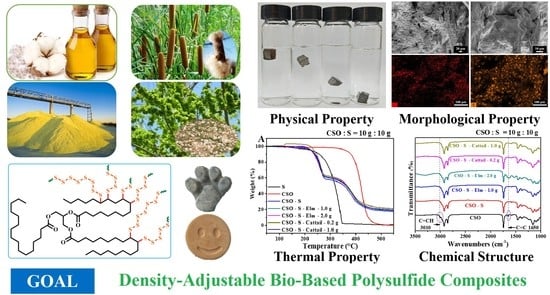
Density-Adjustable Bio-Based Polysulfide Composite Prepared by Inverse Vulcanization and Bio-Based Fillers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Bio-Based Polysulfide Composite
2.3. Density and Hardness Determination of Bio-Based Polysulfide Composite
2.4. Characterizations
3. Results and Discussion
3.1. Synthesis of Bio-Based Polysulfide Composite
3.2. Characterization
3.2.1. SEM and EDX
3.2.2. Elemental Analysis
3.2.3. DSC
3.2.4. FT-IR
3.2.5. Raman Spectra
3.3. Density Regulation of Bio-Based Polysulfide Composite
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Deshpande, A.S.; Khomane, R.B.; Vaidya, B.K.; Joshi, R.M.; Harle, A.S.; Kulkarni, B.D. Sulfur Nanoparticles synthesis and characterization from H2S gas, Using novel biodegradable iron chelates in W/O microemulsion. Nanoscale Res. Lett. 2008, 3, 221. [Google Scholar] [CrossRef] [Green Version]
- Chaudhuri, R.G.; Paria, S. Synthesis of sulfur nanoparticles in aqueous surfactant solutions. J. Colloid Interface Sci. 2010, 343, 439–446. [Google Scholar] [CrossRef]
- Chalker, J.M.; Worthington, M.J.H.; Lundquist, N.A.; Esdaile, L.J. Synthesis and applications of polymers made by inverse vulcanization. Top. Curr. Chem. 2019, 377, 16. [Google Scholar] [CrossRef]
- Wu, X.F.; Smith, J.A.; Petcher, S.; Zhang, B.W.; Parker, D.J.; Griffin, J.M.; Hasell, T. Catalytic inverse vulcanization. Nat. Commun. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Worthington, M.J.H.; Kucera, R.L.; Chalker, J.M. Green chemistry and polymers made from sulfur. Green Chem. 2017, 19, 2748–2761. [Google Scholar] [CrossRef] [Green Version]
- Griebel, J.J.; Glass, R.S.; Char, K.; Pyun, J. Polymerizations with elemental sulfur: A novel route to high sulfur content polymers for sustainability, energy and defense. Polym. Sci. 2016, 58, 90–125. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.; Pyun, J.; Char, K. Recent approaches for the direct use of elemental sulfur in the synthesis and processing of advanced materials. Angew. Chem. Int. Ed. 2015, 54, 3249–3258. [Google Scholar] [CrossRef]
- Hoefling, A.; Theato, P. Polymere auf schwefelbasis: Vulkanisation andersherum. Nachr. Chem. 2016, 64, 9–12. [Google Scholar] [CrossRef]
- Boyd, D.A. Sulfur and its role in modern materials science. Angew. Chem. Int. Ed. 2016, 55, 15486–15502. [Google Scholar] [CrossRef]
- Wręczycki, J.; Bieliński, M.D.; Anyszka, R. Sulfur/organic copolymers as curing agents for rubber. Polymers 2018, 10, 870. [Google Scholar] [CrossRef] [Green Version]
- Chung, W.J.; Griebel, J.J.; Kim, E.T.; Yoon, H.; Simmonds, A.G.; Ji, H.J.; Dirlam, P.T.; Glass, R.S.; Wie, J.J.; Nguyen, N.A. The use of elemental sulfur as an alternative feedstock for polymeric materials. Nat. Chem. 2013, 5, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, S.J.; Wheeler, J.C. Free-radical concentration in doped sulfur. Theory and experiment. J. Phys. Chem. 1983, 87, 3961–3966. [Google Scholar] [CrossRef]
- Meyer, B. Elemental sulfur. Chem. Rev. 1976, 76, 367–388. [Google Scholar] [CrossRef]
