Polyethyleneimine-Oleic Acid Micelles-Stabilized Palladium Nanoparticles as Highly Efficient Catalyst to Treat Pollutants with Enhanced Performance
Abstract
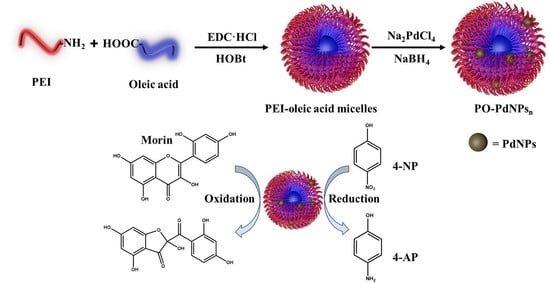
:1. Introduction
2. Materials and Methods
2.1. Preparation of PEI-Oleic Acid Micelles
2.2. Preparation of PO-PdNPsn
2.3. Critical Micelles Concentration Measurement
2.4. Catalytic Reaction on 4-NP
2.5. Catalytic Reaction on Morin
2.6. Statistical Analysis
3. Results
3.1. Characterization of PEI-Oleic Acid Micelles
3.2. Characterization of PO-PdNPsn
3.3. Catalytic Activity of PO-PdNPsn on 4-NP
3.4. Catalytic Activity of PO-PdNPsn on Morin
3.5. Catalytic Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dong, Z.; Le, X.; Dong, C.; Zhang, W.; Li, X.; Ma, J. Ni@Pd core–shell nanoparticles modified fibrous silica nanospheres as highly efficient and recoverable catalyst for reduction of 4-nitrophenol and hydrodechlorination of 4-chlorophenol. Appl. Catal. B 2015, 162, 372–380. [Google Scholar] [CrossRef]
- Zhao, Z.; Ma, X.; Wang, X.; Ma, Y.; Liu, C.; Hang, H.; Zhang, Y.; Du, Y.; Ye, W. Synthesis of amorphous PdP nanoparticles supported on carbon nanospheres for 4-nitrophenol reduction in environmental applications. Appl. Surf. Sci. 2018, 457, 1009–1017. [Google Scholar] [CrossRef]
- Ghosh, P.; Patwari, J.; Dasgupta, S. Complexation with human serum albumin facilitates sustained release of morin from polylactic-co-glycolic acid nanoparticles. J. Phys. Chem. B 2017, 121, 1758–1770. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Zhu, X.-Y.; Feng, J.-J.; Wang, A.-J. Solvothermal synthesis of N-doped graphene supported PtCo nanodendrites with highly catalytic activity for 4-nitrophenol reduction. Appl. Surf. Sci. 2018, 428, 798–808. [Google Scholar] [CrossRef]
- Hao, Y.; Shao, X.; Li, B.; Hu, L.; Wang, T. Mesoporous TiO2 nanofibers with controllable Au loadings for catalytic reduction of 4-nitrophenol. Mater. Sci. Semicond. Process. 2015, 40, 621–630. [Google Scholar] [CrossRef]
- Zhang, H.; Xin, X.; Sun, J.; Zhao, L.; Shen, J.; Song, Z.; Yuan, S. Self-assembled chiral helical nanofibers by amphiphilic dipeptide derived from d- or l-threonine and application as a template for the synthesis of Au and Ag nanoparticles. J. Colloid Interface Sci. 2016, 484, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Xiao, M.; Liang, S.; Zhang, Z.; Zeng, Y.; Xi, J.; Wang, S. Ultrafine palladium nanoparticles supported on nitrogen-doped carbon microtubes as a high-performance organocatalyst. Carbon 2017, 119, 326–331. [Google Scholar] [CrossRef]
- Le, X.; Dong, Z.; Li, X.; Zhang, W.; Le, M.; Ma, J. Fibrous nano-silica supported palladium nanoparticles: An efficient catalyst for the reduction of 4-nitrophenol and hydrodechlorination of 4-chlorophenol under mild conditions. Catal. Commun. 2015, 59, 21–25. [Google Scholar] [CrossRef]
- Deraedt, C.; Salmon, L.; Astruc, D. “Click” dendrimer-stabilized palladium nanoparticles as a green catalyst down to parts per million for efficient CC cross-coupling reactions and reduction of 4-nitrophenol. Adv. Synth. Catal. 2014, 356, 2525–2538. [Google Scholar] [CrossRef]
- Fedorczyk, A.; Ratajczak, J.; Kuzmych, O.; Skompska, M. Kinetic studies of catalytic reduction of 4-nitrophenol with NaBH4 by means of Au nanoparticles dispersed in a conducting polymer matrix. J. Solid State Electrochem. 2015, 19, 2849–2858. [Google Scholar] [CrossRef]
- Ma, T.; Yang, W.; Liu, S.; Zhang, H.; Liang, F. A comparison reduction of 4-nitrophenol by gold nanospheres and gold nanostars. Catalysts 2017, 7, 38. [Google Scholar] [CrossRef] [Green Version]
- Fan, L.; Ji, X.; Lin, G.; Liu, K.; Chen, S.; Ma, G.; Xue, W.; Zhang, X.; Wang, L. Green synthesis of stable platinum nanoclusters with enhanced peroxidase-like activity for sensitive detection of glucose and glutathione. Microchem. J. 2021, 166, 106–202. [Google Scholar] [CrossRef]
