membrane Characterization
2.5. Antibacterial Activity Tests
2.5.1. The Disc Diffusion Method
2.5.2. The Biofouling Resistance Tests
3. Results and Discussion
3.1. Membrane Characterization
3.2. AgNps Releasing Test
3.3. Antibacterial Activity Tests
3.3.1. The Disc Diffusion Method
3.3.2. The Biofouling Resistance Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- BCC Research. The Global Market for Membrane Microfiltration. Available online: https://www.bccresearch.com/market-research/membrane-and-separation-technology/membrane-microfiltration.html (accessed on 20 April 2020).
- Zhang, M.; Zhang, K.; De Gusseme, B.; Verstraete, W. Biogenic silver nanoparticles (bio-Ag 0) decrease biofouling of bio-Ag 0/PES nanocomposite membranes. Water Res. 2012, 46, 2077–2087. [Google Scholar] [PubMed]
- Kochkodan, V.; Hilal, N. A comprehensive review on surface modified polymer membranes for biofouling mitigation. Desalination 2015, 356, 187–207. [Google Scholar] [CrossRef]
- Jiang, S.; Li, Y.; Ladewig, B.P. A review of reverse osmosis membrane fouling and control strategies. Sci. Total Environ. 2017, 595, 567–583. [Google Scholar] [CrossRef] [PubMed]
- Zodrow, K.; Brunet, L.; Mahendra, S.; Li, D.; Zhang, A.; Li, Q.; Alvarez, P.J.J. Polysulfone ultrafiltration membranes impregnated with silver nanoparticles show improved biofouling resistance and virus removal. Water Res. 2009, 43, 715–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basri, H.; Ismail, A.F.; Aziz, M.; Nagai, K.; Matsuura, T.; Abdullah, M.S.; Ng, B.C. Silver-filled polyethersulfone membranes for antibacterial applications—Effect of PVP and TAP addition on silver dispersion. Desalination 2010, 261, 264–271. [Google Scholar] [CrossRef]
- Dong, C.; Wang, Z.; Wu, J.; Wang, Y.; Wang, J.; Wang, S. A green strategy to immobilize silver nanoparticles onto reverse osmosis membrane for enhanced anti-biofouling property. Desalination 2017, 401, 32–41. [Google Scholar] [CrossRef]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.Z.; Kim, T.N.; Kim, J.O. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef]
- Matsumura, Y.; Yoshikata, K.; Kunisaki, S.; Tsuchido, T. Mode of bactericidal action of silver zeolite and its comparison with that of silver nitrate. Appl. Environ. Microbiol. 2003, 69, 4278–4281. [Google Scholar] [CrossRef] [Green Version]
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef] [Green Version]
- Su, H.L.; Chou, C.C.; Hung, D.J.; Lin, S.H.; Pao, I.C.; Lin, J.H.; Huang, F.L.; Dong, R.X.; Lin, J.J. The disruption of bacterial membrane integrity through ROS generation induced by nanohybrids of silver and clay. Biomaterials 2009, 30, 5979–5987. [Google Scholar] [CrossRef] [PubMed]
- Dastjerdi, R.; Montazer, M. A review on the application of inorganic nano-structured materials in the modification of textiles: Focus on anti-microbial properties. Colloids Surf. Biointerfaces 2010, 79, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Ferdous, Z.; Nemmar, A. Health impact of silver nanoparticles: A review of the biodistribution and toxicity following various routes of exposure. Int. J. Mol. Sci. 2020, 21, 2375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Mahendra, S.; Lyon, D.Y.; Brunet, L.; Liga, M.V.; Li, D.; Alvarez, P.J.J. Antimicrobial nanomaterials for water disinfection and microbial control: Potential applications and implications. Water Res. 2008, 42, 4591–4602. [Google Scholar] [CrossRef] [PubMed]
- Nowack, B.; Krug, H.F.; Height, M. 120 years of nanosilver history: Implications for policy makers. Environ. Sci. Technol. 2011, 45, 1177–1183. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality, 4th ed.; World Heathy Organization: Geneve, Switzerland, 2011; ISBN 978-92-4-154815-1. [Google Scholar]
- Siddiqi, K.S.; Husen, A.; Rao, R.A.K. A review on biosynthesis of silver nanoparticles and their biocidal properties. J. Nanobiotechnol. 2018, 16, 14. [Google Scholar] [CrossRef]
