Regulation of Polyvinyl Alcohol/Sulfonated Nano-TiO2 Hybrid Membranes Interface Promotes Diffusion Dialysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Surface Modification of Nano-TiO2
2.3. Preparation of the Hybrid Membranes
2.4. Characterization and Separation Performance of the As-Prepared Membranes
3. Results
3.1. FTIR Spectra of Original Nano-TiO2 and SNT and ATR-FTIR of the As-Prepared Membranes
3.2. IECs
3.3. Water Uptake (WR), Swelling Degree, and Mass Loss
3.4. Mechanical Properties
3.5. Thermal Stabilities
3.6. Microscopic Morphologies
3.7. Separation Performance
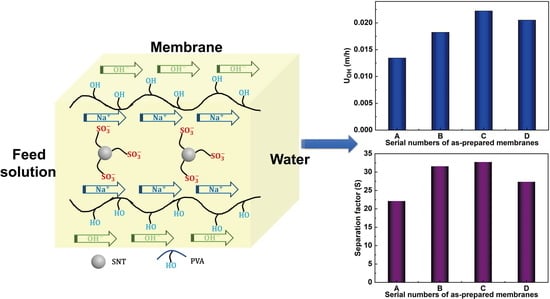
3.7.1. Dialysis Coefficients (UOH)
3.7.2. Separation Factors (S)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Luo, J.; Wu, C.; Xu, T.; Wu, Y. Diffusion dialysis-concept, principle and applications. J. Membr. Sci. 2011, 366, 1–16. [Google Scholar] [CrossRef]
- Naik, N.S.; Padaki, M.; Deon, S.; Murthy, D.H.K. Novel poly (ionic liquid)-based anion exchange membranes for efficient and rapid acid recovery from industrial waste. Chem. Eng. J. 2020, 401. [Google Scholar] [CrossRef]
- Hong, J.G.; Chen, Y. Nanocomposite reverse electrodialysis (RED) ion-exchange membranes for salinity gradient power generation. J. Membr. Sci. 2014, 460, 139–147. [Google Scholar] [CrossRef]
- Yadav, V.; Raj, S.K.; Rathod, N.H.; Kulshrestha, V. Polysulfone/graphene quantum dots composite anion exchange membrane for acid recovery by diffusion dialysis. J. Membr. Sci. 2020, 611. [Google Scholar] [CrossRef]
- Xiao, X.; Wu, C.; Cui, P.; Luo, J.; Wu, Y.; Xu, T. Cation exchange hybrid membranes from SPPO and multi-alkoxy silicon copolymer: Preparation, properties and diffusion dialysis performances for sodium hydroxide recovery. J. Membr. Sci. 2011, 379, 112–120. [Google Scholar] [CrossRef]
- Liu, R.; Wu, L.; Pan, J.; Jiang, C.; Xu, T. Diffusion dialysis membranes with semi-interpenetrating network for alkali recovery. J. Membr. Sci. 2014, 451, 18–23. [Google Scholar] [CrossRef]
- Yadav, V.; Rajput, A.; Kulshrestha, V. Sulfonated Poly(ether sulfone) based sulfonated molybdenum sulfide composite membranes and their applications in salt removal and alkali recovery. J. Membr. Sci. 2020, 603. [Google Scholar] [CrossRef]
- Wang, C.W.; Liang, Y.X.; Miao, J.B.; Wu, B.; Hossain, M.M.; Cao, M.; Ge, Q.Q.; Su, L.F.; Zheng, Z.Z.; Yang, B.; et al. Preparation and properties of polyvinyl alcohol (PVA)/mesoporous silica supported phosphotungstic acid (MS-HPW) hybrid membranes for alkali recovery. J. Membr. Sci. 2019, 592. [Google Scholar] [CrossRef]
- Wang, C.; Wu, C.; Wu, Y.; Gu, J.; Xu, T. Polyelectrolyte complex/PVA membranes for diffusion dialysis. J. Hazard. Mater. 2013, 261, 114–122. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, J.; Zhao, L.; Zhang, G.; Wu, C.; Xu, T. QPPO/PVA anion exchange hybrid membranes from double crosslinking agents for acid recovery. J. Membr. Sci. 2013, 428, 95–103. [Google Scholar] [CrossRef]
- Xiao, X.L.; Shehzad, M.A.; Yasmin, A.; Ge, Z.J.; Liang, X.; Sheng, F.M.; Ji, W.G.; Ge, X.L.; Wu, L.; Xu, T.W. Anion permselective membranes with chemically-bound carboxylic polymer layer for fast anion separation. J. Membr. Sci. 2020, 614. [Google Scholar] [CrossRef]
