Effect of the Two-Dimensional Magnetostrictive Fillers of CoFe2O4-Intercalated Graphene Oxide Sheets in 3-2 Type Poly(vinylidene fluoride)-Based Magnetoelectric Films
Abstract
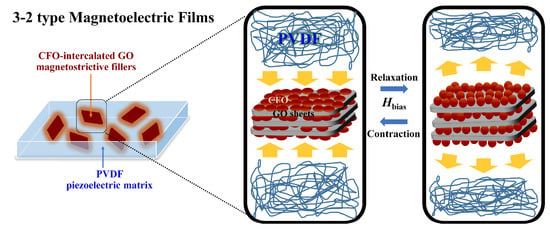
:1. Introduction
2. Experimental
2.1. Synthesis of Graphene Oxide Sheets
2.2. Synthesis of CoFe2O4-Intercalated Graphene Oxide
2.3. Fabrication of Magnetoelectric Films
2.4. Characterization
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhadra, D.; Masud, M.G.; De, S.K.; Chaudhuri, B.K. Large magnetoelectric effect and low-loss high relative permittivity in 0–3 CuO/PVDF composite films exhibiting unusual ferromagnetism at room temperature. J. Phys. D Appl. Phys. 2012, 45, 485002. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, S.C.; Apo, D.J.; Maurya, D.; Priya, S. Tunable self-biased magnetoelectric response in homogenous laminates. Appl. Phys. Lett. 2012, 101, 232905. [Google Scholar] [CrossRef] [Green Version]
- Abdelazim, H. Magneto-electric nanocarriers for drug delivery: An overview. J. Drug Deliv. Sci. Technol. 2017, 37, 46–50. [Google Scholar] [CrossRef]
- Li, M.; Matyushov, A.; Dong, C.; Chen, H.; Lin, H.; Nan, T.; Qian, Z.; Rinaldi, M.; Lin, Y.; Sun, N.X. Ultra-sensitive NEMS magnetoelectric sensor for picotesla DC magnetic field detection. Appl. Phys. Lett. 2017, 110, 143510. [Google Scholar] [CrossRef]
- Noh, B.-I.; Yang, S.C. Individually free-standing piezoelectric nanotubes-based BaTiO3/CoFe2O4 magnetoelectric thin films. Mater. Lett. 2019, 242, 183–186. [Google Scholar] [CrossRef]
- Lu, P.P.; Shen, J.X.; Shang, D.S.; Sun, Y. Nonvolatile Memory and Artificial Synapse Based on the Cu/P(VDF-TrFE)/Ni Organic Memtranstor. ACS Appl. Mater. Interfaces 2020, 12, 4673–4677. [Google Scholar] [CrossRef] [PubMed]
- Martins, P.; Moya, X.; Phillips, L.C.; Kar-Narayan, S.; Mathur, N.D.; Lanceros-Mendez, S. Linear anhysteretic direct magnetoelectric effect in Ni0.5Zn0.5Fe2O4/poly(vinylidene fluoride-trifluoroethylene) 0-3 nanocomposites. J. Phys. D Appl. Phys. 2011, 44, 482001. [Google Scholar] [CrossRef] [Green Version]
- Martins, P.; Moya, X.; Caparrós, C.; Fernandez, J.; Mathur, N.D.; Lanceros-Mendez, S. Large linear anhysteretic magnetoelectric voltage coefficients in CoFe2O4/polyvinylidene fluoride 0–3 nanocomposites. J. Nanoparticle Res. 2013, 15, 1825. [Google Scholar] [CrossRef]
- Martins, P.L.A.; Kolen’Ko, Y.V.; Rivas, J.; Lanceros-Mendez, S. Tailored Magnetic and Magnetoelectric Responses of Polymer-Based Composites. ACS Appl. Mater. Interfaces 2015, 7, 15017–15022. [Google Scholar] [CrossRef] [Green Version]
- Lu, S.G.; Jin, J.Z.; Zhou, X.; Fang, Z.; Wang, Q.; Zhang, Q.M. Large magnetoelectric coupling coefficient in poly(vinylidene fluoride-hexafluoropropylene)/Metglas laminates. J. Appl. Phys. 2011, 110, 104103. [Google Scholar] [CrossRef]
- Jin, J.; Zhao, F.; Han, K.; Haque, M.A.; Dong, L.; Wang, Q. Multiferroic Polymer Laminate Composites Exhibiting High Magnetoelectric Response Induced by Hydrogen-Bonding Interactions. Adv. Funct. Mater. 2013, 24, 1067–1073. [Google Scholar] [CrossRef]
- Chacko, S.K.; Rahul, M.T.; Raneesh, B.; Kalarikkal, N. Enhanced magnetoelectric coupling and dielectric constant in flexible ternary composite electrospun fibers of PVDF-HFP loaded with nanoclay and NiFe2O4 nanoparticles. New J. Chem. 2020, 44, 11356–11364. [Google Scholar] [CrossRef]
