Facile Synthesis of Natural Anise-Based Nanoemulsions and Their Antimicrobial Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
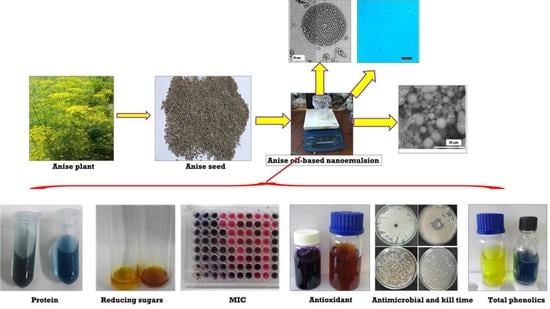
2.2. Preparation of Anise Oil-Based Nanoemulsion
2.3. Characterization of Bulk Anise Oil and Anise Oil-Based Nanoemulsion
2.3.1. Chemical Analysis of Anise Oil Using Gas Chromatography–Mass Spectrometry (GS-MS) Spectroscopy
2.3.2. Physical Characterization of Anise-Based Nanoemulsion
2.3.3. Antimicrobial Activity by Cup Plate Agar Diffusion Method For Macro and Nanoemulsion from Anise Oil
2.3.4. Determination of Minimum Inhibition Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of Different Prepared Nanoemulsions
2.3.5. Kill-Time
2.3.6. Antioxidant Activity of Different Concentrations of Anise Nanoemulsion
2.3.7. Determination of the Total Phenolic Contents of Anise-Based Nanoemulsion
2.3.8. Cytotoxicity Assay
2.3.9. Evaluation of the Mechanism of Action of the Anise Oil Nanoemulsion as Antimicrobial Agent
2.3.10. Morphological Structure of Microbial Cell After Subjecting to Anise Oil-Based Nanoemulsion
2.4. Statistical Analysis
3. Results and Discussion
3.1. Gas Chromatography–Mass Spectroscopy of Anise Oil
3.2. Characterization of Nanoemulsion Using Transmission Electron Microscopy
3.3. Average Hydrodynamic Particle Size and Zeta Potentials of the Formed Anise-Based Nanoemulsion
3.4. Morphological Structure of Anise-Based Nanoemulsion
3.5. Determination of Antimicrobial Activity for Different Concentrations of Anise-Based Micro- and Nanoemulsion
3.6. Kill-Time Assay
3.7. Antioxidant Activity of Different Concentrations of Anise-Based Nanoemulsions Using Different Methods and Evaluations of Their Total Phenolic Contents
3.8. Cytotoxicity Activity of Different Concentrations of Anise Oil-Based Nanoemulsions Against Two Cell Lines, HepG2 and MCF7
3.8.1. Estimation of the Mode of Action of Different Concentrations of Anise Oil-Based Nanoemulsion by Measuring the Release of Reducing Sugars and Proteins from S. aureus and C. albicans Treated with Anise Nanodroplets
3.8.2. Effect of Anise Oil-Based Nanoemulsion on the Morphological Structure of Microbial Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Holley, R.A.; Patel, D. Improvement in shelf-life and safety of perishable foods by plant essential oils and smoke antimicrobials. Food Microbiol. 2005, 22, 273–292. [Google Scholar] [CrossRef]
- Weiss, J.; Gaysinsky, S.; Davidson, M.; McClements, J. Nanostructured encapsulation systems: Food antimicrobials. In Global Issues in Food Science and Technology; Elsevier: Amsterdam, The Netherlands, 2009; pp. 425–479. [Google Scholar]
