Characterization of Bone Marrow and Wharton’s Jelly Mesenchymal Stromal Cells Response on Multilayer Braided Silk and Silk/PLCL Scaffolds for Ligament Tissue Engineering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Silk and the Silk/PLCL Multilayer Braided Scaffold
2.2. Characterization of Physical Properties of Silk and Silk/PLCL Scaffolds
2.2.1. Morphological Characterization by Scanning Electronic Microscopy (SEM)
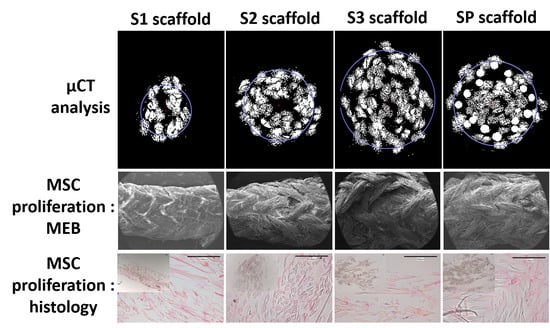
2.2.2. Morphological Characterization and Porosity Calculation by µCT
2.2.3. Mechanical Characterization
2.3. Evaluation of Biocompatibility of MSCs on Scaffolds
2.3.1. MSC Isolation and Expansion
2.3.2. MSCs Culture on Scaffolds
2.3.3. Metabolic Activity of MSCs on Scaffolds
2.3.4. Distribution and Viability of MSCs on Scaffolds
2.3.5. MSCs Chemotaxis Induced by Scaffolds
2.3.6. Histology and Immunohistochemistry
2.4. Statistical Analysis
3. Results
3.1. Physical Properties of Silk-Based Braided Scaffolds
3.1.1. Morphological Characterization of Scaffolds by SEM
3.1.2. Porosity and Pore Size
3.1.3. Mechanical Characterization
3.2. Metabolic Activity of MSCs on Scaffolds
3.2.1. Distribution and Viability of MSCs on Scaffolds
3.2.2. MSCs Chemotaxis Induced by Scaffolds
3.2.3. Histology and Immunohistochemistry
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Khan, W. Ligament tissue engineering. In Regenerative Strategies for the Treatment of Knee Joint Disabilities; Springer: Berlin/Heidelberg, Germany, 2017; pp. 373–389. [Google Scholar]
- Frank, C.B. Ligament structure, physiology and function. J. Musculoskelet. Neuronal Interact. 2004, 4, 199. [Google Scholar] [PubMed]
- Laurencin, C.T.; Freeman, J.W. Ligament tissue engineering: An evolutionary materials science approach. Biomaterials 2005, 26, 7530–7536. [Google Scholar] [CrossRef] [PubMed]
- Doroski, D.M.; Brink, K.S.; Temenoff, J.S. Techniques for biological characterization of tissue-engineered tendon and ligament. Biomaterials 2007, 28, 187–202. [Google Scholar] [CrossRef]
- Laurent, C.P.; Vaquette, C.; Martin, C.; Guedon, E.; Wu, X.; Delconte, A.; Dumas, D.; Hupont, S.; Isla, N.D.; Rahouadj, R. Towards a tissue-engineered ligament: Design and preliminary evaluation of a dedicated multi-chamber tension-torsion bioreactor. Processes 2014, 2, 167–179. [Google Scholar] [CrossRef]
- Alshomer, F.; Chaves, C.; Kalaskar, D.M. Advances in Tendon and Ligament Tissue Engineering: Materials Perspective. J. Mater. 2018, 2018, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Anil, S.; Chalisserry, E.P.; Nam, S.Y.; Venkatesan, J. 24 - Biomaterials for craniofacial tissue engineering and regenerative dentistry. In Advanced Dental Biomaterials; Khurshid, Z., Najeeb, S., Zafar, M.S., Sefat, F., Eds.; Woodhead Publishing: Swaston/Cambridge, UK, 2019; pp. 643–674. ISBN 978-0-08-102476-8. [Google Scholar]
- Ma, D.; Wang, Y.; Dai, W. Silk fibroin-based biomaterials for musculoskeletal tissue engineering. Mater. Sci. Eng. C 2018, 89, 456–469. [Google Scholar] [CrossRef] [PubMed]