- Martins, P.; Lasheras, A.; Gutierrez, J.; Barandiaran, J.M.; Orue, I.; Lanceros-Mendez, S. Optimizing piezoelectric and magnetoelectric responses on CoFe2O4/P(VDF-TrFE) nanocomposites. J. Phys. D Appl. Phys. 2011, 44, 495303. [Google Scholar] [CrossRef] [Green Version]
- Martins, P.; Costa, C.M.; Benelmekki, M.; Botelho, G.; Lanceros-Mendez, S. On the origin of the electroactive poly(vinylidene fluoride) β-phase nucleation by ferrite nanoparticles via surface electrostatic interactions. CrystEngComm 2012, 14, 2807–2811. [Google Scholar] [CrossRef]
- Li, J.; Chen, S.; Liu, W.; Fu, R.; Tu, S.; Zhao, Y.; Dong, L.; Yan, B.; Gu, Y. High Performance Piezoelectric Nanogenerators Based on Electrospun ZnO Nanorods/Poly(vinylidene fluoride) Composite Membranes. J. Phys. Chem. C 2019, 123, 11378–11387. [Google Scholar] [CrossRef]
- Sasmal, A.; Patra, A.; Devi, P.S.; Sen, S. Hydroxylated BiFeO3 as efficient fillers in poly(vinylidene fluoride) for flexible dielectric, ferroelectric, energy storage and mechanical energy harvesting application. Dalton Trans. 2021, 50, 1824–1837. [Google Scholar] [CrossRef]
- Oh, W.J.; Lim, H.S.; Won, J.S.; Lee, S.G. Preparation of PVDF/PAR Composites with Piezoelectric Properties by Post-Treatment. Polymers 2018, 10, 1333. [Google Scholar] [CrossRef] [Green Version]
- Choi, M.H.; Yang, S.C. CoFe2O4 nanofiller effect on β-phase formation of PVDF matrix for polymer-based magnetoelectric composites. Mater. Lett. 2018, 223, 73–77. [Google Scholar] [CrossRef]
- Abdelhamid, E.H.; Jayakumar, O.D.; Kotari, V.; Mandal, B.P.; Rao, R.; Naik, V.M.; Naik, R.; Tyagi, A.K. Multiferroic PVDF–Fe3O4 hybrid films with reduced graphene oxide and ZnO nanofillers. RSC Adv. 2016, 6, 20089–20094. [Google Scholar] [CrossRef]
- Zaaba, N.; Foo, K.; Hashim, U.; Tan, S.; Liu, W.W.; Voon, C. Synthesis of Graphene Oxide using Modified Hummers Method: Solvent Influence. Procedia Eng. 2017, 184, 469–477. [Google Scholar] [CrossRef]
- Xia, H.; Zhu, D.; Fu, Y.; Wang, X. CoFe2O4-graphene nanocomposite as a high-capacity anode material for lithium-ion batteries. Electrochim. Acta 2012, 83, 166–174. [Google Scholar] [CrossRef]
- Sayah, A.; Habelhames, F.; Bahloul, A.; Nessark, B.; Bonnassieux, Y.; Tendelier, D.; El Jouad, M. Electrochemical synthesis of polyaniline-exfoliated graphene composite films and their capacitance properties. J. Electroanal. Chem. 2018, 818, 26–34. [Google Scholar] [CrossRef]
- Feng, Z.; Jin, X.; Zhang, R.; Zhang, C.; Chen, J.; Wang, Y.; Hu, J. An easy and eco-friendly method to prepare reduced graphene oxide with Fe(OH)2 for use as a conductive additive for LiFePO4 cathode materials. RSC Adv. 2013, 3, 4408–4415. [Google Scholar] [CrossRef]
- Pan, S.; Aksay, I.A. Factors Controlling the Size of Graphene Oxide Sheets Produced via the Graphite Oxide Route. ACS Nano 2011, 5, 4073–4083. [Google Scholar] [CrossRef]
- Hidayah, N.M.S.; Liu, W.-W.; Lai, C.-W.; Noriman, N.Z.; Khe, C.-S.; Hashim, U.; Lee, H.C. Comparison on graphite, graphene oxide and reduced graphene oxide: Synthesis and characterization. AIP Conf. Proc. 2017, 150002. [Google Scholar] [CrossRef]