- Crockett, M.P.; Evans, A.M.; Worthington, M.J.H.; Albuquerque, I.S.; Slattery, A.D.; Gibson, C.T.; Campbell, J.A.; Lewis, D.A.; Bernardes, G.J.L.; Chalker, J.M. Sulfur-limonene polysulfide: A material synthesized entirely from industrial by-products and its use in removing toxic metals from water and soil. Angew. Chem. Int. Ed. 2016, 55, 1714–1718. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Romain, C.; Williams, C.K. Sustainable polymers from renewable resources. Nature 2016, 540, 354–362. [Google Scholar] [CrossRef]
- Mutlu, H.; Ceper, E.B.; Li, X.; Yang, J.; Dong, W.; Ozmen, M.M.; Theato, P. Sulfur chemistry in polymer and materials science. Macromol. Rapid Commun. 2019, 40, 1800650. [Google Scholar] [CrossRef]
- Liu, P.; Gardner, J.M.; Kloo, L. Solution processable, cross-linked sulfur polymers as solid electrolytes in dye-sensitized solar cells. Chem. Commun. 2015, 51, 14660–14662. [Google Scholar] [CrossRef] [Green Version]
- Zhuo, S.; Huang, Y.; Liu, C.; Wang, H.; Zhang, B. Sulfur copolymer nanowires with enhanced visible-light photoresponse. Chem. Commun. 2014, 50, 11208–11210. [Google Scholar] [CrossRef]
- Simmonds, A.G.; Griebel, J.J.; Park, J.; Kim, K.R.; Chung, W.J.; Oleshko, V.P.; Kim, J.; Kim, E.T.; Glass, R.S.; Soles, C.L.; et al. Inverse vulcanization of elemental sulfur to prepare polymeric electrode materials for li–s batteries. ACS Macro Lett. 2014, 3, 229–232. [Google Scholar] [CrossRef]
- Manthiram, A.; Fu, Y.; Su, Y.-S. Challenges and prospects of lithium–sulfur batteries. Acc. Chem. Res. 2013, 46, 1125–1134. [Google Scholar] [CrossRef]
- Griebel, J.J.; Li, G.; Glass, R.S.; Char, K.; Pyun, J. Kilogram scale inverse vulcanization of elemental sulfur to prepare high capacity polymer electrodes for Li-S batteries. J. Polym. Sci. Polym. Chem. 2015, 53, 173–177. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Ma, J.; Fan, J.; Pyun, J.; Geng, J. Rational design of sulfur-containing composites for high-performance lithium–sulfur batteries. APL Mater. 2019, 7, 020904. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Peng, Y.; Wang, Y.; Li, J.; Li, H.; Zeng, J.; Wang, J.; Hwang, B.J.; Zhao, J. High sulfur-containing carbon polysulfide polymer as a novel cathode material for lithium-sulfur battery. Sci. Rep. 2017, 7, 11386. [Google Scholar] [CrossRef] [PubMed]
- Bresser, D.; Passerini, S.; Scrosati, B. Recent progress and remaining challenges in sulfur-based lithium secondary batteries—A review. Chem. Commun. 2013, 49, 10545–10562. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Griebel, J.J.; Dirlam, P.T.; Nguyen, N.A.; Glass, R.S.; Mackay, M.E.; Char, K.; Pyun, J. Inverse vulcanization of elemental sulfur and styrene for polymeric cathodes in Li-S batteries. J. Polym. Sci. Polym. Chem. 2017, 55, 107–116. [Google Scholar] [CrossRef]
- Oleshko, V.P.; Kim, J.; Schaefer, J.L.; Hudson, S.D.; Soles, C.L.; Simmonds, A.G.; Griebel, J.J.; Glass, R.S.; Char, K.; Pyun, J. Structural origins of enhanced capacity retention in novel copolymerized sulfur-based composite cathodes for high-energy density Li–S batteries. MRS Commun. 2015, 5, 353–364. [Google Scholar] [CrossRef]