- Altman, G.H.; Horan, R.L.; Lu, H.H.; Moreau, J.; Martin, I.; Richmond, J.C.; Kaplan, D.L. Silk matrix for tissue engineered anterior cruciate ligaments. Biomaterials 2002, 23, 4131–4141. [Google Scholar] [CrossRef]
- Shen, W.; Chen, X.; Hu, Y.; Yin, Z.; Zhu, T.; Hu, J.; Chen, J.; Zheng, Z.; Zhang, W.; Ran, J.; et al. Long-term effects of knitted silk–collagen sponge scaffold on anterior cruciate ligament reconstruction and osteoarthritis prevention. Biomaterials 2014, 35, 8154–8163. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.L.; Yin, Z.; Shen, W.L.; Chen, X.; Heng, B.C.; Zou, X.H.; Ouyang, H.W. Efficacy of hESC-MSCs in knitted silk-collagen scaffold for tendon tissue engineering and their roles. Biomaterials 2010, 31, 9438–9451. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yin, Z.; Qi, Y.-Y.; Wang, L.-L.; Ouyang, H.-W. Synergic Combination of Collagen Matrix with Knitted Silk Scaffold Regenerated Ligament with More Native Microstructure in Rabbit Model. In 13th International Conference on Biomedical Engineering; Lim, C.T., Goh, J.C.H., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; Volume 23, pp. 1195–1198. ISBN 978-3-540-92840-9. [Google Scholar]
- Shen, W.; Chen, J.; Yin, Z.; Chen, X.; Liu, H.; Heng, B.C.; Chen, W.; Ouyang, H.-W. Allogenous Tendon Stem/Progenitor Cells in Silk Scaffold for Functional Shoulder Repair. Cell Transpl. 2012, 21, 943–958. [Google Scholar] [CrossRef] [Green Version]
- Ethicon, I. Wound Closure Manual. The Suture Specific Suturing Materials. Non-Absorbable Suture; Ethicon, Inc.: Somerville, NJ, USA, 2000. [Google Scholar]
- Liu, X.; Laurent, C.; Du, Q.; Targa, L.; Cauchois, G.; Chen, Y.; Wang, X.; de Isla, N. Mesenchymal stem cell interacted with PLCL braided scaffold coated with poly-l-lysine/hyaluronic acid for ligament tissue engineering. J. Biomed. Mater. Res. A 2018, 106, 3042–3052. [Google Scholar] [CrossRef] [PubMed]
- Laurent, C.P.; Vaquette, C.; Liu, X.; Schmitt, J.-F.; Rahouadj, R. Suitability of a PLCL fibrous scaffold for soft tissue engineering applications: A combined biological and mechanical characterisation. J. Biomater. Appl. 2018, 32, 1276–1288. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.; Goh, J.C.H.; Lee, E.H. Selection of cell source for ligament tissue engineering. Cell Transpl. 2005, 14, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Hendrijantini, N.; Hartono, C.K.; Daniati, R.P.; Hong, G.; Sitalaksmi, R.M.; Kuntjoro, M.; Ari, M.D.A. Human Umbilical Cord Mesenchymal Stem Cell-induced Osterix, Bone Morphogenetic Protein-2, and Tartrate-resistant Acid Phosphatase Expression in Osteoporotic Mandibular Bone. Eur. J. Dent. 2020. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Yang, X.; Liu, C.; Dong, Z.; Wang, F.; Wang, X.; Hu, W.; Zhang, X.; Zhao, P.; Xia, Q. Structural and Mechanical Properties of Silk from Different Instars of Bombyx mori. Biomacromolecules 2019, 20, 1203–1216. [Google Scholar] [CrossRef]