- Gurzęda, B.; Buchwald, T.; Nocuń, M.; Bąkowicz, A.; Krawczyk, P. Graphene material preparation through thermal treatment of graphite oxide electrochemically synthesized in aqueous sulfuric acid. RSC Adv. 2017, 7, 19904–19911. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Xie, J.; Fang, C.; Cao, G.; Zhu, T.; Zhao, X. Self-assembly of a CoFe2O4/graphene sandwich by a controllable and general route: Towards a high-performance anode for Li-ion batteries. J. Mater. Chem. 2012, 22, 19738–19743. [Google Scholar] [CrossRef]
- Tabit, R.; Amadine, O.; Essamlali, Y.; Dânoun, K.; Rhihil, A.; Zahouily, M. Magnetic CoFe2O4 nanoparticles supported on graphene oxide (CoFe2O4/GO) with high catalytic activity for peroxymonosulfate activation and degradation of rhodamine B. RSC Adv. 2018, 8, 1351–1360. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Zheng, M.; Chang, X.; Ji, G.; Lu, H.; Xue, L.; Pan, L.; Cao, J. Preparation of magnetic CoFe2O4-functionalized graphene sheets via a facile hydrothermal method and their adsorption properties. J. Solid State Chem. 2011, 184, 953–958. [Google Scholar] [CrossRef]
- Fernandes, M.M.; Mora, H.; Barriga-Castro, E.D.; Luna, C.; Mendoza-Reséndez, R.; Ribeiro, C.; Lanceros-Mendez, S.; Martins, P. Improving Magnetoelectric Contactless Sensing and Actuation through Anisotropic Nanostructures. J. Phys. Chem. C 2018, 122, 19189–19196. [Google Scholar] [CrossRef]
- Sarkar, B.; Dalal, B.; Dev Ashok, V.; Chakrabarti, K.; Mitra, A.; De, S.K. Magnetic properties of mixed spinel BaTiO3-NiFe2O4 composites. J. Appl. Phys. 2014, 115, 123908. [Google Scholar] [CrossRef]
- Pramoda, K.; Mohamed, A.; Phang, I.Y.; Liu, T. Crystal transformation and thermomechanical properties of poly(vinylidene fluoride)/clay nanocomposites. Polym. Int. 2004, 54, 226–232. [Google Scholar] [CrossRef]
- Martins, P.; Lopes, A.; Lanceros-Mendez, S. Electroactive phases of poly(vinylidene fluoride): Determination, processing and applications. Prog. Polym. Sci. 2014, 39, 683–706. [Google Scholar] [CrossRef]
- Ben Osman, C.; Nowak, S.; Garcia-Sanchez, A.; Charles, Y.; Ammar, S.; Mercone, S.; Mammeri, F. In situ monitored stretching induced α to β allotropic transformation of flexible poly(vinylidene fluoride)-CoFe2O4 hybrid films: The role of nanoparticles inclusion. Eur. Polym. J. 2016, 84, 602–611. [Google Scholar] [CrossRef]
- Cai, X.; Lei, T.; Sun, D.; Lin, L. A critical analysis of the α, β and γ phases in poly(vinylidene fluoride) using FTIR. RSC Adv. 2017, 7, 15382–15389. [Google Scholar] [CrossRef] [Green Version]
- Jiang, S.; Wan, H.; Liu, H.; Zeng, Y.; Liu, J.; Wu, Y.; Zhang, G. High β phase content in PVDF/CoFe2O4 nanocomposites induced by DC magnetic fields. Appl. Phys. Lett. 2016, 109, 102904. [Google Scholar] [CrossRef]
- Bae, J.-H.; Chang, S.-H. Characterization of an electroactive polymer (PVDF-TrFE) film-type sensor for health monitoring of composite structures. Compos. Struct. 2015, 131, 1090–1098. [Google Scholar] [CrossRef]
- Gonçalves, R.; Martins, P.; Correia, D.M.; Sencadas, V.; Vilas, J.L.; León, L.M.; Botelho, G.; Lanceros-Méndez, S. Development of magnetoelectric CoFe2O4/poly(vinylidene fluoride) microspheres. RSC Adv. 2015, 5, 35852–35857. [Google Scholar] [CrossRef] [Green Version]