- Li, N.; Zhao, P.; Astruc, D. Anisotropic gold nanoparticles: Synthesis, properties, applications, and toxicity. Angew. Chem. Int. Ed. Engl. 2014, 53, 1756–1789. [Google Scholar] [CrossRef]
- Ye, W.; Yu, J.; Zhou, Y.; Gao, D.; Wang, D.; Wang, C.; Xue, D. Green synthesis of Pt–Au dendrimer-like nanoparticles supported on polydopamine-functionalized graphene and their high performance toward 4-nitrophenol reduction. Appl. Catal. B 2016, 181, 371–378. [Google Scholar] [CrossRef]
- Wang, L.; Yang, Q.; Cui, Y.; Gao, D.; Kang, J.; Sun, H.; Zhu, L.; Chen, S. Highly stable and biocompatible dendrimer-encapsulated gold nanoparticle catalysts for the reduction of 4-nitrophenol. New J. Chem. 2017, 41, 8399–8406. [Google Scholar] [CrossRef]
- Veerakumar, P.; Velayudham, M.; Lu, K.-L.; Rajagopal, S. Silica-supported PEI capped nanopalladium as potential catalyst in Suzuki, Heck and Sonogashira coupling reactions. Appl. Catal. A 2013, 455, 247–260. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.; Cui, Y.; Guo, X.; Chen, S.; Sun, H.; Gao, D.; Yang, Q.; Kang, J. Polyethyleneimine-oleic acid micelle-stabilized gold nanoparticles for reduction of 4-nitrophenol with enhanced performance. Transit. Met. Chem. 2020, 45, 31–39. [Google Scholar] [CrossRef]
- Xu, M.; Qian, J.; Suo, A.; Liu, T.; Liu, X.; Wang, H. A reduction-dissociable PEG-b-PGAH-b-PEI triblock copolymer as a vehicle for targeted co-delivery of doxorubicin and P-gp siRNA. Polym. Chem. 2015, 6, 2445–2456. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, L.; Bernards, M.T.; Chen, S.; Sun, H.; Guo, X. Dendrimer-based biocompatible zwitterionic micelles for efficient cellular internalization and enhanced anti-tumor effects. ACS Appl. Polym. Mater. 2019, 2, 159–171. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.; Guo, X.; Chen, S.; Cui, Y.; Yu, Q.; Yang, L.; Sun, H.; Gao, D.; Xie, D. Highly stable and biocompatible zwitterionic dendrimer-encapsulated palladium nanoparticles that maintain their catalytic activity in bacterial solution. New J. Chem. 2018, 42, 19740–19748. [Google Scholar] [CrossRef]
- Cui, T.; Li, S.; Chen, S.; Liang, Y.; Sun, H.; Wang, L. “Stealth” dendrimers with encapsulation of indocyanine green for photothermal and photodynamic therapy of cancer. Int. J. Pharm. 2021, 600, 120–502. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Li, R.; Wang, L.; Lan, X.; Sun, H.; Zhao, Y.; Wang, L. Green synthesis of platinum nanoclusters using lentinan for sensitively colorimetric detection of glucose. Int. J. Biol. Macromol. 2021, 172, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Lin, W.; Wang, Z.; Zhang, J.; Qian, H.; Xu, L.; Yuan, Z.; Chen, S. Development of polypeptide-based zwitterionic amphiphilic micelles for nanodrug delivery. J. Mater. Chem. B 2016, 4, 5256–5264. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Chang, M.Y.Z.; Cheng, W.I.; Wang, Q.; Commisso, A.; Capeling, M.; Wu, Y.; Cheng, C. Biodegradable zwitterionic sulfobetaine polymer and its conjugate with paclitaxel for sustained drug delivery. Acta Biomater. 2017, 64, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Kaiser, J.; Marzun, G.; Ott, A.; Lu, Y.; Ballauff, M.; Zaccone, A.; Barcikowski, S.; Wagener, P. Ligand-free gold nanoparticles as a reference material for kinetic modelling of catalytic reduction of 4-nitrophenol. Catal. Lett. 2015, 145, 1105–1112. [Google Scholar] [CrossRef]
- Le, X.; Dong, Z.; Liu, Y.; Jin, Z.; Huy, T.-D.; Le, M.; Ma, J. Palladium nanoparticles immobilized on core–shell magnetic fibers as a highly efficient and recyclable heterogeneous catalyst for the reduction of 4-nitrophenol and Suzuki coupling reactions. J. Mater. Chem. A 2014, 2, 19696–19706. [Google Scholar] [CrossRef]
