Stabilization of Palygorskite Aqueous Suspensions Using Bio-Based and Synthetic Polyelectrolytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Clay and Chemical Dispersants
2.2. Characterization of the Palygorskite
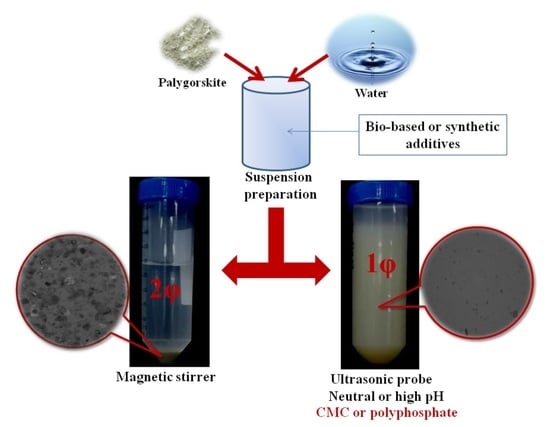
2.3. Preparation of the Palygorskite Suspensions with Different Mechanical Dispersers and Chemical Dispersants
2.4. Characterization of the Palygorskite Suspensions
2.5. Rheology of Aqueous Solutions of the Chemical Dispersants
3. Results
3.1. Characterization of the Palygorskite
3.2. Characterization of the Palygorskite Suspensions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singer, A. Palygorskite and Sepiolite Group Minerals. In Minerals in Soil Environments, 2nd ed.; Dixon, J.B., Weed, S.B., Eds.; Soil Science Society of America Book Series: Madison, WI, USA, 1989; Volume 1, pp. 829–872. [Google Scholar]
- Guggenheim, S.; Krekeler, M.P.S. The Structures and Microtextures of the Palygorskite–Sepiolite Group Minerals. In Developments in Clay Science; Galán, E., Singer, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 3, pp. 3–32. [Google Scholar]
- Wang, W.; Wang, A. Recent progress in dispersion of palygorskite crystal bundles for nanocomposites. Appl. Clay Sci. 2016, 119, 18–30. [Google Scholar] [CrossRef]
- Galán, E. Properties and applications of palygorskite-sepiolite clays. Clay Miner. 1996, 31, 443–453. [Google Scholar] [CrossRef]
- Ruiz-Hitzky, E.; Darder, M.; Fernandes, F.M.; Wicklein, B.; Alcântara, A.C.S.; Aranda, P. Fibrous clays based bionanocomposites. Prog. Polym. Sci. 2013, 38, 1392–1414. [Google Scholar] [CrossRef] [Green Version]
- Zhou, F.-S.; Li, T.; Yan, Y.-H.; Cao, C.; Zhou, L.; Liu, Y. Enhanced Viscosity of Aqueous Palygorskite Suspensions through Physical and Chemical Processing. Adv. Mater. Sci. Eng. 2015, 2015, 941580. [Google Scholar] [CrossRef] [Green Version]
- Novikova, L.; Ayrault, P.; Fontaine, C.; Chatel, G.; Jérôme, F.; Belchinskaya, L. Effect of low frequency ultrasound on the surface properties of natural aluminosilicates. Ultrason. Sonochem. 2016, 31, 598–609. [Google Scholar] [CrossRef]
- Viseras, C.; Meeten, G.H.; Lopez-Galindo, A. Pharmaceutical grade phyllosilicate dispersions: The influence of shear history on floc structure. Int. J. Pharm. 1999, 182, 7–20. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, J.; Wang, Q.; Wang, A. Disaggregation of palygorskite crystal bundles via high-pressure homogenization. Appl. Clay Sci. 2011, 54, 118–123. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, X.; Wang, W.; Wang, A. Ethanol-assisted dispersion of attapulgite and its effect on improving properties of alginate-based superabsorbent nanocomposite. J. Appl. Polym. Sci. 2013, 129, 1080–1088. [Google Scholar] [CrossRef]
- Chen, F.; Lou, D.; Yang, J.; Zhong, M. Mechanical and thermal properties of attapulgite clay reinforced polymethylmethacrylate nanocomposites. Polym. Adv. Technol. 2010, 22, 1912–1918. [Google Scholar] [CrossRef]
- Zhuang, G.; Zhang, Z.; Gao, J.; Zhang, X.; Liao, L. Influences of surfactants on the structures and properties of organo-palygorskite in oil-based drilling fluids. Microporous Mesoporous Mater. 2017, 244, 37–46. [Google Scholar] [CrossRef]
- Wang, C.; Wu, Q.; Liu, F.; An, J.; Lu, R.; Xie, H.; Cheng, R. Synthesis and characterization of soy polyol-based polyurethane nanocomposites reinforced with silylated palygorskite. Appl. Clay Sci. 2014, 101, 246–252. [Google Scholar] [CrossRef]