- Topuz, O.K.; Özvural, E.B.; Zhao, Q.; Huang, Q.; Chikindas, M.; Gölükçü, M. Physical and antimicrobial properties of anise oil loaded nanoemulsions on the survival of foodborne pathogens. Food Chem. 2016, 203, 117–123. [Google Scholar] [CrossRef]
- Gyawali, R.; Ibrahim, S.A. Natural products as antimicrobial agents. Food Control 2014, 46, 412–429. [Google Scholar] [CrossRef]
- Liang, R.; Xu, S.; Shoemaker, C.F.; Li, Y.; Zhong, F.; Huang, Q. Physical and Antimicrobial Properties of Peppermint Oil Nanoemulsions. J. Agric. Food Chem. 2012, 60, 7548–7555. [Google Scholar] [CrossRef] [PubMed]
- Tepe, B.; Eminagaoglu, O.; Akpulat, H.A.; Aydin, E. Antioxidant potentials and rosmarinic acid levels of the methanolic extracts of Salvia verticillata (L.) subsp. verticillata and S. verticillata (L.) subsp. amasiaca (Freyn & Bornm.) Bornm. Food Chem. 2007, 100, 985–989. [Google Scholar]
- Zou, Y.; Lee, H.-Y.; Seo, Y.-C.; Ahn, J. Enhanced Antimicrobial Activity of Nisin-Loaded Liposomal Nanoparticles against Foodborne Pathogens. J. Food Sci. 2012, 77, M165–M170. [Google Scholar] [CrossRef]
- Meleson, K.; Graves, S.; Mason, T.G. Formation of Concentrated Nanoemulsions by Extreme Shear. Soft Mater. 2004, 2, 109–123. [Google Scholar] [CrossRef]
- Canselier, J.P.; Delmas, H.; Wilhelm, A.M.; Abismail, B. Ultrasound emulsification—An overview. J. Dispers. Sci. Technol. 2002, 23, 333–349. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, S.; Zhang, J.; Chi, Y.; Tian, B.; Li, L.; Jiang, B.; Li, D.; Feng, Z.; Liu, C. Preparation of whey protein isolate nanofibrils by microwave heating and its application as carriers of lipophilic bioactive substances. LWT 2020, 125, 109213. [Google Scholar] [CrossRef]
- Ganta, S.; Talekar, M.; Singh, A.; Coleman, T.P.; Amiji, M.M. Nanoemulsions in Translational Research—Opportunities and Challenges in Targeted Cancer Therapy. AAPS PharmSciTech 2014, 15, 694–708. [Google Scholar] [CrossRef] [Green Version]
- de Campos, V.E.B.; Ricci-Júnior, E.; Mansur, C.R.E. Nanoemulsions as delivery systems for lipophilic drugs. J. Nanosci. Nanotechnol. 2012, 12, 2881–2890. [Google Scholar] [CrossRef]
- Simonazzi, A.; Cid, A.G.; Villegas, M.; Romero, A.I.; Palma, S.D.; Bermúdez, J.M. Nanotechnology applications in drug controlled release. In Drug Targeting and Stimuli Sensitive Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2018; pp. 81–116. [Google Scholar]
- Sivakumar, M.; Tang, S.Y.; Tan, K.W. Cavitation technology—A greener processing technique for the generation of pharmaceutical nanoemulsions. Ultrason. Sonochem. 2014, 21, 2069–2083. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, V.; Mukherjee, A.; Chandrasekaran, N. Ultrasonic emulsification of food-grade nanoemulsion formulation and evaluation of its bactericidal activity. Ultrason. Sonochem. 2013, 20, 338–344. [Google Scholar] [CrossRef]