- Wu, Y.; Lin, H.; Zhang, G.; Xu, T.; Wu, C. Non-charged PVA/SiO2 hybrid membranes for potential application in diffusion dialysis. Sep. Purif. Technol. 2013, 118, 359–368. [Google Scholar] [CrossRef]
- Wu, Y.; Hao, J.; Wu, C.; Mao, F.; Xu, T. Cation exchange PVA/SPPO/SiO2 membranes with double organic phases for alkali recovery. J. Membr. Sci. 2012, 424, 383–391. [Google Scholar] [CrossRef]
- Hao, J.; Gong, M.; Wu, Y.; Wu, C.; Luo, J.; Xu, T. Alkali recovery using PVA/SiO2 cation exchange membranes with different -COOH contents. J. Hazard. Mater. 2013, 244, 348–356. [Google Scholar] [CrossRef]
- Lin, Y.F.; Yen, C.Y.; Hung, C.H.; Hsiao, Y.H.; Ma, C.C.M. A novel composite membranes based on sulfonated montmorillonite modified Nafion® for DMFCs. J. Power Sources 2007, 168, 162–166. [Google Scholar] [CrossRef]
- Kim, Y.; Choi, Y.; Kim, H.K.; Lee, J.S. New sulfonic acid moiety grafted on montmorillonite as filler of organic-inorganic composite membrane for non-humidified proton-exchange membrane fuel cells. J. Power Sources 2010, 195, 4653–4659. [Google Scholar] [CrossRef]
- Yu, D.M.; Yoon, Y.J.; Kim, T.H.; Lee, J.Y.; Hong, Y.T. Sulfonated poly(arylene ether sulfone)/sulfonated zeolite composite membrane for high temperature proton exchange membrane fuel cells. Solid State Ionics 2013, 233, 55–61. [Google Scholar] [CrossRef]
- Luo, J.; Wu, C.; Wu, Y.; Xu, T. Diffusion dialysis of hydrochloride acid at different temperatures using PPO/SiO2 hybrid anion exchange membranes. J. Membr. Sci. 2010, 347, 240–249. [Google Scholar] [CrossRef]
- Choi, Y.; Kim, Y.; Kim, H.K.; Lee, J.S. Direct synthesis of sulfonated mesoporous silica as inorganic fillers of proton-conducting organic-inorganic composite membranes. J. Membr. Sci. 2010, 357, 199–205. [Google Scholar] [CrossRef]
- Yang, C.C. Synthesis and characterization of the cross-linked PVA/TiO2 composite polymer membrane for alkaline DMFC. J. Membr. Sci. 2007, 288, 51–60. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, J.S.; Rhee, C.H.; Kim, H.K.; Chang, H. Montmorillonite functionalized with perfluorinated sulfonic acid for proton-conducting organic-inorganic composite membranes. J. Power Sources 2006, 162, 180–185. [Google Scholar] [CrossRef]
- Miao, J.; Yao, L.; Yang, Z.; Pan, J.; Qian, J.; Xu, T. Sulfonated poly(2,6-dimethyl-1,4-phenyleneoxide)/nano silica hybrid membranes for alkali recovery via diffusion dialysis. Sep. Purif. Technol. 2015, 141, 307–313. [Google Scholar] [CrossRef]
- Miao, J.; Li, X.; Yang, X.Z.; Jiang, C.; Qian, J.; Xu, T. Hybrid membranes from sulphonated poly (2, 6-dimethyl-1, 4-phenylene oxide) and sulphonated nano silica for alkali recovery. J. Membr. Sci. 2016, 498, 201–207. [Google Scholar] [CrossRef]
- Li, X.; Miao, J.; Xia, R.; Yang, B.; Chen, P.; Cao, M.; Qian, J. Preparation and properties of sulfonated poly (2, 6-dimethyl-1, 4-phenyleneoxide)/mesoporous silica hybrid membranes for alkali recovery. Micropor. Mesopor. Mater. 2016, 236, 48–53. [Google Scholar] [CrossRef]
