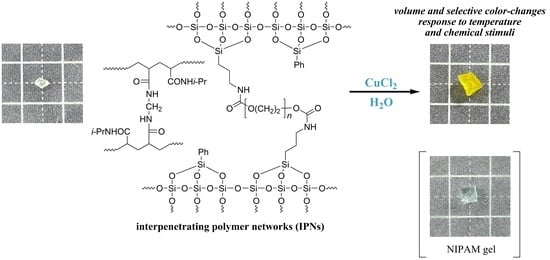
Dual Temperature and Metal Salts-Responsive Interpenetrating Polymer Networks Composed of Poly (N-isopropylacrylamide) and Polyethylene Glycol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Measurements
2.2. Materials
2.3. Preparation of Disilylated Polyethers
2.4. IPN Synthesis
3. Results and Discussion
3.1. Synthesis of IPNs
3.2. Swelling Properties of the Obtained IPNs in Water
3.3. Responses of the Obtained Hydrogels to Metal–Salt Stimuli in Water
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsudome, M.; Kusumi, R.; Wada, M.; Uematsu, K.; Okada, S.; Deguch, S. Moldable crystalline α-chitin hydrogel with toughness and transparency toward ocular applications. ACS Appl. Polym. Mater. 2020, 2, 1656–1663. [Google Scholar]
- Tavakolizadeh, M.; Pourjavadi, A.; Ansari, M.; Tebyanian, H.; Tabaei, S.J.S.; Atarod, M.; Rabiee, N.; Bagherzadeh, M.; Varma, R.S. An environmentally friendly wound dressing based on a self-healing, extensible and compressible antibacterial hydrogel. Green Chem. 2021, 23, 1312–1329. [Google Scholar] [CrossRef]
- Unagolla, J.M.; Jayasuriya, A.C. Hydrogel-based 3D bioprinting: A comprehensive review on cell-laden hydrogels, bioink formulations, and future perspectives. Appl. Mater. Today 2020, 18, 100479. [Google Scholar] [CrossRef] [PubMed]
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, F.; Akil, H.M. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C 2015, 57, 414–433. [Google Scholar] [CrossRef]
- Buwalda, S.J.; Boere, K.W.; Dijkstra, P.J.; Feijen, J.; Vermonden, T.; Hennink, W.E. Hydrogels in a historical perspective: From simple network to smart materials. J. Control. Release 2014, 190, 254–273. [Google Scholar] [CrossRef]
- Kulkarni, R.V.; Biswanath, S.A. Electrically responsive smart hydrogels in drug delivery: A review. J. Appl. Biomater. Biomech. 2007, 5, 125–139. [Google Scholar]
- Alexander, A.; Ajazuddin; Khan, J.; Saraf, S.; Saraf, S. Polyethylene glycol (PEG)-poly(N-isopropylacrylamide) (PNIPAAm) based thermosensitive injectable hydrogels for biomedical applications. Euro. J. Pharm. Biopharm. 2014, 88, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Saikia, A.K.; Mandal, U.K.; Aggarwal, S. Network structure and temperature deoendence swelling behabior of PEG-b-poly(NIPAM-co-AMPSA) hydrogels in water. J. Polym. Res. 2012, 19, 9871. [Google Scholar] [CrossRef]
- Zhang, B.-Y.; He, W.-D.; Li, W.-T.; Li, L.-Y.; Zhang, K.-R.; Zhang, H. Preparation of block-brush PEG-b-P(NIPAM-g-DMAEMA) and its dual stimulus-response. Polymers 2010, 51, 3039–3046. [Google Scholar] [CrossRef]
- Ishida, K.; Uno, T.; Itih, T.; Kubo, M. Synthesis and property of temperature-responsive hydrogel with movable cross-linking points. Macromolecules 2012, 45, 6136–6142. [Google Scholar] [CrossRef]
- Zhao, D.; Kim, J.F.; Ignacz, G.; Pogany, P.; Lee, Y.M.; Szekely, G. Bio-Inspired robust membranes nanoengineered from interpenetrating polymer networks of polybenzimidazole/ polydopamine. ACS Nano 2019, 13, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, N.; Broughton, R.M.; Auad, M.L. Graft semi-interpenetrating polymer network phase change materials for thermal energy storage. ACS Appl. Polym. Mater. 2021, 3, 1785–1794. [Google Scholar] [CrossRef]