- Griebel, J.J.; Nguyen, N.A.; Astashkin, A.V.; Glass, R.S.; Mackay, M.E.; Char, K.; Pyun, J. Preparation of dynamic covalent polymers via inverse vulcanization of elemental sulfur. ACS Macro Lett. 2014, 3, 1258–1261. [Google Scholar] [CrossRef]
- Griebel, J.J.; Nguyen, N.A.; Namnabat, S.; Anderson, L.E.; Glass, R.S.; Norwood, R.A.; Mackay, M.E.; Char, K.; Pyun, J. Dynamic covalent polymers via inverse vulcanization of elemental sulfur for healable infrared optical materials. ACS Macro Lett. 2015, 4, 862–866. [Google Scholar] [CrossRef]
- Kleine, T.S.; Nguyen, N.A.; Anderson, L.E.; Namnabat, S.; LaVilla, E.A.; Showghi, S.A.; Dirlam, P.T.; Arrington, C.B.; Manchester, M.S.; Schwiegerling, J.; et al. High refractive index copolymers with improved thermomechanical properties via the inverse vulcanization of sulfur and 1,3,5-Triisopropenylbenzene. ACS Macro Lett. 2016, 5, 1152–1156. [Google Scholar] [CrossRef]
- Griebel, J.J.; Namnabat, S.; Kim, E.T.; Himmelhuber, R.; Moronta, D.H.; Chung, W.J.; Simmonds, A.G.; Kim, K.-J.; van der Laan, J.; Nguyen, N.A.; et al. New infrared transmitting material via inverse vulcanization of elemental sulfur to prepare high refractive index polymers. Adv. Mater. 2014, 26, 3014–3018. [Google Scholar] [CrossRef]
- Thielke, M.W.B.; Lindsey, A.; Brauer, D. Rapid mercury(ii) removal by electrospun sulfur copolymers. Polymers 2016, 8, 266. [Google Scholar] [CrossRef] [PubMed]
- Worthington, M.J.H.; Kucera, R.L.; Albuquerque, I.S.; Gibson, C.T.; Sibley, A.; Slattery, A.D.; Campbell, J.A.; Alboaiji, S.F.K.; Muller, K.A.; Young, J.; et al. Laying waste to mercury: Inexpensive sorbents made from sulfur and recycled cooking oils. Chem. Eur. J. 2017, 23, 16219–16230. [Google Scholar] [CrossRef] [PubMed]
- Parker, D.J.; Chong, S.T.; Hasell, T. Sustainable inverse-vulcanised sulfur polymers. RSC Adv. 2018, 8, 27892–27899. [Google Scholar] [CrossRef] [Green Version]
- Hasell, T.; Parker, D.J.; Jones, H.A.; McAllister, T.; Howdle, S.M. Porous inverse vulcanised polymers for mercury capture. Chem. Commun. 2016, 52, 5383–5386. [Google Scholar] [CrossRef] [Green Version]
- Parker, D.J.; Jones, H.A.; Petcher, S.; Cervini, L.; Griffin, J.M.; Akhtar, R.; Hasell, T. Low cost and renewable sulfur-polymers by inverse vulcanisation, and their potential for mercury capture. J. Mater. Chem. A 2017, 5, 11682–11692. [Google Scholar] [CrossRef] [Green Version]
- Valle, S.F.; Giroto, A.S.; Klaic, R.; Guimarães, G.G.F.; Ribeiro, C. Sulfur fertilizer based on inverse vulcanization process with soybean oil. Polym. Degrad. Stab. 2019, 162, 102–105. [Google Scholar] [CrossRef]
- Mann, M.; Kruger, J.E.; Andari, F.; McErlean, J.; Gascooke, J.R.; Smith, J.A.; Worthington, M.J.H.; McKinley, C.C.C.; Campbell, J.A.; Lewis, D.A.; et al. Sulfur polymer composites as controlled-release fertilisers. Org. Biomol. Chem. 2019, 17, 1929–1936. [Google Scholar] [CrossRef]