- Tienne, L.G.P.; De Abreu, T.B.; Gondim, F.F.; Cruz, B.D.S.M.D.; Martins, G.R.; Simão, R.A.; Marques, M.D.F.V. Low contents of graphite improving general properties of poly(vinylidene fluoride). Polym. Test. 2020, 91, 106790. [Google Scholar] [CrossRef]
- Sebastian, M.S.; Larrea, A.; Gonçalves, R.; Alejo, T.; Vilas, J.L.; Sebastian, V.; Martins, P.; Lanceros-Mendez, S. Understanding nucleation of the electroactive β-phase of poly(vinylidene fluoride) by nanostructures. RSC Adv. 2016, 6, 113007–113015. [Google Scholar] [CrossRef]
- Hwang, S.; Kim, C.L.; Kim, Y.; Song, M.G.; Lee, J.; Baeck, S.-H.; Shim, S.E.; Qian, Y. Filler size effect in graphite/paraffine wax composite on electromagnetic interference shielding performance. Korean J. Chem. Eng. 2020, 37, 1623–1630. [Google Scholar] [CrossRef]
- Yuan, M.; Brokken-Zijp, J.C.M.; Huijbregts, L.J.; De With, G. Filler size effects on the conductivity of polymer nanocomposites: Semiconductive phthalocyanine nanoparticles in epoxy matrices. J. Polym. Sci. Part B Polym. Phys. 2008, 46, 1079–1093. [Google Scholar] [CrossRef]
- Shi, D.; Zhang, Q.; Ye, Y.M.; Luo, L.; Cheng, E.K.-W.I.; Lin, D.; Lam, K.-H. High-frequency current sensor based on lead-free multiferroic BiFeO 3 -BaTiO 3 -based ceramics. Measurement 2017, 104, 287–293. [Google Scholar] [CrossRef]










| 2D-Fillers | Chemical Composition (wt%) | ||||
|---|---|---|---|---|---|
| Co | Fe | O | C | Others | |
| CFO-i-LGO | 16.3 | 29.4 | 29.3 | 5.0 | 20.0 |
| CFO-i-MGO | 15.8 | 31.0 | 28.4 | 9.0 | 15.8 |
| CFO-i-SGO | 4.6 | 8.1 | 33.8 | 14.4 | 39.1 |
| 2D-Fillers | Ms (emu/g) | Mr (emu/g) | Hc (Oe) |
|---|---|---|---|
| CFO-i-LGO | 60.33 | 26.23 | 1256.81 |
| CFO-i-MGO | 63.98 | 26.73 | 1148.48 |
| CFO-i-SGO | 63.03 | 27.08 | 1095.28 |
| ME Films | β-Phase Content (%) |
|---|---|
| PVDF/CFO-i-LGO | 75.63 |
| PVDF/CFO-i-MGO | 77.53 |
| PVDF/CFO-i-SGO | 74.59 |
| ME Films | Ps (μC/cm2) | Pr (μC/cm2) | Ec (kV/cm) |
|---|---|---|---|
| PVDF/CFO-i-LGO | 0.3333 | 0.032 | 19.50 |
| PVDF/CFO-i-MGO | 0.2591 | 0.012 | 10.73 |
| PVDF/CFO-i-SGO | 0.2394 | 0.012 | 9.94 |
| ME Films | Electric Strength (kV/mm) | ∆d33 (pC/N) | αME (mV/cm·Oe) |
|---|---|---|---|
| PVDF/CFO-i-LGO | 40 | 14 | 3.0 |
| PVDF/CFO-i-MGO | 28 | 7.5 | 1.4 |
| PVDF/CFO-i-SGO | 25 | 6 | 2.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baek, G.; Yang, S.-C. Effect of the Two-Dimensional Magnetostrictive Fillers of CoFe2O4-Intercalated Graphene Oxide Sheets in 3-2 Type Poly(vinylidene fluoride)-Based Magnetoelectric Films. Polymers 2021, 13, 1782. https://doi.org/10.3390/polym13111782
Baek G, Yang S-C. Effect of the Two-Dimensional Magnetostrictive Fillers of CoFe2O4-Intercalated Graphene Oxide Sheets in 3-2 Type Poly(vinylidene fluoride)-Based Magnetoelectric Films. Polymers. 2021; 13(11):1782. https://doi.org/10.3390/polym13111782
Chicago/Turabian StyleBaek, Geunryeol, and Su-Chul Yang. 2021. "Effect of the Two-Dimensional Magnetostrictive Fillers of CoFe2O4-Intercalated Graphene Oxide Sheets in 3-2 Type Poly(vinylidene fluoride)-Based Magnetoelectric Films" Polymers 13, no. 11: 1782. https://doi.org/10.3390/polym13111782
APA StyleBaek, G., & Yang, S. -C. (2021). Effect of the Two-Dimensional Magnetostrictive Fillers of CoFe2O4-Intercalated Graphene Oxide Sheets in 3-2 Type Poly(vinylidene fluoride)-Based Magnetoelectric Films. Polymers, 13(11), 1782. https://doi.org/10.3390/polym13111782