3D Bioprinting in Tissue Engineering for Medical Applications: The Classic and the Hybrid
Abstract
:1. Background
2. 3D Printing Processes and Techniques
2.1. Introduction
2.2. Non-Biological 3D Printing
2.2.1. Fused Deposition Modeling
2.2.2. Stereolithography
2.2.3. Selective Laser Sintering and Electron Beam Melting
2.3. 3D Bioprinting
2.3.1. Inkjet Bioprinting
2.3.2. Laser-Assisted Bioprinting
2.3.3. Extrusion Bioprinting
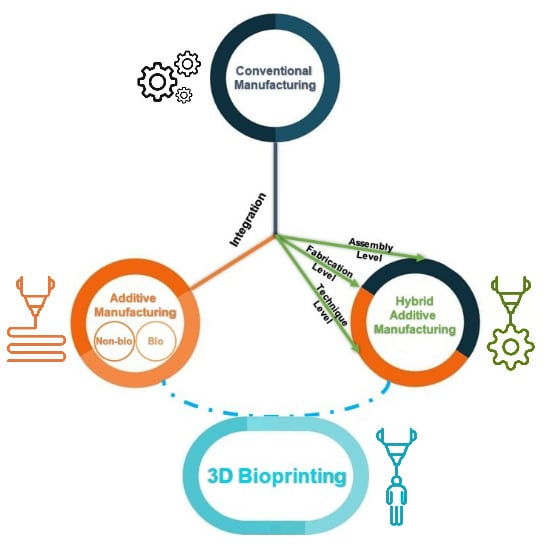
2.4. Hybrid Manufacturing in Tissue Engineering
2.4.1. Hierarchical Integration of Modular Units at the Assembly Level
2.4.2. Multi-feature Integration at the Fabrication Level
2.4.3. Hybrid Additive Manufacturing at the Technique Level
3. Materials for 3D Bioprinting
3.1. Material Characteristics
3.1.1. Printability
3.1.2. Biocompatibility and Control of Degradation and Byproducts
3.1.3. Mechanical Properties
3.2. Biomaterials
3.3. Cell Sources
4. 3D Bioprinting for Medical Applications
4.1. Vessel and Heart Valve Applications
4.2. Bone and Cartilage Applications
4.3. Other Tissue Engineering Applications
4.4. Drug Screening
5. Challenges and Future Prospects
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Whitfield, R.; Farrer, A.S.; Museum, B.; Vainker, S.J.; Rawson, J. Caves of the Thousand Buddhas: Chinese Art from the Silk Route; British Museum Publications for the Trustees of the British Museum: London, UK, 1990; ISBN 9780714114477. [Google Scholar]
- Costas, B.R. Print Culture and Peripheries in Early Modern Europe: A Contribution to the History of Printing and the Book Trade in Small European and Spanish Cities. In Library of the Written Word—The Handpress World; Brill: Leiden, The Netherlands, 2012; ISBN 9789004235755. [Google Scholar]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Baiano, A. 3D Printed Foods: A Comprehensive Review on Technologies, Nutritional Value, Safety, Consumer Attitude, Regulatory Framework, and Economic and Sustainability Issues. Food Rev. Int. 2020. [Google Scholar] [CrossRef]
- Chen, C.; Jiang, J.; He, W.; Lei, W.; Hao, Q.; Zhang, X. 3D Printed High-Loading Lithium-Sulfur Battery Toward Wearable Energy Storage. Adv. Funct. Mater. 2020, 30, 1909469. [Google Scholar] [CrossRef]
- Malone, E.; Lipson, H. Fab@Home: The personal desktop fabricator kit. Rapid Prototyp. J. 2007, 13, 245–255. [Google Scholar] [CrossRef]
- Ellam, R. Editor’s Pick: 3D Printing: You Read It Here First. Available online: https://www.newscientist.com/letter/mg23230991-100-1-editors-pick-3d-printing-you-read-it-here-first/ (accessed on 24 May 2020).
- Kodama, H. Hideo Kodama Automatic method for fabricating a three-dimensional plastic model with photo-hardening polymer. Rev. Sci. Instrum. 1998, 52, 2–6. [Google Scholar]
- Hull, C. Apparatus for Production of Three Dimensional Objects by Stereolithography. U.S. Patent 4,575,330A, 1986. [Google Scholar]
- Nakamura, M.; Iwanaga, S.; Henmi, C.; Arai, K.; Nishiyama, Y. Biomatrices and biomaterials for future developments of bioprinting and biofabrication. Biofabrication 2010, 2. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, M.; Wang, D.; Sun, L.; Webster, T.J. Nanoscale 3D bioprinting for osseous tissue manufacturing. Int. J. Nanomed. 2020, 15, 215–226. [Google Scholar] [CrossRef] [Green Version]
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D.H. 3D bioprinting for engineering complex tissues. Biotechnol. Adv. 2016, 34, 422–434. [Google Scholar] [CrossRef] [Green Version]
- Chia, H.N.; Wu, B.M. Recent advances in 3D printing of biomaterials. J. Biol. Eng. 2015, 9, 4. [Google Scholar] [CrossRef] [Green Version]
- Jamróz, W.; Szafraniec, J.; Kurek, M.; Jachowicz, R. 3D printing in pharmaceutical and medical applications. Pharm. Res. 2018, 35, 176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horn, T.J.; Harrysson, O.L.A. Overview of current additive manufacturing technologies and selected applications. Sci. Prog. 2012, 95, 255–282. [Google Scholar] [CrossRef] [PubMed]
- Rahim, T.N.A.T.; Abdullah, A.M.; Akil, H.M. Recent Developments in Fused Deposition Modeling-Based 3D Printing of Polymers and Their Composites. Polym. Rev. 2019, 59, 589–624. [Google Scholar] [CrossRef]
- Zein, I.; Hutmacher, D.W.; Tan, K.C.; Teoh, S.H. Fused deposition modeling of novel scaffold architectures for tissue engineering applications. Biomaterials 2002, 23, 1169–1185. [Google Scholar] [CrossRef]
- Baca, D.; Ahmad, R. The impact on the mechanical properties of multi-material polymers fabricated with a single mixing nozzle and multi-nozzle systems via fused deposition modeling. Int. J. Adv. Manuf. Technol. 2020, 106, 4509–4520. [Google Scholar] [CrossRef]
- Camposeco-Negrete, C. Optimization of printing parameters in fused deposition modeling for improving part quality and process sustainability. Int. J. Adv. Manuf. Technol. 2020, 108, 2131–2147. [Google Scholar] [CrossRef]