- Diez, S.; Hoefling, A.; Theato, P.; Pauer, W. Mechanical and electrical properties of sulfur-containing polymeric materials prepared via inverse vulcanization. Polymers 2017, 9, 59. [Google Scholar] [CrossRef]
- Dirlam, P.T.; Simmonds, A.G.; Shallcross, R.C.; Arrington, K.J.; Chung, W.J.; Griebel, J.J.; Hill, L.J.; Glass, R.S.; Char, K.; Pyun, J. Improving the charge conductance of elemental sulfur via tandem inverse vulcanization and electropolymerization. ACS Macro Lett. 2015, 4, 111–114. [Google Scholar] [CrossRef]
- Salman, M.K.; Karabay, B.; Karabay, L.C.; Cihaner, A. Elemental sulfur-based polymeric materials: Synthesis and characterization. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Abbasi, A.; Nasef, M.M.; Yahya, W.Z.N. Copolymerization of vegetable oils and bio-based monomers with elemental sulfur: A new promising route for bio-based polymers. Sustain. Chem. Pharm. 2019, 13, 100158. [Google Scholar] [CrossRef]
- Smith, A.M.; Moxon, S.; Morris, G.A. 13—Biopolymers as wound healing materials. In Wound Healing Biomaterials; Ågren, M.S., Ed.; Woodhead Publishing: Shaston, UK, 2016; pp. 261–287. [Google Scholar] [CrossRef]
- Hoefling, A.; Lee, Y.J.; Theato, P. Sulfur-Based Polymer Composites from Vegetable Oils and Elemental Sulfur: A Sustainable Active Material for Li–S Batteries. Macromol. Chem. Phys. 2017, 218, 1600303. [Google Scholar] [CrossRef]
- Lundquist, N.A.; Worthington, M.J.H.; Adamson, N.; Gibson, C.T.; Johnston, M.R.; Ellis, A.V.; Chalker, J.M. Polysulfides made from re-purposed waste are sustainable materials for removing iron from water. RSC Adv. 2018, 8, 1232–1236. [Google Scholar] [CrossRef] [Green Version]
- Qin, X.; He, Y.; Khan, S.; Zhang, B.; Chen, F.; Dong, D.; Wang, Z.; Zhang, L. Controllable synthesis and characterization of soybean-oil-based hyperbranched polymers via one-pot method. ACS Sustain. Chem. Eng. 2018, 6, 12865–12871. [Google Scholar] [CrossRef]
- Worthington, M.J.H.; Shearer, C.J.; Esdaile, L.J.; Campbell, J.A.; Gibson, C.T.; Legg, S.K.; Yin, Y.; Lundquist, N.A.; Gascooke, J.R.; Albuquerque, I.S.; et al. Sustainable polysulfides for oil spill remediation: Repurposing industrial waste for environmental benefit. Adv. Sustain. Syst. 2018, 2, 1800024. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, S.; Anwar, F.; Hussain, A.I.; Ashraf, M.; Awan, A.R. Does soil salinity affect yield and composition of cottonseed oil? J. Am. Oil Chem. Soc. 2007, 84, 845–851. [Google Scholar] [CrossRef]
- Rashid, U.; Anwar, F.; Knothe, G. Evaluation of biodiesel obtained from cottonseed oil. Fuel Process. Technol. 2009, 90, 1157–1163. [Google Scholar] [CrossRef]
- Gunstone, F.D.; Harwood, J.L.; Dijkstra, A.J. The Lipid Handbook with CD-ROM, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Mu, B.; Li, W.; Xu, H.; Emanuel, L.; Yang, Y. Salt-free and environment-friendly reactive dyeing of cotton in cottonseed oil/water system. Cellulose 2019, 26, 6379–6391. [Google Scholar] [CrossRef]
- Bear, J.C.; McGettrick, J.D.; Parkin, I.P.; Dunnill, C.W.; Hasell, T. Porous carbons from inverse vulcanised polymers. Microporous Mesoporous Mater. 2016, 232, 189–195. [Google Scholar] [CrossRef] [Green Version]