- Jiang, B.; Wang, X.; Wang, L.; Wu, S.; Li, D.; Liu, C.; Feng, Z. Fabrication and Characterization of a Microemulsion Stabilized by Integrated Phosvitin and Gallic Acid. J. Agric. Food Chem. 2020, 68, 5437–5447. [Google Scholar] [CrossRef]
- Nikolić, M.; Jovanović, K.K.; Marković, T.; Marković, D.; Gligorijević, N.; Radulović, S.; Soković, M. Chemical composition, antimicrobial, and cytotoxic properties of five Lamiaceae essential oils. Ind. Crops Prod. 2014, 61, 225–232. [Google Scholar] [CrossRef]
- Martucci, J.; Gende, L.; Neira, L.; Ruseckaite, R. Oregano and lavender essential oils as antioxidant and antimicrobial additives of biogenic gelatin films. Ind. Crops Prod. 2015, 71, 205–213. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oils by Gas Chromatography Quadrupole Mass Spectrometry; Allured Publishing Corporation: Carol Stream, IL, USA, 2001. [Google Scholar]
- Ghareeb, M.A.; Refahy, L.A.; Saad, A.M.; Ahmed, W.S. Chemical composition, antioxidant and anticancer activities of the essential oil from Eucalyptus citriodora (Hook.) leaves. Der Pharma Chem. 2016, 8, 192–200. [Google Scholar]
- El-Razek, S.E.A.; El-Gamasy, S.M.; Hassan, M.; Abdel-Aziz, M.S.; Nasr, S.M. Transition metal complexes of a multidentate Schiff base ligand containing guanidine moiety: Synthesis, characterization, anti-cancer effect, and anti-microbial activity. J. Mol. Struct. 2020, 1203, 127381. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Sarker, S.D.; Nahar, L.; Kumarasamy, Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 2007, 42, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Joray, M.B.; Del Rollán, M.R.; Ruiz, G.M.; Palacios, S.; Carpinella, M.C. Antibacterial Activity of Extracts from Plants of Central Argentina—Isolation of an Active Principle from Achyrocline satureioides. Planta Med. 2011, 77, 95–100. [Google Scholar] [CrossRef]
- Maria do Socorro, M.R.; Alves, R.E.; de Brito, E.S.; Pérez-Jiménez, J.; Saura-Calixto, F.; Mancini-Filho, J. Bioactive compounds and antioxidant capacities of 18 non-traditional tropical fruits from Brazil. Food Chem. 2010, 121, 996–1002. [Google Scholar] [CrossRef] [Green Version]
- Karamac, M.; Kosiñska, A.; Pegg, R.B. Comparison of radical-scavenging activities for selected phenolic acids. Pol. J. Food Nutr. Sci. 2005, 14, 165–170. [Google Scholar]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Donizot, F.; Lang, R. Rapid colorimetric assay for cell growth and survival. Modifications to the trerazolium dye procedure giving improve sensitivity and reliability. J. Immunol. Methods 1986, 89, 271–277. [Google Scholar] [CrossRef]
- Zhang, J.; Ye, K.-P.; Zhang, X.; Pan, D.-D.; Sun, Y.-Y.; Cao, J.-X. Antibacterial Activity and Mechanism of Action of Black Pepper Essential Oil on Meat-Borne Escherichia coli. Front. Microbiol. 2017, 7, 2094. [Google Scholar] [CrossRef] [Green Version]