- Vicente, C.M.S.; Martins, T.S.; Leite, M.; Ribeiro, A.; Reis, L. Influence of fused deposition modeling parameters on the mechanical properties of ABS parts. Polym. Adv. Technol. 2020, 31, 501–507. [Google Scholar] [CrossRef]
- Wang, S.; Ma, Y.; Deng, Z.; Zhang, S.; Cai, J. Effects of fused deposition modeling process parameters on tensile, dynamic mechanical properties of 3D printed polylactic acid materials. Polym. Test. 2020, 86, 106483. [Google Scholar] [CrossRef]
- Sahmani, S.; Khandan, A.; Esmaeili, S.; Saber-Samandari, S.; Ghadiri Nejad, M.; Aghdam, M.M. Calcium phosphate-PLA scaffolds fabricated by fused deposition modeling technique for bone tissue applications: Fabrication, characterization and simulation. Ceram. Int. 2020, 46, 2447–2456. [Google Scholar] [CrossRef]
- Tarrés, Q.; Melbø, J.K.; Delgado-Aguilar, M.; Espinach, F.X.; Mutjé, P.; Chinga-Carrasco, G. Bio-polyethylene reinforced with thermomechanical pulp fibers: Mechanical and micromechanical characterization and its application in 3D-printing by fused deposition modelling. Compos. Part B Eng. 2018, 153, 70–77. [Google Scholar] [CrossRef]
- Melchels, F.P.W.; Feijen, J.; Grijpma, D.W. A review on stereolithography and its applications in biomedical engineering. Biomaterials 2010, 31, 6121–6130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.L.; Cheah, C.M.; Fuh, J.Y.H.; Lu, L. Influence of process parameters on stereolithography part shrinkage. Mater. Des. 1996, 17, 205–213. [Google Scholar] [CrossRef]
- Kazemi, M.; Rahimi, A. Stereolithography process optimization for tensile strength improvement of products. Rapid Prototyp. J. 2018, 24, 688–697. [Google Scholar] [CrossRef]
- Cho, Y.H.; Lee, I.H.; Cho, D.W. Laser scanning path generation considering photopolymer solidification in micro-stereolithography. Microsyst. Technol. 2005, 11, 158–167. [Google Scholar] [CrossRef]
- Munprom, R.; Limtasiri, S. Optimization of stereolithographic 3D printing parameters using Taguchi method for improvement in mechanical properties. Mater. Today Proc. 2019, 17, 1768–1773. [Google Scholar] [CrossRef]
- Heller, C.; Schwentenwein, M.; Russmueller, G.; Varga, F.; Stampfl, J.; Liska, R. Vinyl Esters: Low Cytotoxicity Monomers for the Fabrication of Biocompatible 3D Scaffolds by Lithography Based Additive Manufacturing. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 6941–6954. [Google Scholar] [CrossRef]
- Bail, R.; Hong, J.Y.; Chin, B.D. Effect of a Red-Shifted benzotriazole UV absorber on curing depth and kinetics in visible light initiated photopolymer resins for 3D printing. J. Ind. Eng. Chem. 2016, 38, 141–145. [Google Scholar] [CrossRef]
- Zhou, R.; Malval, J.P.; Jin, M.; Spangenberg, A.; Pan, H.; Wan, D.; Morlet-Savary, F.; Knopf, S. A Two-Photon active Chevron-Shaped type i photoinitiator designed for 3D stereolithography. Chem. Commun. 2019, 55, 6233–6236. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, X.N.; Sun, C. Micro-Stereolithography of polymeric and ceramic microstructures. Sens. Actuators A Phys. 1999, 77, 149–156. [Google Scholar] [CrossRef]
- Sun, C.; Fang, N.; Wu, D.M.; Zhang, X. Projection Micro-Stereolithography using digital Micro-Mirror dynamic mask. Sens. Actuators A Phys. 2005, 121, 113–120. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, M.; Zhou, Z.; Gou, J.; Hui, D. 3D printing of polymer matrix composites: A review and prospective. Compos. Part B Eng. 2017, 110, 442–458. [Google Scholar] [CrossRef]
- Anna, M.Z. Effect of laser energy density, internal porosity and heat treatment on mechanical behavior of biomedical Ti6Al4V alloy obtained with DMLS technology. Materials 2019, 12, 2331. [Google Scholar] [CrossRef] [Green Version]
- Iveković, A.; Montero-Sistiaga, M.L.; Vanmeensel, K.; Kruth, J.P.; Vleugels, J. Effect of processing parameters on microstructure and properties of tungsten heavy alloys fabricated by SLM. Int. J. Refract. Met. Hard Mater. 2019, 82, 23–30. [Google Scholar] [CrossRef]
- Lee, H.; Lim, C.H.J.; Low, M.J.; Tham, N.; Murukeshan, V.M.; Kim, Y.J. Lasers in additive manufacturing: A review. Int. J. Precis. Eng. Manuf. Green Technol. 2017, 4, 307–322. [Google Scholar] [CrossRef]
- Goodridge, R.D.; Shofner, M.L.; Hague, R.J.M.; McClelland, M.; Schlea, M.R.; Johnson, R.B.; Tuck, C.J. Processing of a Polyamide-12/carbon nanofibre composite by laser sintering. Polym. Test. 2011, 30, 94–100. [Google Scholar] [CrossRef]
- Singh, M.; Haverinen, H.M.; Dhagat, P.; Jabbour, G.E. Inkjet Printing-Process and its applications. Adv. Mater. 2010, 22, 673–685. [Google Scholar] [CrossRef]
- Cui, X.; Dean, D.; Ruggeri, Z.M.; Boland, T. Cell damage evaluation of thermal inkjet printed chinese hamster ovary cells. Biotechnol. Bioeng. 2010, 106, 963–969. [Google Scholar] [CrossRef]