- Mu, B.; Liu, L.; Li, W.; Yang, Y. High sorption of reactive dyes onto cotton controlled by chemical potential gradient for reduction of dyeing effluents. J. Environ. Manag. 2019, 239, 271–278. [Google Scholar] [CrossRef]










| Entry | CSO (g) | S (g) | Density (g cm−3) | Shore Hardness (Shore A) | Gelation Time (min) |
|---|---|---|---|---|---|
| 1 | 10.0 | 2.0 | - | - | - |
| 2 | 10.0 | 2.5 | 1.01 | - | 120–130 |
| 3 | 10.0 | 3.0 | 1.02 | 4.5 | 60–65 |
| 4 | 10.0 | 4.0 | 1.08 | 12.3 | 50–55 |
| 5 | 10.0 | 6.0 | 1.12 | 19.5 | 40–43 |
| 6 | 10.0 | 8.0 | 1.12 | 28.9 | 38–42 |
| 7 | 10.0 | 10.0 | 1.27 | 29.3 | 30–33 |
| 8 | 10.0 | 12.0 | 1.27 | 29.4 | 28–30 |
| 9 | 10.0 | 15.0 | 1.34 | 30.0 | 24–26 |
| Entry | CSO (g) | S (g) | Filler | Density (g cm−3) | |
|---|---|---|---|---|---|
| Material | Content (g) | ||||
| 1 | 10.0 | 10.0 | No Filler | 0 | 1.27 |
| 2 | 10.0 | 10.0 | Active Carbon | 1.0 | 1.26 |
| 3 | 10.0 | 10.0 | Active Clay | 1.0 | 1.29 |
| 4 | 10.0 | 10.0 | 300–800 CMC | 1.0 | 1.25 |
| 5 | 10.0 | 10.0 | 800–1200 CMC | 1.0 | 1.25 |
| 6 | 10.0 | 10.0 | Elm | 1.0 | 1.23 |
| 7 | 10.0 | 10.0 | Cattail | 1.0 | 1.16 |
| Entry | CSO(g) | S(g) | Filler | Elemental | ||||
|---|---|---|---|---|---|---|---|---|
| Material | Content (g) | S (%) | C (%) | H (%) | N (%) | |||
| 1 | 10.0 | 10.0 | No Filler | 0 | 52.70 | 38.02 | 5.57 | 0.07 |
| 2 | 10.0 | 10.0 | Elm | 1.0 | 50.46 | 39.09 | 5.63 | 0.13 |
| 3 | 10.0 | 10.0 | Elm | 2.0 | 48.69 | 39.05 | 5.60 | 0.19 |
| 4 | 10.0 | 10.0 | Cattail | 0.2 | 52.35 | 39.15 | 5.57 | 0.10 |
| 5 | 10.0 | 10.0 | Cattail | 1.0 | 52.26 | 38.80 | 5.44 | 0.10 |
| 6 | 10.0 | 3.0 | No Filler | 0 | 23.31 | 59.49 | 8.48 | 0.05 |
| 7 | 10.0 | 3.0 | Elm | 0.15 | 22.98 | 59.86 | 8.50 | 0.12 |
| 8 | 10.0 | 3.0 | Elm | 0.45 | 22.57 | 59.90 | 8.50 | 0.07 |
| 9 | 10.0 | 3.0 | Cattail | 0.06 | 22.29 | 60.24 | 8.52 | 0.03 |
| 10 | 10.0 | 3.0 | Cattail | 0.3 | 21.98 | 59.82 | 8.45 | 0.07 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Chen, Y.; Zhang, Y.; Chen, Y.; Wang, L.; Zan, X.; Zhang, L. Density-Adjustable Bio-Based Polysulfide Composite Prepared by Inverse Vulcanization and Bio-Based Fillers. Polymers 2020, 12, 2127. https://doi.org/10.3390/polym12092127
Liu Y, Chen Y, Zhang Y, Chen Y, Wang L, Zan X, Zhang L. Density-Adjustable Bio-Based Polysulfide Composite Prepared by Inverse Vulcanization and Bio-Based Fillers. Polymers. 2020; 12(9):2127. https://doi.org/10.3390/polym12092127
Chicago/Turabian StyleLiu, Yanxia, Yidan Chen, Yagang Zhang, Yurong Chen, Lulu Wang, Xingjie Zan, and Letao Zhang. 2020. "Density-Adjustable Bio-Based Polysulfide Composite Prepared by Inverse Vulcanization and Bio-Based Fillers" Polymers 12, no. 9: 2127. https://doi.org/10.3390/polym12092127
APA StyleLiu, Y., Chen, Y., Zhang, Y., Chen, Y., Wang, L., Zan, X., & Zhang, L. (2020). Density-Adjustable Bio-Based Polysulfide Composite Prepared by Inverse Vulcanization and Bio-Based Fillers. Polymers, 12(9), 2127. https://doi.org/10.3390/polym12092127