- Al-Askar, A.A.; Rashad, Y.M. Efficacy of some plant extracts against Rhizoctonia solani on pea. J. Plant Prot. Res. 2010, 50. [Google Scholar] [CrossRef]
- Emerich, D.F.; Thanos, C.G. Nanotechnology and medicine. Expert Opin. Biol. Ther. 2003, 3, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, E.S.; Player, A. Nanotechnology, nanomedicine, and the development of new, effective therapies for cancer. Nanomed. Nanotechnol. Biol. Med. 2005, 1, 101–109. [Google Scholar] [CrossRef]
- Abdelsalam, N.R.; Fouda, M.M.G.; Abdel-Megeed, A.; Ajarem, J.; Allam, A.A.; El-Naggar, M.E. Assessment of silver nanoparticles decorated starch and commercial zinc nanoparticles with respect to their genotoxicity on onion. Int. J. Biol. Macromol. 2019, 133, 1008–1018. [Google Scholar] [CrossRef]
- Hussein, J.; Attia, M.F.; El Bana, M.; El-Daly, S.M.; Mohamed, N.; El-Khayat, Z.; El-Naggar, M.E. Solid state synthesis of docosahexaenoic acid-loaded zinc oxide nanoparticles as a potential antidiabetic agent in rats. Int. J. Biol. Macromol. 2019, 140, 1305–1314. [Google Scholar] [CrossRef] [PubMed]
- Hussein, J.S.; Rasheed, W.; Ramzy, T.; Nabeeh, M.; Harvy, M.; El-Toukhy, S.; Ali, O.; Raafat, J.; El-Naggar, M. Synthesis of docosahexaenoic acid-loaded silver nanoparticles for improving endothelial dysfunctions in experimental diabetes. Hum. Exp. Toxicol. 2019, 38, 962–973. [Google Scholar] [CrossRef] [PubMed]
- Hussein, J.; El Naggar, M.E.; Latif, Y.A.; Medhat, D.; El Bana, M.; Refaat, E.; Morsy, S. Solvent-free and one pot synthesis of silver and zinc nanoparticles: Activity toward cell membrane component and insulin signaling pathway in experimental diabetes. Colloids Surf. B Biointerfaces 2018, 170, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Hussein, J.; El-Naggar, M.E.; Fouda, M.M.G.; Morsy, O.M.; Ajarem, J.S.; Almalki, A.M.; Allam, A.A.; Mekawi, E.M. The efficiency of blackberry loaded AgNPs, AuNPs and Ag@AuNPs mediated pectin in the treatment of cisplatin-induced cardiotoxicity in experimental rats. Int. J. Biol. Macromol. 2020, 159, 1084–1093. [Google Scholar] [CrossRef] [PubMed]
- Abu-Taweel, G.M.; Attia, M.F.; Hussein, J.; Mekawi, E.M.; Galal, H.M.; Ahmed, E.I.; Allam, A.A.; El-Naggar, M.E. Curcumin nanoparticles have potential antioxidant effect and restore tetrahydrobiopterin levels in experimental diabetes. Biomed. Pharmacother. 2020, 131, 110688. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, M.E.; Abdelsalam, N.R.; Fouda, M.M.G.; Mackled, M.I.; Al-Jaddadi, M.A.M.; Ali, H.M.; Siddiqui, M.H.; Kandil, E.E. Soil Application of Nano Silica on Maize Yield and Its Insecticidal Activity against Some Stored Insects after the Post-Harvest. Nanomaterials 2020, 10, 739. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, M.E.; Hussein, J.; El-sayed, S.M.; Youssef, A.M.; El Bana, M.; Latif, Y.A.; Medhat, D. Protective effect of the functional yogurt based on Malva parviflora leaves extract nanoemulsion on acetic acid-induced ulcerative colitis in rats. J. Mater. Res. Technol. 2020, 9, 14500–14508. [Google Scholar] [CrossRef]