- Morère, J.; Tenorio, M.J.; Torralvo, M.J.; Pando, C.; Renuncio, J.A.R.; Cabañas, A. Deposition of Pd into mesoporous silica SBA-15 using supercritical carbon dioxide. J. Supercrit. Fluids 2011, 56, 213–222. [Google Scholar] [CrossRef]
- Liu, B.; Yu, S.; Wang, Q.; Hu, W.; Jing, P.; Liu, Y.; Jia, W.; Liu, Y.; Liu, L.; Zhang, J. Hollow mesoporous ceria nanoreactors with enhanced activity and stability for catalytic application. Chem. Commun. 2013, 49, 3757–3759. [Google Scholar] [CrossRef]
- Ncube, P.; Hlabathe, T.; Meijboom, R. The preparation of well-defined dendrimer-encapsulated palladium and platinum nanoparticles and their catalytic evaluation in the oxidation of morin. Appl. Surf. Sci. 2015, 357, 1141–1149. [Google Scholar] [CrossRef]
- Nemanashi, M.; Meijboom, R. Catalytic behavior of different sizes of dendrimer-encapsulated Au(n) nanoparticles in the oxidative degradation of morin with H2O2. Langmuir 2015, 31, 9041–9053. [Google Scholar] [CrossRef]
- Ilunga, A.K.; Meijboom, R. Catalytic and kinetic investigation of the encapsulated random alloy (Pdn-Au110-n) nanoparticles. Appl. Catal. B 2016, 189, 86–98. [Google Scholar] [CrossRef]
- Ndolomingo, M.J.; Meijboom, R. Kinetics of the catalytic oxidation of morin on γ-Al2O3 supported gold nanoparticles and determination of gold nanoparticles surface area and sizes by quantitative ligand adsorption. Appl. Catal. B 2016, 199, 142–154. [Google Scholar] [CrossRef]
- Polzer, F.; Wunder, S.; Lu, Y.; Ballauff, M. Oxidation of an organic dye catalyzed by MnOx nanoparticles. J. Catal. 2012, 289, 80–87. [Google Scholar] [CrossRef]
- Xaba, M.S.; Meijboom, R. Kinetic and catalytic analysis of mesoporous Co3O4 on the oxidation of morin. Appl. Surf. Sci. 2017, 423, 53–62. [Google Scholar] [CrossRef]
- Gu, S.; Wunder, S.; Lu, Y.; Ballauff, M.; Fenger, R.; Rademann, K.; Jaquet, B.; Zaccone, A. Kinetic analysis of the catalytic reduction of 4-nitrophenol by Metallic Nanoparticles. J. Phys. Chem. C 2014, 118, 18618–18625. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Baran, T.; Baran, N.Y.; Sajjadi, M.; Tahsili, M.R.; Shokouhimehr, M. Pd nanocatalyst stabilized on amine-modified zeolite: Antibacterial and catalytic activities for environmental pollution remediation in aqueous medium. Sep. Purif. Technol. 2020, 239, 116–542. [Google Scholar] [CrossRef]
- Xiao, H.; Wang, R.; Dong, L.; Cui, Y.; Chen, S.; Sun, H.; Ma, G.; Gao, D.; Wang, L. Biocompatible Dendrimer-Encapsulated Palladium Nanoparticles for Oxidation of Morin. ACS Omega 2019, 4, 18685–18691. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, X.; Zhang, X.; Li, S.; Zhang, J.; Lin, W.; Wang, L. Polyethyleneimine-Oleic Acid Micelles-Stabilized Palladium Nanoparticles as Highly Efficient Catalyst to Treat Pollutants with Enhanced Performance. Polymers 2021, 13, 1890. https://doi.org/10.3390/polym13111890
Lai X, Zhang X, Li S, Zhang J, Lin W, Wang L. Polyethyleneimine-Oleic Acid Micelles-Stabilized Palladium Nanoparticles as Highly Efficient Catalyst to Treat Pollutants with Enhanced Performance. Polymers. 2021; 13(11):1890. https://doi.org/10.3390/polym13111890
Chicago/Turabian StyleLai, Xiang, Xuan Zhang, Shukai Li, Jie Zhang, Weifeng Lin, and Longgang Wang. 2021. "Polyethyleneimine-Oleic Acid Micelles-Stabilized Palladium Nanoparticles as Highly Efficient Catalyst to Treat Pollutants with Enhanced Performance" Polymers 13, no. 11: 1890. https://doi.org/10.3390/polym13111890
APA StyleLai, X., Zhang, X., Li, S., Zhang, J., Lin, W., & Wang, L. (2021). Polyethyleneimine-Oleic Acid Micelles-Stabilized Palladium Nanoparticles as Highly Efficient Catalyst to Treat Pollutants with Enhanced Performance. Polymers, 13(11), 1890. https://doi.org/10.3390/polym13111890