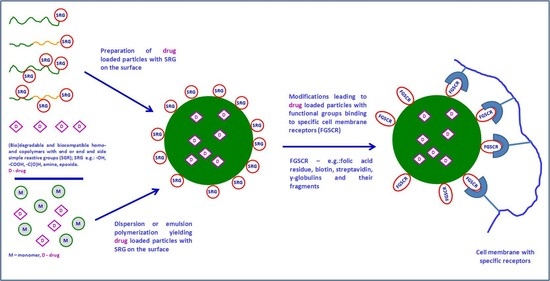
Functionalized Particles Designed for Targeted Delivery
Abstract
:1. Introduction
2. Polymers for Preparation of Drug Delivery Carriers
2.1. Polyanhydrides
2.2. Polycarbonates
2.3. Aliphatic Polyesters
2.4. Polyorthoesters
2.5. Polyalkylcyanoacrylates
2.6. Biopolymers
3. Preparation of Functionalized Nano- and Microparticles
3.1. Functional Nano- and Microparticles Prepared by Polymerization
3.2. Nano- and Microparticles by Self-Assembly of Functional (Co)Polymers
- -
- Nanoprecipitation covering “classical” nanoprecipitation and “reverse” nanoprecipitation;
- -
- Flash nanoprecipitation;
- -
- Solvent evaporation/dialysis;
- -
- Salting out;
- -
- Miscellaneous methods including spray-drying.
3.3. Hybrid Inorganic and Organic Nano- and Microparticles by Multistep Functionalization of Parent Particles
3.4. Nano- and Microparticles with Immobilized Ligands Specific for Nanoparticle-Selected Cell Interactions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Evans, D.F.; Pye, G.; Bramley, R.; Clark, A.G.; Dyson, T.J.; Hardcastle, J.D. Measurement of Gastrointestinal pH Profiles in Normal Ambulant Human Subjects. Gut 1988, 29, 1035–1041. [Google Scholar] [CrossRef] [Green Version]
- Newby, J.M.; Seim, I.; Lysy, M.; Ling, Y.; Huckaby, J.; Lai, S.K.; Forest, M.G. Technological Strategies to Estimate and Control Diffusive Passage Times Through the Mucus Barrier in Mucosal Drug Delivery. Adv. Drug Deliv. Rev. 2018, 124, 64–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slomkowski, S.; Alemán, J.V.; Gilbert, R.G.; Hess, M.; Horie, K.; Jones, R.G.; Kubisa, P.; Meisel, I.; Mormann, W.; Penczek, S.; et al. Terminology of Polymers and Polymerization Processes in Dispersed Systems (IUPAC Recommendations 2011). Pure Appl. Chem. 2011, 83, 2229–2259. [Google Scholar] [CrossRef] [Green Version]
- Münch, S.; Wohlrab, J.; Neubert, R.H.H. Dermal and Transdermal Delivery of Pharmaceutically Relevant Macromolecules. Eur. J. Pharm. Biopharm. 2017, 119, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.C.; Shukla, S.; Skoog, S.A.; Boehm, R.D.; Narayan, R.J. Current Advancements in Transdermal Biosensing and Targeted Drug Delivery. Sensors 2019, 19, 1028. [Google Scholar] [CrossRef] [Green Version]
- Osman, N.; Kaneko, K.; Carini, V.; Saleem, I. Carriers for the Targeted Delivery of Aerosolized Macromolecules for Pulmonary Pathologies. Expert Opin. Drug Deliv. 2018, 15, 821–834. [Google Scholar] [CrossRef] [PubMed]
- Kuzmov, A.; Minko, T. Nanotechnology Approaches for Inhalation Treatment for Lung Diseases. J. Control. Release 2015, 219, 500–518. [Google Scholar] [CrossRef] [Green Version]
- Gizurarson, S. Anatomical and Histological Factors Affecting Intranasal Drug and Vaccine Delivery. Curr. Drug Deliv. 2012, 9, 566–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghadiri, M.; Young, P.M.; Traini, D. Strategies to Enhance Drug Absorption via Nasal and Pulmonary Routes. Pharmaceutics 2019, 11, 113. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Q.; Li, H.M.; Jiang, H.; Yu, J.; Wang, Y.; Ke, H.; Gong, T.; Zhang, Z.R.; Sun, X. Tailoring Polymeric Hybrid Micelles with Lymph Node Targeting Ability to Improve the Potency of Cancer Vaccines. Biomaterials 2017, 122, 105–113. [Google Scholar] [CrossRef]
- Jiang, D.; Mu, W.; Pang, X.; Liu, Y.; Zhang, N.; Song, Y.; Garg, S. Cascade Cytosol Delivery of Dual-sensitive Micelle-tailored Vaccine for Enhancing Cancer Immunotherapy. ACS Appl. Mater. Interfaces 2018, 10, 37797–37811. [Google Scholar] [CrossRef]