- Goldmann, T.; Gonzalez, J.S. DNA-printing: Utilization of a standard inkjet printer for the transfer of nucleic acids to solid supports. J. Biochem. Biophys. Methods 2000, 42, 105–110. [Google Scholar] [CrossRef]
- Okamoto, T.; Suzuki, T.; Yamamoto, N. Microarray fabrication with covalent attachment of DNA using Bubble Jet technology. Nat. Biotechnol. 2000, 18, 438–441. [Google Scholar] [CrossRef]
- Xu, T.; Gregory, C.A.; Molnar, P.; Cui, X.; Jalota, S.; Bhaduri, S.B.; Boland, T. Viability and electrophysiology of neural cell structures generated by the inkjet printing method. Biomaterials 2006, 27, 3580–3588. [Google Scholar] [CrossRef]
- Xu, T.; Jin, J.; Gregory, C.; Hickman, J.J.; Boland, T. Inkjet printing of viable mammalian cells. Biomaterials 2005, 26, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Tekin, E.; Smith, P.J.; Schubert, U.S. Inkjet printing as a deposition and patterning tool for polymers and inorganic particles. Soft Matter 2008, 4, 703–713. [Google Scholar] [CrossRef]
- Orloff, N.D.; Truong, C.; Cira, N.; Koo, S.; Hamilton, A.; Choi, S.; Wu, V.; Riedel-Kruse, I.H. Integrated bioprinting and imaging for scalable, networkable desktop experimentation. RSC Adv. 2014, 4, 34721–34728. [Google Scholar] [CrossRef]
- Mohammadi, Z.; Rabbani, M. Bacterial bioprinting on a flexible substrate for fabrication of a colorimetric temperature indicator by using a commercial inkjet printer. J. Med. Signals Sens. 2018, 8, 170. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Ma, X.; Gou, M.; Mei, D.; Zhang, K.; Chen, S. 3D printing of functional biomaterials for tissue engineering. Curr. Opin. Biotechnol. 2016, 40, 103–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chrisey, D.B. The power of direct writing. Science 2000, 289, 879–881. [Google Scholar] [CrossRef] [PubMed]
- Bohandy, J.; Kim, B.F.; Adrian, F.J. Metal deposition from a supported metal film using an excimer laser. J. Appl. Phys. 1986, 60, 1538–1539. [Google Scholar] [CrossRef]
- Barron, J.A.; Ringeisen, B.R.; Kim, H.; Spargo, B.J.; Chrisey, D.B. Application of laser printing to mammalian cells. Thin Solid Films 2004, 453, 383–387. [Google Scholar] [CrossRef]
- Duocastella, M.; Colina, M.; Fernández-Pradas, J.M.; Serra, P.; Morenza, J.L. Study of the laser-induced forward transfer of liquids for laser bioprinting. Appl. Surf. Sci. 2007, 253, 7855–7859. [Google Scholar] [CrossRef]
- Kattamis, N.T.; Purnick, P.E.; Weiss, R.; Arnold, C.B. Thick film laser induced forward transfer for deposition of thermally and mechanically sensitive materials. Appl. Phys. Lett. 2007, 91, 171120. [Google Scholar] [CrossRef] [Green Version]
- Koch, L.; Brandt, O.; Deiwick, A.; Chichkov, B. Laser-assisted bioprinting at different wavelengths and pulse durations with a metal dynamic release layer: A parametric study. Int. J. Bioprint. 2017, 3, 42–53. [Google Scholar] [CrossRef] [Green Version]
- Guillemot, F.; Souquet, A.; Catros, S.; Guillotin, B. Laser-assisted cell printing: Principle, physical parameters versus cell fate and perspectives in tissue engineering. Nanomedicine 2010, 5, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Koch, L.; Kuhn, S.; Sorg, H.; Gruene, M.; Schlie, S.; Gaebel, R.; Polchow, B.; Reimers, K.; Stoelting, S.; Ma, N.; et al. Laser printing of skin cells and human stem cells. Tissue Eng. Part C Methods 2010, 16, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Hopp, B.; Smausz, T.; Kresz, N.; Barna, N.; Bor, Z.; Kolozsvári, L.; Chrisey, D.B.; Szabó, A.; Nógrádi, A. Survival and proliferative ability of various living cell types after laser-induced forward transfer. Tissue Eng. 2005, 11, 1817–1823. [Google Scholar] [CrossRef]
- Gruene, M.; Deiwick, A.; Koch, L.; Schlie, S.; Unger, C.; Hofmann, N.; Bernemann, I.; Glasmacher, B.; Chichkov, B. Laser printing of stem cells for biofabrication of scaffold-free autologous grafts. Tissue Eng. Part C Methods 2010, 17, 79–87. [Google Scholar] [CrossRef]
- Sears, N.A.; Seshadri, D.R.; Dhavalikar, P.S.; Cosgriff-Hernandez, E. A Review of Three-Dimensional Printing in Tissue Engineering. Tissue Eng. Part B Rev. 2016, 22, 298–310. [Google Scholar] [CrossRef]
- Chang, C.C.; Boland, E.D.; Williams, S.K.; Hoying, J.B. Direct-write bioprinting three-dimensional biohybrid systems for future regenerative therapies. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 98, 160–170. [Google Scholar] [CrossRef] [Green Version]
- Emmermacher, J.; Spura, D.; Cziommer, J.; Kilian, D.; Wollborn, T.; Fritsching, U.; Steingroewer, J.; Walther, T.; Gelinsky, M.; Lode, A. Engineering considerations on extrusion-based bioprinting: Interactions of material behavior, mechanical forces and cells in the printing needle. Biofabrication 2020, 12, 025022. [Google Scholar] [CrossRef]
- Is Hybrid Manufacturing Technology the Future of Additive Manufacturing? Available online: https://amfg.ai/2018/07/10/hybrid-technology-the-future-of-manufacturing/ (accessed on 7 June 2020).