- Fan, H.; Liu, H.; Toh, S.L.; Goh, J.C.H. Anterior cruciate ligament regeneration using mesenchymal stem cells and silk scaffold in large animal model. Biomaterials 2009, 30, 4967–4977. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, B. Biodegradation of silk biomaterials. Int. J. Mol. Sci. 2009, 10, 1514–1524. [Google Scholar] [CrossRef] [Green Version]
- Jeong, S.I.; Kim, B.-S.; Kang, S.W.; Kwon, J.H.; Lee, Y.M.; Kim, S.H.; Kim, Y.H. In vivo biocompatibilty and degradation behavior of elastic poly(l-lactide-co-ε-caprolactone) scaffolds. Biomaterials 2004, 25, 5939–5946. [Google Scholar] [CrossRef]
- Yin, A.; Li, J.; Bowlin, G.L.; Li, D.; Rodriguez, I.A.; Wang, J.; Wu, T.; EI-Hamshary, H.A.; Al-Deyab, S.S.; Mo, X. Fabrication of cell penetration enhanced poly (l-lactic acid-co-ɛ-caprolactone)/silk vascular scaffolds utilizing air-impedance electrospinning. Colloids Surf. B Biointerfaces 2014, 120, 47–54. [Google Scholar] [CrossRef]
- Bhutto, M.A.; Wu, T.; Sun, B.; EI-Hamshary, H.; Al-Deyab, S.S.; Mo, X. Fabrication and characterization of vitamin B5 loaded poly (l-lactide-co-caprolactone)/silk fiber aligned electrospun nanofibers for schwann cell proliferation. Colloids Surf. B Biointerfaces 2016, 144, 108–117. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, M.; Xie, Q.; Sun, H.; Huang, Y.; Zhang, D.; Yu, Z.; Bi, X.; Chen, J.; Wang, J.; et al. Electrospun silk fibroin/poly(lactide-co-ε-caprolactone) nanofibrous scaffolds for bone regeneration. Int. J. Nanomed. 2016, 11, 1483–1500. [Google Scholar] [CrossRef] [Green Version]
- Laurent, C.P.; Latil, P.; Durville, D.; Rahouadj, R.; Geindreau, C.; Orgéas, L.; Ganghoffer, J.-F. Mechanical behaviour of a fibrous scaffold for ligament tissue engineering: Finite elements analysis vs. X-ray tomography imaging. J. Mech. Behav. Biomed. Mater. 2014, 40, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-I.; Wang, Y. Cell Responses to Surface and Architecture of Tissue Engineering Scaffolds. Regen. Med. Tissue Eng. Cells Biomater. 2011. [Google Scholar] [CrossRef] [Green Version]
- Ge, Z.; Yang, F.; Goh, J.C.; Ramakrishna, S.; Lee, E.H. Biomaterials and scaffolds for ligament tissue engineering. J. Biomed. Mater. Res. Part A 2006, 77, 639–652. [Google Scholar] [CrossRef] [PubMed]
- Nehrer, S.; Breinan, H.A.; Ramappa, A.; Young, G.; Shortkroff, S.; Louie, L.K.; Sledge, C.B.; Yannas, I.V.; Spector, M. Matrix collagen type and pore size influence behaviour of seeded canine chondrocytes. Biomaterials 1997, 18, 769–776. [Google Scholar] [CrossRef]
- Stenhamre, H.; Nannmark, U.; Lindahl, A.; Gatenholm, P.; Brittberg, M. Influence of pore size on the redifferentiation potential of human articular chondrocytes in poly (urethane urea) scaffolds. J. Tissue Eng. Regen. Med. 2011, 5, 578–588. [Google Scholar] [CrossRef]
- Griffon, D.J.; Sedighi, M.R.; Schaeffer, D.V.; Eurell, J.A.; Johnson, A.L. Chitosan scaffolds: Interconnective pore size and cartilage engineering. Acta Biomater. 2006, 2, 313–320. [Google Scholar] [CrossRef]
- Lien, S.-M.; Ko, L.-Y.; Huang, T.-J. Effect of pore size on ECM secretion and cell growth in gelatin scaffold for articular cartilage tissue engineering. Acta Biomaterialia 2009, 5, 670–679. [Google Scholar] [CrossRef]