- Ni, L.; Zhang, P.; Chen, J.; Jiang, J.; Ding, S. Iso-propanol assisted preparation of individualized functional palygorskite fibers and its impact on improving dispersion abilities in polymer nanocomposites. Korean J. Chem. Eng. 2017, 34, 1827–1833. [Google Scholar] [CrossRef]
- Benobeidallah, B.; Benhamida, A.; Dorigato, A.; Sola, A.; Messori, M.; Pegoretti, A. Structure and Properties of Polyamide 11 Nanocomposites Filled with Fibrous Palygorskite Clay. J. Renew. Mater. 2019, 7, 89–102. [Google Scholar] [CrossRef] [Green Version]
- Alves, L.; Ferraz, E.; Gamelas, J.A.F. Composites of nanofibrillated cellulose with clay minerals: A review. Adv. Colloid Interface Sci. 2019, 272, 101994. [Google Scholar] [CrossRef]
- Gamelas, J.A.F.; Ferraz, E. Composite Films Based on Nanocellulose and Nanoclay Minerals as High Strength Materials with Gas Barrier Capabilities: Key Points and Challenges. BioResources 2015, 10, 6310–6313. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Wang, A. Nanocomposite of carboxymethyl cellulose and attapulgite as a novel pH-sensitive superabsorbent: Synthesis, characterization and properties. Carbohydr. Polym. 2010, 82, 83–91. [Google Scholar] [CrossRef]
- Ding, J.; Huang, D.; Wang, W.; Wang, Q.; Wang, A. Effect of removing coloring metal ions from the natural brick-red palygorskite on properties of alginate/palygorskite nanocomposite film. Int. J. Biol. Macromol. 2019, 122, 684–694. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, Y.; Zhang, X.-F.; Zhang, X.; Jiang, J.; Yao, J. Alginate-based attapulgite foams as efficient and recyclable adsorbents for the removal of heavy metals. J. Colloid Interface Sci. 2018, 514, 190–198. [Google Scholar] [CrossRef]
- Alves, L.; Ferraz, E.; Santarén, J.; Rasteiro, M.G.; Gamelas, J.A.F. Improving Colloidal Stability of Sepiolite Suspensions: Effect of the Mechanical Disperser and Chemical Dispersant. Minerals 2020, 10, 779. [Google Scholar] [CrossRef]
- Middea, A.; Fernandes, T.L.; Neumann, R.; Gomes, O.D.F.M.; Spinelli, L.S. Evaluation of Fe(III) adsorption onto palygorskite surfaces. Appl. Surf. Sci. 2013, 282, 253–258. [Google Scholar] [CrossRef] [Green Version]
- Frost, R.L.; Ding, Z. Controlled rate thermal analysis and differential scanning calorimetry of sepiolites and palygorskites. Thermochim. Acta 2003, 397, 119–128. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.; Yang, J.; Frost, R.L. Thermogravimetric analysis-mass spectrometry (TG-MS) of selected Chinese palygorskites—Implications for structural water. Thermochim. Acta 2011, 512, 202–207. [Google Scholar] [CrossRef]
- Post, J.E.; Heaney, P.J. Synchrotron powder X-ray diffraction study of the structure and dehydration behavior of palygorskite. Am. Miner. 2008, 93, 667–675. [Google Scholar] [CrossRef]
- Faust, G.T. Thermal analysis studies on carbonates I. aragonite and calcite1. Am. Mineral. 1950, 35, 207–224. [Google Scholar]
- Olszak-Humienik, M.; Jablonski, M. Thermal behavior of natural dolomite. J. Therm. Anal. Calorim. 2015, 119, 2239–2248. [Google Scholar] [CrossRef] [Green Version]
- Augsburger, M.; Strasser, E.; Perino, E.; Mercader, R.; Pedregosa, J. Ftir and mössbauer investigation of a substituted palygorskite: Silicate with a channel structure. J. Phys. Chem. Solids 1998, 59, 175–180. [Google Scholar] [CrossRef]
- Farmer, V.C. The Infrared Spectra of Minerals; Mineralogical Society of Great Britain and Ireland: London, UK, 1974; Volume 4. [Google Scholar]
- Frost, R.L.; Locos, O.B.; Ruan, H.; Kloprogge, J.T. Near-infrared and mid-infrared spectroscopic study of sepiolites and palygorskites. Vib. Spectrosc. 2001, 27, 1–13. [Google Scholar] [CrossRef]