- Fatyeyeva, K.; Bigarré, J.; Blondel, B.; Galiano, H.; Gaud, D.; Lecardeur, M.; Poncin-Epaillard, F. Grafting of p-styrene sulfonate and 1,3-propane sultone onto laponite for proton exchange membrane fuel cell application. J. Membr. Sci. 2011, 366, 33–42. [Google Scholar] [CrossRef]
- Munakata, H.; Chiba, H.; Kanamura, K. Enhancement on proton conductivity of inorganic-organic composite electrolyte membrane by addition of sulfonic acid group. Solid State Ionics 2005, 176, 2445–2450. [Google Scholar] [CrossRef]
- Kabiri, K.; Zohuriaan-Mehr, M.J.; Mirzadeh, H.; Kheirabadi, M. Solvent-, ion- and pH-specific swelling of poly(2-acrylamido-2-methylpropane sulfonic acid) superabsorbing gels. J. Polym. Res. 2010, 17, 203–212. [Google Scholar] [CrossRef]
- Hao, J.; Wu, Y.; Ran, J.; Wu, B.; Xu, T. A simple and green preparation of PVA-based cation exchange hybrid membranes for alkali recovery. J. Membr. Sci. 2013, 433, 10–16. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, C.; Li, Y.; Xu, T.; Fu, Y. PVA-silica anion-exchange hybrid membranes prepared through a copolymer crosslinking agent. J. Membr. Sci. 2010, 350, 322–332. [Google Scholar] [CrossRef]
- Vinod, V.T.P.; Nguyen, N.H.A.; Sevcu, A.; Cernik, M. Fabrication, Characterization, and Antibacterial Properties of Electrospun Membrane Composed of Gum Karaya, Polyvinyl Alcohol, and Silver Nanoparticles. J. Nanomater. 2015. [Google Scholar] [CrossRef]
- Chong, F.; Wang, C.; Miao, J.; Xia, R.; Cao, M.; Chen, P.; Yang, B.; Zhou, W.; Qian, J. Preparation and properties of cation-exchange membranes based on commercial chlorosulfonated polyethylene (CSM) for diffusion dialysis. J. Taiwan Inst. Chem. Eng. 2017, 78, 561–565. [Google Scholar] [CrossRef]










| Membranes | 0% | 1% | 3% | 5% |
|---|---|---|---|---|
| IEC (mmol/g) | 0 | 0.0033 | 0.0096 | 0.0157 |
| Thickness (μm) | 68 | 73 | 76 | 69 |
| Membranes | 0% | 1% | 3% | 5% |
|---|---|---|---|---|
| TS (MPa) | 53.38 | 38.41 | 31.46 | 33.1 |
| Eb (%) | 146 | 108.28 | 90.02 | 74.48 |
| Membrane | 0% | 1% | 3% | 5% |
|---|---|---|---|---|
| IDT1 (°C) | 220 | 247 | 253 | 250 |
| IDT2 (°C) | 402 | 425 | 431 | 427 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Y.; Huang, X.; Yao, L.; Xia, R.; Cao, M.; Ge, Q.; Zhou, W.; Qian, J.; Miao, J.; Wu, B. Regulation of Polyvinyl Alcohol/Sulfonated Nano-TiO2 Hybrid Membranes Interface Promotes Diffusion Dialysis. Polymers 2021, 13, 14. https://doi.org/10.3390/polym13010014
Liang Y, Huang X, Yao L, Xia R, Cao M, Ge Q, Zhou W, Qian J, Miao J, Wu B. Regulation of Polyvinyl Alcohol/Sulfonated Nano-TiO2 Hybrid Membranes Interface Promotes Diffusion Dialysis. Polymers. 2021; 13(1):14. https://doi.org/10.3390/polym13010014
Chicago/Turabian StyleLiang, Yuxia, Xiaonan Huang, Lanzhong Yao, Ru Xia, Ming Cao, Qianqian Ge, Weibin Zhou, Jiasheng Qian, Jibin Miao, and Bin Wu. 2021. "Regulation of Polyvinyl Alcohol/Sulfonated Nano-TiO2 Hybrid Membranes Interface Promotes Diffusion Dialysis" Polymers 13, no. 1: 14. https://doi.org/10.3390/polym13010014
APA StyleLiang, Y., Huang, X., Yao, L., Xia, R., Cao, M., Ge, Q., Zhou, W., Qian, J., Miao, J., & Wu, B. (2021). Regulation of Polyvinyl Alcohol/Sulfonated Nano-TiO2 Hybrid Membranes Interface Promotes Diffusion Dialysis. Polymers, 13(1), 14. https://doi.org/10.3390/polym13010014