- Deshmukh, S.P.; Patil, S.M.; Mullani, S.B.; Delekar, S.D. Silver nanoparticles as an effective disinfectant: A review. Mater. Sci. Eng. 2019, 97, 954–965. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, S.H.; Park, S.J.; Ryoo, S.; Woo, K.; Lee, J.S.; Kim, T.S.; Park, H.D.; Park, H.; Park, Y.I.; et al. Direct incorporation of silver nanoparticles onto thin-film composite membranes via arc plasma deposition for enhanced antibacterial and permeation performance. J. Memb. Sci. 2016, 513, 226–235. [Google Scholar] [CrossRef]
- Cao, G.; Wang, Y. Nanostructures and Nanomaterials: Synthesis, Properties and Applications, 2nd ed.; World Scientific: London, UK, 2011; ISBN 978-981-4322-50-8. [Google Scholar]
- Wasa, K.; Kanno, I.; Kotera, H. Handbook of Sputter Deposition Technology: Fundamentals and Applications for Functional Thin Films, Nano-Materials and MEMS, 2nd ed.; Elsevier: Oxford, UK, 2012; ISBN 978-143-7734-836. [Google Scholar]
- Liu, S.; Fang, F.; Wu, J.; Zhang, K. The anti-biofouling properties of thin-film composite nanofiltration membranes grafted with biogenic silver nanoparticles. Desalination 2015, 375, 121–128. [Google Scholar] [CrossRef] [Green Version]
- Dong, X.; Shannon, H.D.; Amirsoleimani, A.; Brion, G.M.; Escobar, I.C. Thiol-affinity immobilization of casein-coated silver nanoparticles on polymeric membranes for biofouling control. Polymers 2019, 11, 2057. [Google Scholar] [CrossRef] [Green Version]
- Alpatova, A.; Kim, E.S.; Sun, X.; Hwang, G.; Liu, Y.; Gamal El-Din, M. Fabrication of porous polymeric nanocomposite membranes with enhanced anti-fouling properties: Effect of casting composition. J. Memb. Sci. 2013, 444, 449–460. [Google Scholar] [CrossRef]
- Li, X.; Sotto, A.; Li, J.; Van der Bruggen, B. Progress and perspectives for synthesis of sustainable antifouling composite membranes containing in situ generated nanoparticles. J. Memb. Sci. 2017, 524, 502–528. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Roque, É.B.; da Fonseca, F.V.; Borges, C.P. High flux microfiltration membranes with silver nanoparticles for water disinfection. Desalin. Water Treat. 2015, 56, 3590–3598. [Google Scholar] [CrossRef]
- Kuzminova, A.; Beranová, J.; Polonskyi, O.; Shelemin, A.; Kylián, O.; Choukourov, A.; Slavínská, D.; Biederman, H. Antibacterial nanocomposite coatings produced by means of gas aggregation source of silver nanoparticles. Surf. Coat. Technol. 2016, 294, 225–230. [Google Scholar] [CrossRef]
- Haider, M.S.; Shao, G.N.; Imran, S.M.; Park, S.S.; Abbas, N.; Tahir, M.S.; Hussain, M.; Bae, W.; Kim, H.T. Aminated polyethersulfone-silver nanoparticles (AgNPs-APES) composite membranes with controlled silver ion release for antibacterial and water treatment applications. Mater. Sci. Eng. 2016, 62, 732–745. [Google Scholar] [CrossRef]
- Pasmore, M.; Todd, P.; Smith, S.; Baker, D.; Silverstein, J.A.; Coons, D.; Bowman, C.N. Effects of ultrafiltration membrane surface properties on Pseudomonas aeruginosa biofilm initiation for the purpose of reducing biofouling. J. Memb. Sci. 2001, 194, 15–32. [Google Scholar] [CrossRef]
- De Prijck, K.; Nelis, H.; Coenye, T. Efficacy of silver-releasing rubber for the prevention of Pseudomonas aeruginosa biofilm formation in water. Biofouling 2007, 23, 405–411. [Google Scholar] [CrossRef]
- Vaughan, J.; Benson, R.; Vaughan, K. Assessing the effectiveness of antimicrobial wound dressings in vitro. In Advanced Wound Repair Therapies; Woodhead Publishing Limited: Cambridge, UK, 2011; pp. 227–246. ISBN 978-184-5697-006. [Google Scholar]
- Khanna, P.K.; Singh, N.; Charan, S.; Subbarao, V.V.V.S.; Gokhale, R.; Mulik, U.P. Synthesis and characterization of Ag/PVA nanocomposite by chemical reduction method. Mater. Chem. Phys. 2005, 93, 117–121. [Google Scholar] [CrossRef]
- RRUFF Database. Available online: http://rruff.info/silver/display=default/R070416 (accessed on 4 April 2018).