- Mei, H.; Liu, H.; Shang, Q.; Dong, Y.; Pedersen-Bjergaard, S.; Huang, C.; Shen, X. Organic-solvent-free electromembrane extraction based on semi-interpenetrating polymer networks. Green Chem. 2021, 23, 1782–1793. [Google Scholar] [CrossRef]
- Dragan, E.S. Design and applications of interpenetrating polymer network hydrogels. A review. Chem. Eng. J. 2014, 243, 572–590. [Google Scholar] [CrossRef]
- Myung, D.; Waters, D.; Wiseman, M.; Duhamel, P.-E.; Noolandi, J.; Ta, C.N.; Frank, C.W. Progress in the development of interpenetrating polymer network hydrogels. Polym. Adv. Technol. 2008, 19, 647–657. [Google Scholar] [CrossRef] [Green Version]
- Sperling, J.H.; Mishra, V. The current status of interpenetrating polymer networks. Polym. Adv. Technol. 1996, 7, 197–208. [Google Scholar] [CrossRef]
- Dumitriu, R.P.; Mitchell, G.R.; Vasile, C. Multi-responsive hydrogels based on N-isopropylacrylamide and sodium alginate. Polym. Int. 2011, 60, 222–233. [Google Scholar] [CrossRef]
- Sun, S.; Wu, P. A one-step strategy for thermal- and pH-responsive graphene oxide interpenetrating polymer hydrogel networks. J. Mater. Chem. 2011, 20, 4095–4097. [Google Scholar] [CrossRef]
- Dragan, E.S.; Cocatra, A.I. Smart macroporous IPN hydrogels responsive to pH, temperature, and ionic strength: Synthesis, characterization, and evaluation of controlled release of drugs. ACS Appl. Mater. Interfaces 2016, 8, 12018–12030. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, J.; Cai, P.; Xiao, H. Dual-responsive IPN hydrogel based on sugarcane bagasse cellulose as drug carrier. Int. J. Bio. Maclomol. 2018, 118, 132–140. [Google Scholar] [CrossRef]
- Waters, D.J.; Engberg, K.; Parke-Houben, R.; Ta, C.N.; Jackson, A.J.; Toney, M.F.; Frank, C.W. Structure and mechanism of strength enhancement in interpenetrating polymer network hydrogels. Macromolecules 2011, 44, 5776–5787. [Google Scholar] [CrossRef]
- Myung, D.; Koh, W.; Ko, J.; Hu, Y.; Carrasco, M.; Noolandi, J.; Ta, C.N.; Frank, C.W. BViomimetic strain hardening in interpenetrating polymer network hydrogels. Polymers 2007, 48, 5376–5387. [Google Scholar] [CrossRef]
- Sano, J.; Habaue, S. Multi-responsive polysiloxane/poly(N-isopropylacrylamide) interpenetrating networks containing urea and thiourea groups. Polymers 2020, 12, 1175. [Google Scholar] [CrossRef] [PubMed]
- Sano, J.; Habaue, S. Synthesis of interpenetrating polymer networks using silicone polymer with silanol redue. React. Funct. Polym. 2019, 137, 21–26. [Google Scholar] [CrossRef]
- Abe, Y.; Misono, T. Preparation of polysiloxanes from silicic acid. II. Esterification of silicic acid with various alcohols and isolation of esterification products by silylation. J. Polym. Sci. Polym. Lett. Ed. 1982, 20, 205–210. [Google Scholar] [CrossRef]
- Gunji, T.; Nagao, Y.; Misono, T.; Abe, Y. Condensation and structure of silicic acid in tetrahydrofuran. J. Polym. Sci. Part A Polym. Chem. 1992, 30, 1779–1787. [Google Scholar] [CrossRef]