- Flynn, J.M.; Shokrani, A.; Newman, S.T.; Dhokia, V. Hybrid additive and subtractive machine tools - Research and industrial developments. Int. J. Mach. Tools Manuf. 2016, 101, 79–101. [Google Scholar] [CrossRef] [Green Version]
- Bae, W.G.; Kim, H.N.; Kim, D.; Park, S.H.; Jeong, H.E.; Suh, K.Y. 25th anniversary article: Scalable multiscale patterned structures inspired by nature: The role of hierarchy. Adv. Mater. 2014, 26, 675–700. [Google Scholar] [CrossRef]
- Liu, C.; Xia, Z.; Czernuszka, J.T. Design and development of three-dimensional scaffolds for tissue engineering. Chem. Eng. Res. Des. 2007, 85, 1051–1064. [Google Scholar] [CrossRef]
- Giannitelli, S.M.; Mozetic, P.; Trombetta, M.; Rainer, A. Combined additive manufacturing approaches in tissue engineering. Acta Biomater. 2015, 24, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.R.C.; Almeida, H.A.; Bártolo, P.J. Additive manufacturing techniques for scaffold-based cartilage tissue engineering: A review on various additive manufacturing technologies in generating scaffolds for cartilage tissue engineering. Virtual Phys. Prototyp. 2013, 8, 175–186. [Google Scholar] [CrossRef]
- Fomby, P.; Cherlin, A.J.; Hadjizadeh, A.; Doillon, C.J.; Sueblinvong, V.; Weiss, D.J.; Bates, J.H.T.; Gilbert, T.; Liles, W.C.; Lutzko, C.; et al. Stem cells and cell therapies in lung biology and diseases: Conference report. Ann. Am. Thorac. Soc. 2010, 12, 181–204. [Google Scholar] [CrossRef]
- Ding, C.; Qiao, Z.; Jiang, W.; Li, H.; Wei, J.; Zhou, G.; Dai, K. Regeneration of a goat femoral head using a tissue-specific, biphasic scaffold fabricated with CAD/CAM technology. Biomaterials 2013, 34, 6706–6716. [Google Scholar] [CrossRef] [PubMed]
- Vaquette, C.; Fan, W.; Xiao, Y.; Hamlet, S.; Hutmacher, D.W.; Ivanovski, S. A biphasic scaffold design combined with cell sheet technology for simultaneous regeneration of alveolar bone/periodontal ligament complex. Biomaterials 2012, 33, 5560–5573. [Google Scholar] [CrossRef]
- Schek, R.M.; Taboas, J.M.; Segvich, S.J.; Hollister, S.J.; Krebsbach, P.H. Engineered Osteochondral Grafts Using Biphasic Composite Solid Free-Form Fabricated Scaffolds. Tissue Eng. 2004, 10, 1376–1385. [Google Scholar] [CrossRef] [Green Version]
- Saniei, H.; Mousavi, S. Surface modification of PLA 3D-printed implants by electrospinning with enhanced bioactivity and cell affinity. Polymer 2020, 196, 122467. [Google Scholar] [CrossRef]
- Moroni, L.; Schotel, R.; Hamann, D.; De Wijn, J.R.; Van Blitterswijk, C.A. 3D fiber-deposited electrospun integrated scaffolds enhance cartilage tissue formation. Adv. Funct. Mater. 2008, 18, 53–60. [Google Scholar] [CrossRef]
- Sooriyaarachchi, D.; Minière, H.J.; Maharubin, S.; Tan, G.Z. Hybrid Additive Microfabrication Scaffold Incorporated with Highly Aligned Nanofibers for Musculoskeletal Tissues. Tissue Eng. Regen. Med. 2019, 16, 29–38. [Google Scholar] [CrossRef]
- Arafat, M.T.; Gibson, I.; Li, X. State of the art and future direction of additive manufactured scaffolds-based bone tissue engineering. Rapid Prototyp. J. 2014, 20, 13–26. [Google Scholar] [CrossRef]
- Oliveira, S.M.; Silva, T.H.; Reis, R.L.; Mano, J.F. Hierarchical Fibrillar Scaffolds Obtained by Non-conventional Layer-By-Layer Electrostatic Self-Assembly. Adv. Healthc. Mater. 2013, 2, 422–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, R.; Morsi, Y.; Patel, S.; Ke, Q.F.; Mo, X.M. A novel approach via combination of electrospinning and FDM for tri-leaflet heart valve scaffold fabrication. Front. Mater. Sci. China 2009, 3, 359–366. [Google Scholar] [CrossRef]
- Centola, M.; Rainer, A.; Spadaccio, C.; De Porcellinis, S.; Genovese, J.A.; Trombetta, M. Combining electrospinning and fused deposition modeling for the fabrication of a hybrid vascular graft. Biofabrication 2010, 2, 014102. [Google Scholar] [CrossRef] [Green Version]
- Peña, J.; Román, J.; Victoria Cabañas, M.; Vallet-Regí, M. An alternative technique to shape scaffolds with hierarchical porosity at physiological temperature. Acta Biomater. 2010, 6, 1288–1296. [Google Scholar] [CrossRef]
- Tan, J.Y.; Chua, C.K.; Leong, K.F. Fabrication of channeled scaffolds with ordered array of micro-pores through microsphere leaching and indirect Rapid Prototyping technique. Biomed. Microdevices 2013, 15, 83–96. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, H.; Zhao, L.; Hu, Q.; Fang, M. Design and preparation of bone tissue engineering scaffolds with porous controllable structure. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2009, 24, 174–180. [Google Scholar] [CrossRef]
- Taboas, J.M.; Maddox, R.D.; Krebsbach, P.H.; Hollister, S.J. Indirect solid free form fabrication of local and global porous, biomimetic and composite 3D polymer-ceramic scaffolds. Biomaterials 2003, 24, 181–194. [Google Scholar] [CrossRef]
- Jensen, J.; Rölfing, J.H.D.; Le Svend, D.Q.; Kristiansen, A.A.; Nygaard, J.V.; Hokland, L.B.; Bendtsen, M.; Kassem, M.; Lysdahl, H.; Bünger, C.E. Surface-Modified functionalized polycaprolactone scaffolds for bone repair: In vitro and in vivo experiments. J. Biomed. Mater. Res. Part A 2014, 102, 2993–3003. [Google Scholar] [CrossRef] [Green Version]
- Dorj, B.; Park, J.H.; Kim, H.W. Robocasting chitosan/nanobioactive glass dual-pore structured scaffolds for bone engineering. Mater. Lett. 2012, 73, 119–122. [Google Scholar] [CrossRef]
- Yen, H.J.; Hsu, S.H.; Tseng, C.S.; Huang, J.P.; Tsai, C.L. Fabrication of precision scaffolds using liquid-frozen deposition manufacturing for cartilage tissue engineering. Tissue Eng. Part A 2009, 15, 965–975. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Yan, Y.; Wang, S.; Zhang, R.; Zhang, C. Fabrication of porous scaffolds for bone tissue engineering via low-temperature deposition. Scr. Mater. 2002, 46, 771–776. [Google Scholar] [CrossRef]