- Amer, A.M.; Aly, U.I. Antioxidant and antibacterial properties of anise (Pimpinella anisum L.). Egypt. Pharm. J. 2019, 18, 68–73. [Google Scholar] [CrossRef]
- Mashareq, M.K.; Amira, M.E.; Zenab, A.A.; Ali, I.A.; Fathy, I.R. Evaluating antimicrobial and antioxidant activities of volatile oils extracted from anise, fennel and spearmint plants. J. Sustain. Agric. Sci. 2016, 42, 196–209. [Google Scholar]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial Activity of Some Essential Oils—Present Status and Future Perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]










| Sample No. | Rt | % | M.W. | M.F. | Identified Compounds |
|---|---|---|---|---|---|
| 1 | 8.25 | 0.12 | 136 | C10H16 | Sabinene |
| 2 | 13.75 | 0.20 | 178 | C12H18O | Bicyclo[3.3.1]nonan-2-one, 9-isopropylidene |
| 3 | 14.22 | 0.17 | 154 | C10H18O | Linalool |
| 4 | 14.27 | 0.12 | 136 | C10H16 | Camphene |
| 5 | 14.38 | 0.21 | 140 | C9H16O | Nonadien-1-ol |
| 6 | 14.80 | 0.22 | 154 | C10H18O | 3-Decen-2-one |
| 7 | 14.95 | 0.21 | 154 | C10H18O | Cyclohexanol,1-methyl-4-(1-methylethenyl)-,cis-6 |
| 8 | 15.53 | 11.22 | 154 | C10H18O | 1-Menthone |
| 9 | 15.85 | 0.17 | 154 | C9H14O2 | 2-Acetylcycloheptaneone |
| 14 | 16.12 | 0.19 | 154 | C10H18O | 3-Hepten-2-one, 3-ethyl-4-methyl |
| 15 | 16.35 | 0.16 | 256 | C14H24O4 | 2,5-Dimethoxy-2,3:5,6-bis(tetramethylene)-1,4-dioxane |
| 16 | 16.71 | 0.24 | 150 | C9H10O2 | 2-Methyl-2-(2′-propynyloxy)-4-pentynal |
| 17 | 16.84 | 1.09 | 154 | C10H18O | Cyclohexanone, 5-methyl-2-(1-methylethyl)-,(2R-cis)- |
| 18 | 18.13 | 0.22 | 156 | C10H20O | α-Citronellol |
| 19 | 18.24 | 0.73 | 250 | C17H30O | 2,6-Dimethyl1cyclohexyl-2-ethylenehept-5-enol |
| 20 | 18.29 | 0.49 | 138 | C10H18 | 1,6-Octadiene,2,7-dimethyl- |
| 21 | 18.63 | 4.19 | 140 | C9H16O | 2-Nonyn-1-ol |
| 22 | 18.71 | 8.35 | 124 | C9H16 | 1,6-Heptadiene,3,3-dimethyl- |
| 23 | 20.61 | 1.02 | 138 | C10H18 | 1,4-Hexadiene,3-ethyl-4,5-dimethyl |
| 24 | 21.04 | 3.48 | 154 | C10H18O | Geraniol |
| 25 | 21.13 | 0.12 | 182 | C11H18O2 | Geraniol formate |
| 26 | 22.11 | 0.26 | 204 | C15H24 | α-Ylangene |
| 27 | 22.30 | 4.03 | 196 | C12H20O2 | Geranyl acetate |
| 28 | 22.38 | 0.75 | 204 | C15H24 | (-)-α-Bourbonene |
| 29 | 23.05 | 4.77 | 144 | C8H16O2 | 1-Propanol,2-methyl-1-[1-(hydroxymethyl)cyclopropyl] |
| 30 | 23.38 | 1.24 | 204 | C15H24 | Cadinene |
| 31 | 23.58 | 1.41 | 204 | C15H24 | Calarene |