- Habaue, S.; Hirasa, T.; Akagi, Y.; Yamashita, K.; Kajiwara, M. Synthesis and property of silicone polymer from chrysotile asbestos by acid-leaching and silylation. J. Inorg. Organomet. Polym. Mater. 2006, 16, 155–160. [Google Scholar] [CrossRef]
- Habaue, S.; Sato, K.; Yamashita, K.; Shimamura, T.; Kaito, M.; Masuda, T.; Kajiwara, M. Polysiloxanes derived from chrysotile asbestos via acid-leaching and silylation processes. J. Appl. Polym. Sci. 2008, 110, 2891–2897. [Google Scholar] [CrossRef]
- Jo, S.; Park, K. Novel poly(ethylene glycol) hydrogels from silylated PEGs. J. Bioact. Compat. Polym. 1999, 14, 457–473. [Google Scholar] [CrossRef]
- Subramani, S.; Lee, J.M.; Cheong, I.W.; Kim, J.H. Synthesis and characterization of water-borne crosslinked silylated polyurethane dispersions. J. Appl. Polym. Sci. 2005, 98, 620–631. [Google Scholar] [CrossRef]
- Rivas, B.L.; Pereira, E.D.; Moreno-Villoslada, I. Water-soluble polymer-metal ion interactions. Prog. Polym. Sci. 2003, 28, 173–208. [Google Scholar] [CrossRef]
- Zhong, C.; Wu, Q.; Guo, R.; Zhang, H. Synthesis and luminescence properties of polymeric complexes of Cu(II), Zn(II) and Al(III) with functionalized polybenzimidazole containing 8-hydroxyquinoline side group. Opt. Mater. 2008, 30, 870–875. [Google Scholar] [CrossRef]
- Baskar, C.; Lai, Y.-H.; Valiyaveettil, S. Synthesis of a novel optically tunable amphiphilic poly(p-phenylene): Influence of hydrogen bonding and metal complexation on optical properties. Macromolecules 2001, 34, 6255–6260. [Google Scholar] [CrossRef]
- Choudhury, N.; Mete, S.; Kambalapalli, S.; De, P. Side-chain glycylglycine-based polymer for simultaneous sensing and removal of copper(II) from aqueous medium. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 914–921. [Google Scholar] [CrossRef]








| Run | Polysiloxane | Polyether | Gel Abbreviation | Yield (g) 1 | Q2 |
|---|---|---|---|---|---|
| 1 | none | DS-PEG | IPNPEG | 1.12 | 6.2 |
| 2 | poly-SOLPh | DS-PEG | IPNSOL + PEG | 0.88 | 6.0 |
| 3 | none | DS-PTMO | IPNPTMO | 1.15 | 3.9 |
| 4 | poly-SOLPh | DS-PTMO | IPNSOL + PTMO | 1.10 | 3.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sano, J.; Habaue, S. Dual Temperature and Metal Salts-Responsive Interpenetrating Polymer Networks Composed of Poly (N-isopropylacrylamide) and Polyethylene Glycol. Polymers 2021, 13, 1750. https://doi.org/10.3390/polym13111750
Sano J, Habaue S. Dual Temperature and Metal Salts-Responsive Interpenetrating Polymer Networks Composed of Poly (N-isopropylacrylamide) and Polyethylene Glycol. Polymers. 2021; 13(11):1750. https://doi.org/10.3390/polym13111750
Chicago/Turabian StyleSano, Junta, and Shigeki Habaue. 2021. "Dual Temperature and Metal Salts-Responsive Interpenetrating Polymer Networks Composed of Poly (N-isopropylacrylamide) and Polyethylene Glycol" Polymers 13, no. 11: 1750. https://doi.org/10.3390/polym13111750
APA StyleSano, J., & Habaue, S. (2021). Dual Temperature and Metal Salts-Responsive Interpenetrating Polymer Networks Composed of Poly (N-isopropylacrylamide) and Polyethylene Glycol. Polymers, 13(11), 1750. https://doi.org/10.3390/polym13111750