- Kim, G.; Ahn, S.; Yoon, H.; Kim, Y.; Chun, W. A cryogenic direct-plotting system for fabrication of 3D collagen scaffolds for tissue engineering. J. Mater. Chem. 2009, 19, 8817–8823. [Google Scholar] [CrossRef]
- Pham, C.B.; Leong, K.F.; Lim, T.C.; Chian, K.S. Rapid freeze prototyping technique in bio-plotters for tissue scaffold fabrication. Rapid Prototyp. J. 2008, 14, 246–253. [Google Scholar] [CrossRef] [Green Version]
- Zhu, N.; Li, M.G.; Cooper, D.; Chen, X.B. Development of novel hybrid poly(l-lactide)/chitosan scaffolds using the rapid freeze prototyping technique. Biofabrication 2011, 3, 034105. [Google Scholar] [CrossRef] [PubMed]
- Mota, C.; Puppi, D.; Dinucci, D.; Gazzarri, M.; Chiellini, F. Additive manufacturing of star poly(ε-caprolactone) wet-spun scaffolds for bone tissue engineering applications. J. Bioact. Compat. Polym. 2013, 28, 320–340. [Google Scholar] [CrossRef]
- Puppi, D.; Mota, C.; Gazzarri, M.; Dinucci, D.; Gloria, A.; Myrzabekova, M.; Ambrosio, L.; Chiellini, F. Additive manufacturing of wet-spun polymeric scaffolds for bone tissue engineering. Biomed. Microdevices 2012, 14, 1115–1127. [Google Scholar] [CrossRef]
- Alexander, F.A.; Johnson, L.; Williams, K.; Packer, K. A Parameter Study for 3D-Printing Organized Nanofibrous Collagen Scaffolds Using Direct-Write Electrospinning Frank. Materials 2019, 12, 4131. [Google Scholar] [CrossRef] [Green Version]
- Abdal-hay, A.; Abbasi, N.; Gwiazda, M.; Hamlet, S.; Ivanovski, S. Novel polycaprolactone/hydroxyapatite nanocomposite fibrous scaffolds by direct melt-electrospinning writing. Eur. Polym. J. 2018, 105, 257–264. [Google Scholar] [CrossRef] [Green Version]
- Farrugia, B.L.; Brown, T.D.; Upton, Z.; Hutmacher, D.W.; Dalton, P.D.; Dargaville, T.R. Dermal fibroblast infiltration of poly(ε-caprolactone) scaffolds fabricated by melt electrospinning in a direct writing mode. Biofabrication 2013, 5, 025001. [Google Scholar] [CrossRef]
- Muerza-Cascante, M.L.; Haylock, D.; Hutmacher, D.W.; Dalton, P.D. Melt electrospinning and its technologization in tissue engineering. Tissue Eng. Part B Rev. 2015, 21, 187–202. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Dong, J. Direct fabrication of high-resolution three-dimensional polymeric scaffolds using electrohydrodynamic hot jet plotting. J. Micromech. Microeng. 2013, 23. [Google Scholar] [CrossRef]
- Li, J.L.; Cai, Y.L.; Guo, Y.L.; Fuh, J.Y.H.; Sun, J.; Hong, G.S.; Lam, R.N.; Wong, Y.S.; Wang, W.; Tay, B.Y.; et al. Fabrication of three-dimensional porous scaffolds with controlled filament orientation and large pore size via an improved E-jetting technique. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Talbot, E.L.; Berson, A.; Brown, P.S.; Bain, C.D. Evaporation of picoliter droplets on surfaces with a range of wettabilities and thermal conductivities. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2012, 85, 061604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hopp, B. Femtosecond laser printing of living cells using absorbing film-assisted laser-induced forward transfer. Opt. Eng. 2012, 51, 014302. [Google Scholar] [CrossRef]
- Duymaz, B.T.; Erdiler, F.B.; Alan, T.; Aydogdu, M.O.; Inan, A.T.; Ekren, N.; Uzun, M.; Sahin, Y.M.; Bulus, E.; Oktar, F.N.; et al. 3D bio-printing of levan/polycaprolactone/gelatin blends for bone tissue engineering: Characterization of the cellular behavior. Eur. Polym. J. 2019, 119, 426–437. [Google Scholar] [CrossRef]
- Discher, D.E.; Janmey, P.; Wang, Y.L. Tissue cells feel and respond to the stiffness of their substrate. Science 2005, 310, 1139–1143. [Google Scholar] [CrossRef] [Green Version]
- Bauer, M.; Kim, K.; Qiu, Y.; Calpe, B.; Khademhosseini, A.; Liao, R.; Wheeldon, I. Spot identification and quality control in cell-based microarrays. ACS Comb. Sci. 2012, 14, 471–477. [Google Scholar] [CrossRef] [Green Version]
- Nikkhah, M.; Eshak, N.; Zorlutuna, P.; Annabi, N.; Castello, M.; Kim, K.; Dolatshahi-Pirouz, A.; Edalat, F.; Bae, H.; Yang, Y.; et al. Directed endothelial cell morphogenesis in micropatterned gelatin methacrylate hydrogels. Biomaterials 2012, 33, 9009–9018. [Google Scholar] [CrossRef] [Green Version]
- Polyzois, G.L. In vitro evaluation of dental materials. Clin. Mater. 1994, 16, 21–60. [Google Scholar] [CrossRef]
- Williams, D.F. On the mechanisms of biocompatibility. Biomaterials 2008, 29, 2941–2953. [Google Scholar] [CrossRef] [PubMed]
- West, J.L.; Hubbell, J.A. Polymeric biomaterials with degradation sites for proteases involved in cell migration. Macromolecules 1999, 32, 241–244. [Google Scholar] [CrossRef]
- Limpanuphap, S.; Derby, B. Manufacture of biomaterials by a novel printing process. J. Mater. Sci. Mater. Med. 2002, 13, 1163–1166. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Skardal, A.; Atala, A. Evaluation of hydrogels for bio-printing applications. J. Biomed. Mater. Res. Part A 2013, 101 A, 272–284. [Google Scholar] [CrossRef]
- Benson, K.; Galla, H.J.; Kehr, N.S. Cell adhesion behavior in 3D hydrogel scaffolds functionalized with D-Or L-aminoacids. Macromol. Biosci. 2014, 14, 793–798. [Google Scholar] [CrossRef]
- Kim, H.N.; Kang, D.H.; Kim, M.S.; Jiao, A.; Kim, D.H.; Suh, K.Y. Patterning methods for polymers in cell and tissue engineering. Ann. Biomed. Eng. 2012, 40, 1339–1355. [Google Scholar] [CrossRef]
- Hunt, N.C.; Grover, L.M. Cell encapsulation using biopolymer gels for regenerative medicine. Biotechnol. Lett. 2010, 32, 733–742. [Google Scholar] [CrossRef]