| 32 | 23.71 | 0.37 | 204 | C15H24 | Humulene |
| 33 | 23.82 | 0.27 | 204 | C15H24 | Trans-Caryophyllene |
| 34 | 24.15 | 3.13 | 222 | C15H26O | Nerolidol |
| 35 | 24.35 | 6.78 | 204 | C15H24 | Gurjunene |
| 36 | 24.63 | 3.93 | 204 | C15H24 | Elemene |
| 37 | 25.01 | 1.54 | 220 | C15H24O | Alloaromadendrene oxide (2) |
| 38 | 25.16 | 5.19 | 204 | C13H16O2 | Cyclopentanecarboxylic acid, 1-(4-methylphenyl)- |
| 39 | 25.28 | 6.79 | 222 | C15H26O | 3(R)-Hydroxy-4-acorene |
| 40 | 25.79 | 1.30 | 220 | C15H24O | Cedrene oxide |
| 41 | 25.90 | 0.18 | 224 | C14H24O2 | Butanoic acid, 3,7-dimethyl-2,6-octadienyl ester, (E)- |
| 42 | 26.55 | 0.22 | 276 | C18H28O2 | 10,12-Octadecadiynoic acid |
| 43 | 26.74 | 0.64 | 204 | C13H16O2 | 2-Phenylethyl tiglate |
| 44 | 26.85 | 0.61 | 204 | C15H24 | Trans-Caryophyllene |
| 45 | 27.49 | 2.97 | 222 | C15H26O | gamma-Eudesmol |
| 46 | 27.59 | 6.29 | 204 | C12H16N2O | 2-Pyrrolidinone,1-(4-amino-3,5-dimethylphenyl) |
| 47 | 28.08 | 2.71 | 326 | C21H26O3 | Benzoic acid,5-methylene-1,1,4a-trimethyldecalin-6-on-2-ylester (8aá) |
| 48 | 28.16 | 0.66 | 222 | C15H26O | Agarospirol |
| 49 | 28.22 | 0.62 | 222 | C15H26O | Cubebol |
| 50 | 28.57 | 6.41 | 138 | C9H14O | 1H-Inden-1-one,octahydro-,cis |
| 51 | 29.33 | 3.53 | 236 | C15H24O2 | Geranyl tiglate |
| T% | 99.52% |
| Sample No. | Anise Oil-Based Nanoemulsion Concentration (mg/mL) | Clear Zone (ϕmm) | |||
|---|---|---|---|---|---|
| S. aureus | A. niger | C. albicans | E. coli | ||
| 1 | 0.055 | 27.5 ± 0.50 | 0 | 17.93 ± 0.11 | 21.96 ± 0.15 |
| 2 | 0.115 | 22.16 ± 0.28 | 0 | 12.46 ± 0.50 | 20.26 ± 0.25 |
| 3 | 0.165 | 27.83 ± 0. 76 | 0 | 19.26 ± 0.30 | 24.40 ± 0.40 |
| 4 | 0.096 | 23.33 ± 0.57 | 0 | 20.63 ± 0.55 | 13.93 ± 0.11 |
| 5 | 0.072 | 12.40 ± 0.36 | 0 | 0 | 0 |
| 6 | 0.048 | 13.60 ± 0.52 | 0 | 15.03 ± 0.25 | 20.16 ± 0.29 |
| Neomycin Cyclohexamide | 0.1 | 26.53 ± 0.47 | 21.86 ± 0.81 | 28.16 ± 0.28 | 0 |
| 0.1 | 0 | 0 | 0 | 31.20 ± 0.20 | |
| Sample No. | Sample conc.(mg /mL) | A. niger | E. coli | S. aureus | C. albicans | ||||
|---|---|---|---|---|---|---|---|---|---|
| MIC (µg/mL) | MBC (µg/mL) | MIC (µg/mL) | MBC (µg/mL) | MIC (µg/mL) | MBC (µg/mL) | MIC (µg/mL) | MBC (µg/mL) | ||
| 1 | 0.055 | 13.50 ± 0.25 | 27.16 ± 0.29 | 27.18 ± 0.27 | 55.35 ± 0.31 | 13.76 ± 0.02 | 27.33 ± 0.28 | 6.70 ± 0.26 | 27.80 ± 0.26 |
| 2 | 0.115 | 14.25 ± 0.25 | 28.73 ± 0.25 | 28.70 ± 0.13 | 57.76 ± 0.25 | 14.55 ± 0.25 | 57.56 ± 0.26 | 7.23 ± 0.15 | 57.29 ± 0.26 |
| 3 | 0.165 | 20.25 ± 0.25 | 41.33 ± 0.14 | 41.50 ± 0.25 | 82.85 ± 0.78 | 20.79 ± 0.19 | 20.79 ± 0.19 | 5.25 ± 0.13 | 41.31 ± 0.16 |
| 4 | 0.096 | 24.06 ± 0.11 | 48.33 ± 0.38 | 24.0 ± 0.25 | 48.43 ± 0.39 | 23.58 ± 0.52 | 24.20 ± 0.18 | 1.50 ± 0.25 | 24.0 ± 0.0 |