- Wang, W.; Tian, G.; Zhang, Z.; Wang, A. From naturally low-grade palygorskite to hybrid silicate adsorbent for efficient capture of Cu(II) ions. Appl. Clay Sci. 2016, 132–133, 438–448. [Google Scholar] [CrossRef]
- Tan, L.; Tan, X.; Ren, X.; Mei, H.; Wang, X. Influence of pH, soil humic acid, ionic strength and temperature on sorption of U(VI) onto attapulgite. J. Radioanal. Nucl. Chem. 2018, 316, 981–991. [Google Scholar] [CrossRef]
- Xu, J.; Wang, A. Electrokinetic and Colloidal Properties of Homogenized and Unhomogenized Palygorskite in the Presence of Electrolytes. J. Chem. Eng. Data 2012, 57, 1586–1593. [Google Scholar] [CrossRef]
- Zhang, J.; Cai, D.; Zhang, G.; Cai, C.; Zhang, C.; Qiu, G.; Zheng, K.; Wu, Z. Adsorption of methylene blue from aqueous solution onto multiporous palygorskite modified by ion beam bombardment: Effect of contact time, temperature, pH and ionic strength. Appl. Clay Sci. 2013, 83–84, 137–143. [Google Scholar] [CrossRef]
- Rusmin, R.; Sarkar, B.; Biswas, B.; Churchman, J.; Liu, Y.; Naidu, R. Structural, electrokinetic and surface properties of activated palygorskite for environmental application. Appl. Clay Sci. 2016, 134, 95–102. [Google Scholar] [CrossRef]
- Del Campo, M.M.G.; Caja-Munoz, B.; Darder, M.; Aranda, P.; Vázquez, L.; Ruiz-Hitzky, E. Ultrasound-assisted preparation of nanocomposites based on fibrous clay minerals and nanocellulose from microcrystalline cellulose. Appl. Clay Sci. 2020, 189, 105538. [Google Scholar] [CrossRef]
- Chuang, J.-J.; Huang, Y.-Y.; Lo, S.-H.; Hsu, T.-F.; Huang, W.-Y.; Huang, S.-L.; Lin, Y.-S. Effects of pH on the Shape of Alginate Particles and Its Release Behavior. Int. J. Polym. Sci. 2017, 2017, 3902704. [Google Scholar] [CrossRef]
- Alves, L.; Lindman, B.; Klotz, B.; Böttcher, A.; Haake, H.-M.; Antunes, F.E. Rheology of polyacrylate systems depends strongly on architecture. Colloid Polym. Sci. 2015, 293, 3285–3293. [Google Scholar] [CrossRef]
- Neaman, A.; Singer, A. Rheological Properties of Aqueous Suspensions of Palygorskite. Soil Sci. Soc. Am. J. 2000, 64, 427–436. [Google Scholar] [CrossRef] [Green Version]
- Vleugels, L.F.; Ricois, S.; Voets, I.K.; Tuinier, R. Determination of the ‘apparent pKa’ of selected food hydrocolloids using ortho-toluidine blue. Food Hydrocoll. 2018, 81, 273–283. [Google Scholar] [CrossRef] [Green Version]
- Lindman, B.; Medronho, B.; Alves, L.; Costa, C.; Edlund, H.; Norgren, M. The relevance of structural features of cellulose and its interactions to dissolution, regeneration, gelation and plasticization phenomena. Phys. Chem. Chem. Phys. 2017, 19, 23704–23718. [Google Scholar] [CrossRef] [Green Version]
- Stewart, M.B.; Myat, D.T.; Kuiper, M.J.; Manning, R.J.; Gray, S.; Orbell, J.D. A structural basis for the amphiphilic character of alginates—Implications for membrane fouling. Carbohydr. Polym. 2017, 164, 162–169. [Google Scholar] [CrossRef] [Green Version]
- Manfredini, T.; Pellacani, G.C.; Pozzi, P.; Corradi, A.B. Monomeric and oligomeric phosphates as deflocculants of concentrated aqueous clay suspensions. Appl. Clay Sci. 1990, 5, 193–201. [Google Scholar] [CrossRef]
- Younes, M.; Aquilina, G.; Castle, L.; Engel, K.; Fowler, P.; Fernandez, M.J.F.; Fürst, P.; Gürtler, R.; Husøy, T.; Mennes, W.; et al. Re-evaluation of phosphoric acid–phosphates-di-, tri- and polyphosphates (E 338–341, E 343, E 450–452) as food additives and the safety of proposed extension of use. EFSA J. 2019, 17, e05674. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| SiO2 | 53.0 |
| MgO | 8.9 |
| Al2O3 | 7.0 |