- Spiller, K.L.; Maher, S.A.; Lowman, A.M. Hydrogels for the repair of articular cartilage defects. Tissue Eng. Part B Rev. 2011, 17, 281–299. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Kawashita, M. Current progress in inorganic artificial biomaterials. J. Artif. Organs 2011, 14, 163–170. [Google Scholar] [CrossRef]
- Zorlutuna, P.; Vrana, N.E.; Khademhosseini, A. The expanding world of tissue engineering: The building blocks and new applications of tissue engineered constructs. IEEE Rev. Biomed. Eng. 2013, 6, 47–62. [Google Scholar] [CrossRef]
- Pati, F.; Jang, J.; Ha, D.H.; Won Kim, S.; Rhie, J.W.; Shim, J.H.; Kim, D.H.; Cho, D.W. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat. Commun. 2014, 5, 3935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donderwinkel, I.; Van Hest, J.C.M.; Cameron, N.R. Bio-inks for 3D bioprinting: Recent advances and future prospects. Polym. Chem. 2017, 8, 4451–4471. [Google Scholar] [CrossRef] [Green Version]
- Suntornnond, R.; An, J.; Chua, C.K. Bioprinting of Thermoresponsive Hydrogels for Next Generation Tissue Engineering: A Review. Macromol. Mater. Eng. 2017, 302, 1–15. [Google Scholar] [CrossRef]
- Müller, M.; Becher, J.; Schnabelrauch, M.; Zenobi-Wong, M. Printing thermoresponsive reverse molds for the creation of patterned two-component hydrogels for 3D cell culture. J. Vis. Exp. 2013, 77, e50632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kesti, M.; Eberhardt, C.; Pagliccia, G.; Kenkel, D.; Grande, D.; Boss, A.; Zenobi-Wong, M. Bioprinting Complex Cartilaginous Structures with Clinically Compliant Biomaterials. Adv. Funct. Mater. 2015, 25, 7406–7417. [Google Scholar] [CrossRef] [Green Version]
- Ambesi-Impiombato, F.S.; Parks, L.A.M.; Coon, H.G. Culture of hormone-dependent functional epithelial cells from rat thyroids. Proc. Natl. Acad. Sci. USA 1980, 77, 3455–3459. [Google Scholar] [CrossRef] [Green Version]
- Hamm, A.; Krott, N.; Breibach, I.; Blindt, R.; Bosserhoff, A.K. Efficient transfection method for primary cells. Tissue Eng. 2002, 8, 235–245. [Google Scholar] [CrossRef]
- Okumura, N.; Ueno, M.; Koizumi, N.; Sakamoto, Y.; Hirata, K.; Hamuro, J.; Kinoshita, S. Enhancement on primate corneal endothelial cell survival in vitro by a rock inhibitor. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3680–3687. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, M.; Fu, P.; Xie, M.; Wang, W.; Luo, X. ROCK inhibition with Y27632 promotes the proliferation and cell cycle progression of cultured astrocyte from spinal cord. Neurochem. Int. 2012, 61, 1114–1120. [Google Scholar] [CrossRef]
- Ozbolat, I.T.; Yu, Y. Bioprinting toward organ fabrication: Challenges and future trends. IEEE Trans. Biomed. Eng. 2013, 60, 691–699. [Google Scholar] [CrossRef]
- Dimri, G.P.; Lee, X.; Basile, G.; Acosta, M.; Scott, G.; Roskelley, C.; Medrano, E.E.; Linskens, M.; Rubelj, I.; Pereira-Smith, O.; et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 1995, 92, 9363–9367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.M.; Elabd, C.; Amri, E.Z.; Ailhaud, G.; Dani, C. The human adipose tissue is a source of multipotent stem cells. Biochimie 2005, 87, 125–128. [Google Scholar] [CrossRef]
- De Coppi, P.; Bartsch, G.; Siddiqui, M.M.; Xu, T.; Santos, C.C.; Perin, L.; Mostoslavsky, G.; Serre, A.C.; Snyder, E.Y.; Yoo, J.J.; et al. Isolation of amniotic stem cell lines with potential for therapy. Nat. Biotechnol. 2007, 25, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Derakhshanfar, S.; Mbeleck, R.; Xu, K.; Zhang, X.; Zhong, W.; Xing, M. 3D bioprinting for biomedical devices and tissue engineering: A review of recent trends and advances. Bioact. Mater. 2018, 3, 144–156. [Google Scholar] [CrossRef]
- Borden, W.B.; Bravata, D.M.; Dai, S.; Gillespie, C.; Hailpern, S.M.; Heit, J.A.; Kittner, S.J.; Lackland, D.T.; Judith, H. Heart Disease and Stroke Statistics—2013 Update: A Report From the American Heart Association. Circulation 2017, 127, 498. [Google Scholar] [CrossRef]
- Bertassoni, L.E.; Cecconi, M.; Manoharan, V.; Nikkhah, M.; Hjortnaes, J.; Cristino, A.L.; Barabaschi, G.; Demarchi, D.; Dokmeci, M.R.; Yang, Y.; et al. Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs. Lab Chip 2014, 14, 2202–2211. [Google Scholar] [CrossRef] [Green Version]
- Kolesky, D.B.; Truby, R.L.; Gladman, A.S.; Busbee, T.A.; Homan, K.A.; Lewis, J.A. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv. Mater. 2014, 26, 3124–3130. [Google Scholar] [CrossRef]
- Duan, B.; Kapetanovic, E.; Hockaday, L.A.; Butcher, J.T. Three-dimensional printed trileaflet valve conduits using biological hydrogels and human valve interstitial cells. Acta Biomater. 2014, 10, 1836–1846. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Chen, M.; Fan, X.; Zhou, H. Recent advances in bioprinting techniques: Approaches, applications and future prospects. J. Transl. Med. 2016, 14, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Zhou, H.; Tang, M.; Weir, M.D.; Bao, C.; Xu, H.H.K. Gas-foaming calcium phosphate cement scaffold encapsulating human umbilical cord stem cells. Tissue Eng. Part A 2012, 18, 816–827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thein-Han, W.; Xu, H.H.K. Prevascularization of a gas-foaming macroporous calcium phosphate cement scaffold via coculture of human umbilical vein endothelial cells and osteoblasts. Tissue Eng. Part A 2013, 19, 1675–1685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, T.G.; Chung, H.J.; Park, T.G. Macroporous and nanofibrous hyaluronic acid/collagen hybrid scaffold fabricated by concurrent electrospinning and deposition/leaching of salt particles. Acta Biomater. 2008, 4, 1611–1619. [Google Scholar] [CrossRef]