- Toroghi, M.; Raisi, A.; Aroujalian, A. Preparation and characterization of polyethersulfone/silver nanocomposite ultrafiltration membrane for antibacterial applications. Polym. Adv. Technol. 2014, 25, 711–722. [Google Scholar] [CrossRef]
- Gracia-Pinilla, M.Á.; Ferrer, D.; Mejía-Rosales, S.; Pérez-Tijerina, E. Size-selected Ag nanoparticles with five-fold symmetry. Nanoscale Res. Lett. 2009, 4, 896–902. [Google Scholar] [CrossRef] [Green Version]
- Asanithi, P.; Chaiyakun, S.; Limsuwan, P. Growth of silver nanoparticles by DC magnetron sputtering. J. Nanomater. 2012, 2012, 1–8. [Google Scholar] [CrossRef]
- Dutka, M.V.; Turkin, A.A.; Vainchtein, D.I.; De Hosson, J.T.M. On the formation of copper nanoparticles in nanocluster aggregation source. J. Vac. Sci. Technol. 2015, 33, 031509. [Google Scholar] [CrossRef]
- Morel, R.; Brenac, A.; Bayle-Guillemaud, P.; Portement, C.; La Rizza, F. Growth and properties of cobalt clusters made by sputtering gas-aggregation. Eur. Phys. J. 2003, 24, 287–290. [Google Scholar] [CrossRef]
- Bray, K.R.; Jiao, C.Q.; Decerbo, J.N. Nucleation and growth of Nb nanoclusters during plasma gas condensation. J. Appl. Phys. 2013, 113, 234307. [Google Scholar] [CrossRef]
- Ayesh, A.I.; Qamhieh, N.; Ghamlouche, H.; Thaker, S.; El-Shaer, M. Fabrication of size-selected Pd nanoclusters using a magnetron plasma sputtering source. J. Appl. Phys. 2010, 107, 034317. [Google Scholar] [CrossRef]
- Hirsch, U.M.; Teuscher, N.; Rühl, M.; Heilmann, A. Plasma-enhanced magnetron sputtering of silver nanoparticles on reverse osmosis membranes for improved antifouling properties. Surf. Interfaces 2019, 16, 1–7. [Google Scholar] [CrossRef]
- Cruz, M.C.; Ruano, G.; Wolf, M.; Hecker, D.; Castro Vidaurre, E.; Schmittgens, R.; Rajal, V.B. Plasma deposition of silver nanoparticles on ultrafiltration membranes: Antibacterial and anti-biofouling properties. Chem. Eng. Res. Des. 2015, 94, 524–537. [Google Scholar] [CrossRef] [Green Version]
- Saxena, N.; Prabhavathy, C.; De, S.; DasGupta, S. Flux enhancement by argon-oxygen plasma treatment of polyethersulfone membranes. Sep. Purif. Technol. 2009, 70, 160–165. [Google Scholar] [CrossRef]
- Sprick, C.; Chede, S.; Oyanedel-Craver, V.; Escobar, I.C. Bio-inspired immobilization of casein-coated silver nanoparticles on cellulose acetate membranes for biofouling control. J. Environ. Chem. Eng. 2018, 6, 2480–2491. [Google Scholar] [CrossRef]
- Belfer, S.; Fainchtain, R.; Purinson, Y.; Kedem, O. Surface characterization by FTIR-ATR spectroscopy of polyethersulfone membranes-unmodified, modified and protein fouled. J. Memb. Sci. 2000, 172, 113–124. [Google Scholar] [CrossRef]
- Vatsha, B.; Ngila, J.C.; Moutloali, R.M. Preparation of antifouling polyvinylpyrrolidone (PVP 40K) modified polyethersulfone (PES) ultrafiltration (UF) membrane for water purification. Phys. Chem. Earth 2014, 67–69, 125–131. [Google Scholar] [CrossRef]
- Miyano, T.; Matsuura, T.; Carlsson, D.J.; Sourirajan, S. Retention of polyvinylpyrrolidone swelling agent in the poly(ether p-phenylenesulfone) ultrafiltration membrane. J. Appl. Polym. Sci. 1990, 41, 407–417. [Google Scholar] [CrossRef]