- Kuai, R.; Sun, X.; Yuan, W.; Xu, Y.; Schwendeman, A.; Moon, J. Subcutaneous Nanodisc Vaccination with Neoantigens for Combination Cancer Immunotherapy. Bioconjug. Chem. 2018, 29, 771–775. [Google Scholar] [CrossRef]
- Lucke, M.; Mottas, I.; Herbst, T.; Hotz, C.; Römer, L.; Schierling, M.; Herold, H.M.; Slotta, U.; Spinetti, T.; Scheibel, T.; et al. Engineered Hybrid Spider Silk Particles as Delivery System for Peptide Vaccines. Biomaterials 2018, 172, 105–115. [Google Scholar] [CrossRef] [Green Version]
- Howard, G.P.; Verma, G.; Ke, X.; Thayer, W.M.; Hamerly, T.; Baxter, V.K.; Lee, J.E.; Dinglasan, R.R.; Mao, H.-Q. Critical Size Limit of Biodegradable Nanoparticles for Enhanced Lymph Node Trafficking and Paracortex Penetration. Nano Res. 2019, 12, 837–844. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, S.; Qin, Y.; Fan, F.; Zhang, Z.; Huang, C.; Ji, W.; Lu, L.; Wang, C.; Sun, H.; et al. Targeted Codelivery of an Antigen and Dual Agonists by Hybrid Nanoparticles for Enhanced Cancer Immunotherapy. Nano Lett. 2019, 19, 4237–4249. [Google Scholar] [CrossRef]
- Gea, Y.; Chen, D.; Xie, L.; Zhang, R. Optimized Preparation of Daidzein-Loaded Chitosan Microspheres and In Vivo Evaluation After Intramuscular Injection in Rats. Int. J. Pharm. 2007, 338, 142–151. [Google Scholar] [CrossRef]
- Sang, L.; Luo, D.; Wei, Z.; Qi, M. X-Ray Visible and Doxorubicin-loaded Beads Based on Inherently Radiopaque Poly(Lactic Acid)-Polyurethane for Chemoembolization Therapy. Mater. Sci. Eng. C 2017, 75, 1389–1398. [Google Scholar] [CrossRef]
- Guo, Y.; Yang, Y.; He, L.; Sun, R.; Pu, C.; Xie, B.; He, H.; Zhang, Y.; Yin, T.; Wang, Y.; et al. Injectable Sustained-Release Depots of PLGA Microspheres for Insoluble Drugs Prepared by Hot-Melt Extrusion. Pharm. Res. 2017, 34, 2211–2222. [Google Scholar] [CrossRef]
- Tomic, I.; Mueller-Zsigmondy, M.; Vidis-Millward, A.; Cardot, J.-M. In Vivo Release of Peptide-loaded PLGA Microspheres Assessed Through Deconvolution Coupled with Mechanistic Approach. Eur. J. Pharm. Biopharm. 2018, 125, 21–27. [Google Scholar] [CrossRef]
- Andhariya, J.V.; Jog, R.; Shen, J.; Choi, S.; Wang, Y.; Zou, Y. Development of Level A In Vitro-In Vivo Correlations for Peptide Loaded PLGA Microspheres. J. Control. Release 2019, 308, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Qi, P.; Bu, R.; Zhang, H.; Yin, J.; Chen, J.; Zhang, A.; Gou, J.; Yin, T.; Zhang, Y.; He, H.; et al. Goserelin Acetate Loaded Poloxamer Hydrogel in PLGA Microspheres: Core-Shell Di-Depot Intramuscular Sustained Release Delivery System. Mol. Pharm. 2019, 16, 3502–3513. [Google Scholar] [CrossRef]
- Ahmed, O.A.A.; Zidan, A.S.; Khayat, M. Mechanistic Analysis of Zein Nanoparticles/PLGA Triblock In Situ Forming Implants for Glimepiride. Int. J. Nanomed. 2016, 11, 543–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duchi, S.; Ramos-Romero, S.; Dozza, B.; Guerra-Rebollo, M.; Cattini, L.; Ballestri, M.; Dambruoso, P.; Guerrini, A.; Sotgiu, G.; Varchi, G.; et al. Development of Near-infrared Photoactivable Phthalocyanine-loaded Nanoparticles to Kill Tumor Cells: An Improved Tool for Photodynamic Therapy of Solid Cancers. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1885–1897. [Google Scholar] [CrossRef] [PubMed]
- Domb, A.J. Polymeric Site-Specific Pharmacotherapy; John Wiley & Sons: Chichester, UK, 1994. [Google Scholar]