- Mehrabanian, M.; Nasr-Esfahani, M. HA/nylon 6,6 porous scaffolds fabricated by salt-leaching/solvent casting technique: Effect of nano-sized filler content on scaffold properties. Int. J. Nanomed. 2011, 6, 1651–1659. [Google Scholar]
- Alizadeh, M.; Abbasi, F.; Khoshfetrat, A.B.; Ghaleh, H. Microstructure and characteristic properties of gelatin/chitosan scaffold prepared by a combined freeze-drying/leaching method. Mater. Sci. Eng. C 2013, 33, 3958–3967. [Google Scholar] [CrossRef] [PubMed]
- Gerçek, I.; Tiǧli, R.S.; Gumusderelioglu, M. A novel scaffold based on formation and agglomeration of PCL microbeads by freeze-drying. J. Biomed. Mater. Res. Part A 2008, 86, 1012–1022. [Google Scholar] [CrossRef] [PubMed]
- Castilho, M.; Moseke, C.; Ewald, A.; Gbureck, U.; Groll, J.; Pires, I.; Teßmar, J.; Vorndran, E. Direct 3D powder printing of biphasic calcium phosphate scaffolds for substitution of complex bone defects. Biofabrication 2014, 6, 015006. [Google Scholar] [CrossRef] [PubMed]
- Pati, F.; Song, T.H.; Rijal, G.; Jang, J.; Kim, S.W.; Cho, D.W. Ornamenting 3D printed scaffolds with cell-laid extracellular matrix for bone tissue regeneration. Biomaterials 2015, 37, 230–241. [Google Scholar] [CrossRef]
- Wang, M.; Favi, P.; Cheng, X.; Golshan, N.H.; Ziemer, K.S.; Keidar, M.; Webster, T.J. Cold atmospheric plasma (CAP) surface nanomodified 3D printed polylactic acid (PLA) scaffolds for bone regeneration. Acta Biomater. 2016, 46, 256–265. [Google Scholar] [CrossRef]
- Lee, J.S.; Hong, J.M.; Jung, J.W.; Shim, J.H.; Oh, J.H.; Cho, D.W. 3D printing of composite tissue with complex shape applied to ear regeneration. Biofabrication 2014, 6, 024103. [Google Scholar] [CrossRef]
- Antich, C.; de Vicente, J.; Jiménez, G.; Chocarro, C.; Carrillo, E.; Montañez, E.; Gálvez-Martín, P.; Marchal, J.A. Bio-inspired hydrogel composed of hyaluronic acid and alginate as a potential bioink for 3D bioprinting of articular cartilage engineering constructs. Acta Biomater. 2020, 106, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, K.; Zhou, X.; Li, T.; Xu, Y.; Qiang, L.; Peng, M.; Xu, Y.; Xie, L.; He, C.; et al. Controllable fabrication of hydroxybutyl chitosan/oxidized chondroitin sulfate hydrogels by 3D bioprinting technique for cartilage tissue engineering. Biomed. Mater. 2019, 14, 025006. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Xie, R.; Zhang, J.; Liu, Y.; Li, Z.; Zhang, Y.; Zhang, X.; Yuan, T.; Chen, Y.; Fan, W. Low-Temperature Three-Dimensional Printing of Tissue Cartilage Engineered with Gelatin Methacrylamide. Tissue Eng. Part C Methods 2020, 26, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Michael, S.; Sorg, H.; Peck, C.T.; Koch, L.; Deiwick, A.; Chichkov, B.; Vogt, P.M.; Reimers, K. Tissue Engineered Skin Substitutes Created by Laser-Assisted Bioprinting Form Skin-Like Structures in the Dorsal Skin Fold Chamber in Mice. PLoS ONE 2013, 8, e57741. [Google Scholar] [CrossRef]
- Bale, S.S.; Vernetti, L.; Senutovitch, N.; Jindal, R.; Hegde, M.; Gough, A.; McCarty, W.J.; Bakan, A.; Bhushan, A.; Shun, T.Y.; et al. In vitro platforms for evaluating liver toxicity. Exp. Biol. Med. 2014, 239, 1180–1191. [Google Scholar] [CrossRef] [Green Version]
- Ikegami, T.; Maehara, Y. Transplantation: 3D printing of the liver in living donor liver transplantation. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 697–698. [Google Scholar] [CrossRef]
- Nakao, Y.; Kimura, H.; Sakai, Y.; Fujii, T. Bile canaliculi formation by aligning rat primary hepatocytes in a microfluidic device. Biomicrofluidics 2011, 5, 022212. [Google Scholar] [CrossRef] [Green Version]
- Huh, D.D.; Kim, D.H. JALA Special Issue: Microengineered Cell- and Tissue-Based Assays for Drug Screening and Toxicology Applications. J. Lab. Autom. 2015, 20, 79–81. [Google Scholar] [CrossRef]
- Nam, K.H.; Smith, A.S.T.; Lone, S.; Kwon, S.; Kim, D.H. Biomimetic 3D Tissue Models for Advanced High-Throughput Drug Screening. J. Lab. Autom. 2015, 20, 201–215. [Google Scholar] [CrossRef] [Green Version]
- Chang, R.; Emami, K.; Wu, H.; Sun, W. Biofabrication of a three-dimensional liver micro-organ as an in vitro drug metabolism model. Biofabrication 2010, 2, 045004. [Google Scholar] [CrossRef]
- Lee, V.; Singh, G.; Trasatti, J.P.; Bjornsson, C.; Xu, X.; Tran, T.N.; Yoo, S.S.; Dai, G.; Karande, P. Design and fabrication of human skin by three-dimensional bioprinting. Tissue Eng. Part C Methods 2014, 20, 473–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Samanipour, R.; Koo, K.I.; Kim, K. Organ-on-a-chip platforms for drug delivery and cell characterization: A review. Sens. Mater. 2015, 27, 487–506. [Google Scholar] [CrossRef]
- Connell, J.L.; Ritschdorff, E.T.; Whiteley, M.; Shear, J.B. 3D printing of microscopic bacterial communities. Proc. Natl. Acad. Sci. USA 2013, 110, 18380–18385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soman, P.; Chung, P.H.; Zhang, A.P.; Chen, S. Digital microfabrication of user-defined 3D microstructures in cell-laden hydrogels. Biotechnol. Bioeng. 2013, 110, 3038–3047. [Google Scholar] [CrossRef] [Green Version]
- Tumbleston, J.R.; Shirvanyants, D.; Ermoshkin, N.; Janusziewicz, R.; Johnson, A.R.; Kelly, D.; Chen, K.; Pinschmidt, R.; Rolland, J.P.; Ermoshkin, A.; et al. Continuous liquid interface production of 3D objects. Science 2015, 347, 1349–1352. [Google Scholar] [CrossRef]