| CaO | 4.7 |
| Fe2O3 | 4.3 |
| Na2O | 0.14 |
| K2O | 0.30 |
| MnO | 0.02 |
| TiO2 | 0.34 |
| P2O5 | 1.8 |
| SO3 | 0.04 |
| F | 0.47 |
| Cl | 0.04 |
| Cr | 0.06 |
| V | 0.03 |
| LOI a | 18.8 |
| Mechanical Treatment | pH 3 | pH 8 | pH 12 | ||||
|---|---|---|---|---|---|---|---|
| Di50 (nm) | PDI | Di50 (nm) | PDI | Di50 (nm) | PDI | ||
| Without dispersing agent | Magnetic stirring | 2φ | - | 2φ | - | 2φ | - |
| High-speed homogenization | 2φ | - | 2φ | - | 2φ | - | |
| Ultrasonication | 313 | 1.00 | 827 | 0.71 | 563 | 0.30 | |
| With 0.1% polyphosphate | Magnetic stirring | 2φ | - | 2φ | - | 2φ | - |
| High-speed homogenization | 2φ | - | 544 * | 0.25 | 286 * | 0.26 | |
| Ultrasonication | 837 | 0.96 | 506 | 0.27 | 330 | 0.25 | |
| With 0.1% polyacrylate | Magnetic stirring | 2φ | - | 2φ | - | 2φ | - |
| High-speed homogenization | 2φ | - | 2φ | - | 1502 | 0.48 | |
| Ultrasonication | 290 | 1.00 | 2φ | - | 509 | 0.27 | |
| With 0.1% CMC | Magnetic stirring | 2φ | - | 2φ | - | 2φ | - |
| High-speed homogenization | 2φ | - | 1172 * | 0.34 | 937 | 0.43 | |
| Ultrasonication | 2φ | - | 485 | 0.27 | 552 | 0.34 | |
| With 0.1% alginate | Magnetic stirring | 2φ | - | 2φ | - | 2φ | - |
| High-speed homogenization | 2φ | - | 1680 * | 0.60 | 2φ | - | |
| Ultrasonication | 659 | 0.71 | 2φ | - | 242 | 0.23 | |
| Mechanical Treatment | pH 3 | pH 8 | pH 12 | ||||
|---|---|---|---|---|---|---|---|
| Zeta potential (mV) | Zeta Deviation | Zeta Potential (mV) | Zeta Deviation | Zeta Potential (mV) | Zeta Deviation | ||
| Without dispersing agent | Magnetic stirring | 2φ | - | 2φ | - | 2φ | - |
| High-speed homogenization | 2φ | - | 2φ | - | 2φ | - | |
| Ultrasonication | −18 | 3.0 | −20 | 3.1 | −44 | 4.7 | |
| With 0.1% polyphosphate | Magnetic stirring | 2φ | - | 2φ | - | 2φ | - |
| High-speed homogenization | 2φ | - | −47 | 3.2 | −57 | 5.9 | |
| Ultrasonication | −24 | 3.0 | −35 | 4.7 | −49 | 7.6 | |
| With 0.1% polyacrylate | Magnetic stirring | 2φ | - | 2φ | - | 2φ | - |
| High-speed homogenization | 2φ | - | 2φ | - | −45 | 3.7 | |
| Ultrasonication | −16 | 2.8 | 2φ | - | −50 | 5.0 | |
| With 0.1% CMC | Magnetic stirring | 2φ | - | 2φ | - | 2φ | - |
| High-speed homogenization | 2φ | - | −47 | 3.8 | −59 | 4.2 | |
| Ultrasonication | 2φ | - | −53 | 4.3 | −54 | 4.2 | |
| With 0.1% alginate | Magnetic stirring | 2φ | - | 2φ | - | 2φ | - |
| High-speed homogenization | 2φ | - | −53 | 3.8 | 2φ | - | |
| Ultrasonication | −23 | 3.7 | 2φ | - | −49 | 4.4 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferraz, E.; Alves, L.; Sanguino, P.; Santarén, J.; Rasteiro, M.G.; Gamelas, J.A.F. Stabilization of Palygorskite Aqueous Suspensions Using Bio-Based and Synthetic Polyelectrolytes. Polymers 2021, 13, 129. https://doi.org/10.3390/polym13010129
Ferraz E, Alves L, Sanguino P, Santarén J, Rasteiro MG, Gamelas JAF. Stabilization of Palygorskite Aqueous Suspensions Using Bio-Based and Synthetic Polyelectrolytes. Polymers. 2021; 13(1):129. https://doi.org/10.3390/polym13010129
Chicago/Turabian StyleFerraz, Eduardo, Luís Alves, Pedro Sanguino, Julio Santarén, Maria G. Rasteiro, and José A. F. Gamelas. 2021. "Stabilization of Palygorskite Aqueous Suspensions Using Bio-Based and Synthetic Polyelectrolytes" Polymers 13, no. 1: 129. https://doi.org/10.3390/polym13010129
APA StyleFerraz, E., Alves, L., Sanguino, P., Santarén, J., Rasteiro, M. G., & Gamelas, J. A. F. (2021). Stabilization of Palygorskite Aqueous Suspensions Using Bio-Based and Synthetic Polyelectrolytes. Polymers, 13(1), 129. https://doi.org/10.3390/polym13010129