| 5 | 0.072 | 9.0 ± 0.20 | 18.29 ± 0.26 | 36.05 ± 0.18 | 72.26 ± 0.25 | 4.48 ± 0.22 | 36.20 ± 0.20 | 18.18 ± 0.16 | 36.15 ± 0.14 |
| 6 | 0.048 | 24.08 ± 0.14 | 24.28 ± 0.30 | 12.21 ± 0.22 | 24.23 ± 0.32 | 3.23 ± 0.21 | 12.15 ± 0.18 | 24.11 ± 0.12 | 12.16 ± 0.15 |
| Sample No. | Sample Description | Concentration Used(µg/mL) | No. of Colonies (cfu) | |
|---|---|---|---|---|
| After 2 h | After 24 h | |||
| 1 | MIC | 6.8 | 37 | 12 |
| 1B | One conc above MIC | 13.6 | 19 | 11 |
| 2 | MIC | 7.1 | 41 | 37 |
| 2B | One conc above MIC | 14.2 | 13 | 30 |
| 3 | MIC | 5.16 | 40 | 55 |
| 3B | One conc above MIC | 10.3 | 15 | 21 |
| 4 | MIC | 3 | 40 | 8 |
| 4B | One conc above MIC | 6 | 18 | 15 |
| 5 | MIC | 18 | 57 | 8 |
| 5B | One conc above MIC | 36 | 42 | 3 |
| 6 | MIC | 24 | 44 | 7 |
| 6B | One conc above MIC | 48 | 21 | 2 |
| Sample No. | Sample Description | Concentration Used(µg/mL) | No. of Colonies (cfu) | |
|---|---|---|---|---|
| After 2 h | After 24 h | |||
| 1 | MIC | 6.87 | 25 | 14 |
| 1B | One conc above MIC | 13.74 | 17 | 18 |
| 2 | MIC | 14.3 | 96 | 22 |
| 2B | One conc above MIC | 28.6 | 26 | 14 |
| 3 | MIC | 20.6 | 17 | 13 |
| 3B | One conc above MIC | 41.2 | 12 | 12 |
| 4 | MIC | 3 | 50 | 20 |
| 4B | One conc above MIC | 6 | 25 | 3 |
| 5 | MIC | 0.56 | 15 | 8 |
| 5B | One conc above MIC | 1.12 | 12 | 3 |
| 6 | MIC | 6 | 28 | 1 |
| 6B | One conc above MIC | 12 | 10 | 0 |
| Sample No. | Concentration of Anise Oil-Based Nanoemulsion (µg/mL) | IC50 (µg) | |
|---|---|---|---|
| HepG-2 | MCF-7 | ||
| 1 | 55 | 9.61 | 7.78 |
| 2 | 115 | 21.48 | 23.31 |
| 3 | 165 | 125.60 | 127.80 |
| 4 | 96 | 50.03 | 43.82 |
| 5 | 72 | 43.20 | 23.68 |
| 6 | 48 | 40.65 | 31.40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu Ali, O.A.; El-Naggar, M.E.; Abdel-Aziz, M.S.; Saleh, D.I.; Abu-Saied, M.A.; El-Sayed, W.A. Facile Synthesis of Natural Anise-Based Nanoemulsions and Their Antimicrobial Activity. Polymers 2021, 13, 2009. https://doi.org/10.3390/polym13122009
Abu Ali OA, El-Naggar ME, Abdel-Aziz MS, Saleh DI, Abu-Saied MA, El-Sayed WA. Facile Synthesis of Natural Anise-Based Nanoemulsions and Their Antimicrobial Activity. Polymers. 2021; 13(12):2009. https://doi.org/10.3390/polym13122009
Chicago/Turabian StyleAbu Ali, Ola A., Mehrez E. El-Naggar, Mohamed S. Abdel-Aziz, Dalia I. Saleh, Mohamed. A. Abu-Saied, and Wael A. El-Sayed. 2021. "Facile Synthesis of Natural Anise-Based Nanoemulsions and Their Antimicrobial Activity" Polymers 13, no. 12: 2009. https://doi.org/10.3390/polym13122009
APA StyleAbu Ali, O. A., El-Naggar, M. E., Abdel-Aziz, M. S., Saleh, D. I., Abu-Saied, M. A., & El-Sayed, W. A. (2021). Facile Synthesis of Natural Anise-Based Nanoemulsions and Their Antimicrobial Activity. Polymers, 13(12), 2009. https://doi.org/10.3390/polym13122009