- Ousterout, D.G.; Perez-Pinera, P.; Thakore, P.I.; Kabadi, A.M.; Brown, M.T.; Qin, X.; Fedrigo, O.; Mouly, V.; Tremblay, J.P.; Gersbach, C.A. Reading frame correction by targeted genome editing restores dystrophin expression in cells from duchenne muscular dystrophy patients. Mol. Ther. 2013, 21, 1718–1726. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Xu, B.; Puperi, D.S.; Yonezawa, A.L.; Wu, Y.; Tseng, H.; Cuchiara, M.L.; West, J.L.; Grande-Allen, K.J. Integrating valve-inspired design features into poly(ethylene glycol) hydrogel scaffolds for heart valve tissue engineering. Acta Biomater. 2015, 14, 11–21. [Google Scholar] [CrossRef] [Green Version]
- Shin, S.R.; Jung, S.M.; Zalabany, M.; Kim, K.; Zorlutuna, P.; Kim, S.B.; Nikkhah, M.; Khabiry, M.; Azize, M.; Kong, J.; et al. Carbon-nanotube-embedded hydrogel sheets for engineering cardiac constructs and bioactuators. ACS Nano 2013, 7, 2369–2380. [Google Scholar] [CrossRef] [Green Version]
- Kim, P.; Yuan, A.; Nam, K.H.; Jiao, A.; Kim, D.H. Fabrication of poly(ethylene glycol): Gelatin methacrylate composite nanostructures with tunable stiffness and degradation for vascular tissue engineering. Biofabrication 2014, 6, 024112. [Google Scholar] [CrossRef]
- Kawazoe, Y. Synthetic small molecules that control stem cell fate. Tanpakushitsu Kakusan Koso 2007, 52, 1800–1801. [Google Scholar]
- Li, X.-J.; Hu, B.-Y.; Jones, S.A.; Zhang, Y.-S.; LaVaute, T.; Du, Z.-W.; Zhang, S.-C. Directed Differentiation of Ventral Spinal Progenitors and Motor Neurons from Human Embryonic Stem Cells by Small Molecules. Stem Cells 2008, 26, 886–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Zhang, Q.; Wu, X.; Schultz, P.G.; Ding, S. Dedifferentiation of Lineage-Committed Cells by a Small Molecule. J. Am. Chem. Soc. 2004, 126, 410–411. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Borowiak, M.; Fox, J.L.; Maehr, R.; Osafune, K.; Davidow, L.; Lam, K.; Peng, L.F.; Schreiber, S.L.; Rubin, L.L.; et al. A small molecule that directs differentiation of human ESCs into the pancreatic lineage. Nat. Chem. Biol. 2009, 5, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Mattox, J.M. Additive Manufacturing and its Implications for Military Ethics. J. Mil. Ethics 2013, 12, 225–234. [Google Scholar] [CrossRef]
- Gilbert, F.; O’Connell, C.D.; Mladenovska, T.; Dodds, S. Print Me an Organ? Ethical and Regulatory Issues Emerging from 3D Bioprinting in Medicine. Sci. Eng. Ethics 2018, 24, 73–91. [Google Scholar] [CrossRef]
- van Schothorst, M.; Weeda, J.; Schiffers, K.; Oortwijn, W.; Hoekman, J.; Coppens, D.; De Bruin, M.L. Study on the Regulation of Advanced Therapies in Selected Jurisdictions; The Publications Office of the European Union: Luxembourg City, Luxembourg, 2016; ISBN 9789292007317. [Google Scholar]







| Methods | Advantages | Disadvantages | Characteristics | References | |
|---|---|---|---|---|---|
| Non-biological 3D printing | Fused deposition modeling (FDM) | Low cost, fast and easy process | High processing temperature | Continuous filaments of thermoplastics are heated into a semi-liquid state for extrusion | [14,17] [20,21,22] |
| Stereolithography (SLA) | Extremely high resolution, good for complex structures | Cytotoxicity, weak mechanical properties | UV light or electron beams to initiate polymerization reactions, nozzle-free | [33,34,35] | |
| Selective laser sintering (SLS) | Superior mechanical properties | Limited material selection, low resolution | Powder bed fusion process, high energy input, nozzle-free | [38,39] | |
| Electron beam melting (EBM) | |||||
| Direct energy deposition (DED) | Bulk metal repair and retrofit | [4] | |||
| 3D bioprinting | Inkjet bioprinting | Low cost, fast printing, widely accessible | Nozzle clogging | Conventional inkjet printing based technique | [47,48,49] |
| Laser-assisted bioprinting (LAB) | Non-contact, high cell viability | Complex operation, time consuming preparation | “Ribbon” structure preparation needed for printing material, nozzle-free | [3,13] [57,58,59] | |
| Extrusion bioprinting | Deposition of high-density cells | Low cell viability | Continuous filaments of bioink extruded by various driving forces | [3,62] |
| Advantages | Disadvantages | Advances | References | |
|---|---|---|---|---|
| Natural polymers | Close to native ECM and inherent bioactivities | Limited tunability | dECM in bioinks to closely resemble natural tissues | [115,116] |
| Synthetic polymers | Adjustable chemical and mechanical properties | Poor biocompatibility | Pluronics as a sacrificial material with outstanding bioprintability | [117,118,119] |
| PNIPAAM incorporated with other polymers for excellent printing resolution and high cell viability | [120] | |||
| Cells | Good proliferation and differentiation capability by STEM cells | Finite lifespan and culturing difficulties by many primary cell types | Stem cells from bone marrow and fat; perinatal stem cells from amniotic fluid | [125,126,127,128,129] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Z.; Gao, M.; Lobo, A.O.; Webster, T.J. 3D Bioprinting in Tissue Engineering for Medical Applications: The Classic and the Hybrid. Polymers 2020, 12, 1717. https://doi.org/10.3390/polym12081717
Xie Z, Gao M, Lobo AO, Webster TJ. 3D Bioprinting in Tissue Engineering for Medical Applications: The Classic and the Hybrid. Polymers. 2020; 12(8):1717. https://doi.org/10.3390/polym12081717
Chicago/Turabian StyleXie, Zelong, Ming Gao, Anderson O. Lobo, and Thomas J. Webster. 2020. "3D Bioprinting in Tissue Engineering for Medical Applications: The Classic and the Hybrid" Polymers 12, no. 8: 1717. https://doi.org/10.3390/polym12081717
APA StyleXie, Z., Gao, M., Lobo, A. O., & Webster, T. J. (2020). 3D Bioprinting in Tissue Engineering for Medical Applications: The Classic and the Hybrid. Polymers, 12(8), 1717. https://doi.org/10.3390/polym12081717