- Moarefian, A.; Golestani, H.A.; Bahmanpour, H. Removal of amoxicillin from wastewater by self-made polyethersulfone membrane using nanofiltration. J. Environ. Health Sci. Eng. 2014, 12, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koloti, L.E.; Gule, N.P.; Arotiba, O.A.; Malinga, S.P. Laccase-immobilized dendritic nanofibrous membranes as a novel approach towards the removal of bisphenol A. Environ. Technol. 2018, 39, 392–404. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Tang, M.; Liu, F.; Nie, Y.; Zhao, C. Immobilization of silver nanoparticles onto sulfonated polyethersulfone membranes as antibacterial materials. Colloids Surf. Biointerfaces 2010, 81, 555–562. [Google Scholar] [CrossRef]
- Yin, J.; Yang, Y.; Hu, Z.; Deng, B. Attachment of silver nanoparticles (AgNPs) onto thin-film composite (TFC) membranes through covalent bonding to reduce membrane biofouling. J. Memb. Sci. 2013, 441, 73–82. [Google Scholar] [CrossRef]
- Liu, Y.; Rosenfield, E.; Hu, M.; Mi, B. Direct observation of bacterial deposition on and detachment from nanocomposite membranes embedded with silver nanoparticles. Water Res. 2013, 47, 2949–2958. [Google Scholar] [CrossRef]
- Agnihotri, S.; Mukherji, S.; Mukherji, S. Size-controlled silver nanoparticles synthesized over the range 5–100 nm using the same protocol and their antibacterial efficacy. RSC Adv. 2014, 4, 3974–3983. [Google Scholar] [CrossRef] [Green Version]
- Cobos, M.; De-La-Pinta, I.; Guillermo, Q.; Fernández, M.J.; Fernández, M.D. Synthesis, physical, mechanical and antibacterial properties of nanocomposites based on poly(vinyl alcohol)/graphene oxide–silver nanoparticles. Polymers 2020, 12, 723. [Google Scholar] [CrossRef] [Green Version]









| Membrane | Water Permeability (L h−1 m−2 bar−1) | Rejection (%) |
|---|---|---|
| MF-membrane | 6349.9 ± 475.1 | 26.4 |
| MF-15mA-15s | 6455.0 ± 519.9 | - |
| MF-50mA-120s | 6388.0 ± 564.8 | 78.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Linhares, A.M.F.; Borges, C.P.; Fonseca, F.V. Investigation of Biocidal Effect of Microfiltration Membranes Impregnated with Silver Nanoparticles by Sputtering Technique. Polymers 2020, 12, 1686. https://doi.org/10.3390/polym12081686
Linhares AMF, Borges CP, Fonseca FV. Investigation of Biocidal Effect of Microfiltration Membranes Impregnated with Silver Nanoparticles by Sputtering Technique. Polymers. 2020; 12(8):1686. https://doi.org/10.3390/polym12081686
Chicago/Turabian StyleLinhares, Aline M. F., Cristiano P. Borges, and Fabiana V. Fonseca. 2020. "Investigation of Biocidal Effect of Microfiltration Membranes Impregnated with Silver Nanoparticles by Sputtering Technique" Polymers 12, no. 8: 1686. https://doi.org/10.3390/polym12081686
APA StyleLinhares, A. M. F., Borges, C. P., & Fonseca, F. V. (2020). Investigation of Biocidal Effect of Microfiltration Membranes Impregnated with Silver Nanoparticles by Sputtering Technique. Polymers, 12(8), 1686. https://doi.org/10.3390/polym12081686