- Klawitter, J.J.; Bagwell, J.G.; Weinstein, A.M.; Sauer, B.W.; Pruitt, J.R. An evaluation of bone growth into porous high density polyethylene. J. Biomed. Mater. Res. 1976, 10, 311–323. [Google Scholar] [CrossRef]
- Kuboki, Y.; Jin, Q.; Takita, H. Geometry of carriers controlling phenotypic expression in BMP-induced osteogenesis and chondrogenesis. JBJS 2001, 83, S105–S115. [Google Scholar] [CrossRef]
- Roosa, S.M.M.; Kemppainen, J.M.; Moffitt, E.N.; Krebsbach, P.H.; Hollister, S.J. The pore size of polycaprolactone scaffolds has limited influence on bone regeneration in an in vivo model. J. Biomed. Mater. Res. Part A 2010, 92, 359–368. [Google Scholar] [CrossRef]
- Tsuruga, E.; Takita, H.; Itoh, H.; Wakisaka, Y.; Kuboki, Y. Pore size of porous hydroxyapatite as the cell-substratum controls BMP-induced osteogenesis. J. Biochem. 1997, 121, 317–324. [Google Scholar] [CrossRef] [Green Version]
- Yannas, I.V. Tissue regeneration by use of collagen-glycosaminoglycan copolymers. Clin. Mater. 1992, 9, 179–187. [Google Scholar] [CrossRef]
- Kendoff, D.; Morgan-Jones, R.; Haddad, F.S. Periprosthetic Joint Infections: Changing Paradigms; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 978-3-319-30091-7. [Google Scholar]
- Bružauskaitė, I.; Bironaitė, D.; Bagdonas, E.; Bernotienė, E. Scaffolds and cells for tissue regeneration: Different scaffold pore sizes—different cell effects. Cytotechnology 2016, 68, 355–369. [Google Scholar] [CrossRef] [Green Version]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Baldit, A.; de Brosses, E.; Velard, F.; Cauchois, G.; Chen, Y.; Wang, X.; de Isla, N.; Laurent, C. Characterization of Bone Marrow and Wharton’s Jelly Mesenchymal Stromal Cells Response on Multilayer Braided Silk and Silk/PLCL Scaffolds for Ligament Tissue Engineering. Polymers 2020, 12, 2163. https://doi.org/10.3390/polym12092163
Liu X, Baldit A, de Brosses E, Velard F, Cauchois G, Chen Y, Wang X, de Isla N, Laurent C. Characterization of Bone Marrow and Wharton’s Jelly Mesenchymal Stromal Cells Response on Multilayer Braided Silk and Silk/PLCL Scaffolds for Ligament Tissue Engineering. Polymers. 2020; 12(9):2163. https://doi.org/10.3390/polym12092163
Chicago/Turabian StyleLiu, Xing, Adrien Baldit, Emilie de Brosses, Frédéric Velard, Ghislaine Cauchois, Yun Chen, Xiong Wang, Natalia de Isla, and Cédric Laurent. 2020. "Characterization of Bone Marrow and Wharton’s Jelly Mesenchymal Stromal Cells Response on Multilayer Braided Silk and Silk/PLCL Scaffolds for Ligament Tissue Engineering" Polymers 12, no. 9: 2163. https://doi.org/10.3390/polym12092163
APA StyleLiu, X., Baldit, A., de Brosses, E., Velard, F., Cauchois, G., Chen, Y., Wang, X., de Isla, N., & Laurent, C. (2020). Characterization of Bone Marrow and Wharton’s Jelly Mesenchymal Stromal Cells Response on Multilayer Braided Silk and Silk/PLCL Scaffolds for Ligament Tissue Engineering. Polymers, 12(9), 2163. https://doi.org/10.3390/polym12092163