- Zhang, Y.-N.; Poon, W.; Tavares, A.J.; McGilvray, J.D.; Warren, C.W.; Chana, W.C.W. Nanoparticle–Liver Interactions: Cellular Uptake and Hepatobiliary Elimination. J. Control. Release 2016, 240, 332–348. [Google Scholar] [CrossRef] [PubMed]
- Karabasz, A.; Szczepanowicz, K.; Cierniak, A.; Mezyk-Kopec, A.; Dyduch, G.; Szczęch, M.; Bereta, J.; Bzowska, M. In Vivo Studies on Pharmacokinetics, Toxicity and Immunogenicity of Polyelectrolyte Nanocapsules Functionalized with Two Different Polymers: Poly-L-Glutamic Acid or PEG. Int. J. Nanomed. 2019, 14, 9587–9602. [Google Scholar] [CrossRef] [Green Version]
- Yessine, M.A.; Lafleur, M.; Meier, G.; Petereit, H.-U.; Leroux, J.-C. Characterization of the Membrane-Destabilizing Properties of Different pH-sensitive Methacrylic Acid Copolymers. Biochim. Biophys. Acta 2003, 1613, 28–38. [Google Scholar] [CrossRef] [Green Version]
- Duncan, R. Polymer Conjugates as Anticancer Nanomedicines. Nat. Rev. Cancer 2006, 6, 688–701. [Google Scholar] [CrossRef]
- Nam, J.; Won, N.; Bang, J.; Jin, H.; Park, J.; Jung, S.; Jung, S.; Park, Y.; Kim, S. Surface Engineering of Inorganic Nanoparticles for Imaging and Therapy. Adv. Drug Deliv. Rev. 2013, 65, 622–648. [Google Scholar] [CrossRef]
- Shen, S.; Jiang, D.; Cheng, L.; Chao, Y.; Nie, K.; Dong, Z.; Kutyreff, C.J.; Engle, J.W.; Huang, P.; Cai, W.; et al. Renal-Clearable Ultrasmall Coordination Polymer Nanodots for Chelator-free 64Cu-labeling and Imaging-guided Enhanced Radiotherapy of Cancer. ACS Nano 2017, 11, 9103–9111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, L.; Wang, Z.; Wang, F.; Kang, L.; Cao, F.; Dong, K.; Ren, J.; Qu, X. Renal-clearable Ultrasmall Covalent Organic Framework Nanodots as Photodynamic Agents for Effective Cancer Therapy. Biomaterials 2019, 223, 119462. [Google Scholar] [CrossRef] [PubMed]
- Rosen, H.B.; Chang, J.; Wnek, G.E.; Linhard, R.J.; Langer, R. Bioerodible Polyanhydrides for Controlled Drug Delivery. Biomaterials 1983, 4, 131–133. [Google Scholar] [CrossRef]
- Leong, K.W.; Brott, B.C.; Langer, R. Bioerodible Polyanhydrides as Drug-carrier Matrices. I: Characterization, Degradation, and Release Characteristics. J. Biomed. Mater. Res. 1985, 19, 941–955. [Google Scholar] [CrossRef]
- Leong, K.W.; D’Amore, P.; Marletta, M.; Langer, R. Bioerodible Polyanhydrides as Drug-carrier Matrices. II. Biocompatibility and Chemical Reactivity. J. Biomed. Mater. Res. 1986, 20, 51–64. [Google Scholar] [CrossRef]
- Mathiowitz, E.; Saltzman, W.M.; Domb, A.; Dor, P.; Langer, R. Polyanhydride Microspheres as Drug Carriers. 2. Microencapsulation by Solvent Removal. J. Appl. Polym. Sci. 1988, 35, 755–774. [Google Scholar] [CrossRef]
- Bindschaedler, C.; Leong, K.L.; Mathiowitz, E.; Langer, R. Polyanhydride Microsphere Formulation by Solvent Extraction. J. Pharm. Sci. 1988, 77, 696–698. [Google Scholar] [CrossRef]
- Mathiowitz, E.; Amato, C.; Dor, P.; Langer, R. Polyanhydride Microspheres: 3. Morphology and Characterization of Systems Made by Solvent Removal. Polymer 1990, 31, 547–555. [Google Scholar] [CrossRef]
- Mathiowitz, E.; Bernstein, H.; Giannos, S.; Dor, P.; Turek, T.; Langer, R. Polyanhydride Microspheres. IV. Morphology and Characterization of Systems Made by Spray Drying. J. Appl. Polym. Sci. 1992, 45, 125–134. [Google Scholar] [CrossRef]
- Berkland, C.; Kipper, M.J.; Narasimhan, B.; Kim, K.; Pack, D.W. Microsphere Size, Precipitation Kinetics and Drug Distribution Control Drug Release from Biodegradable Polyanhydride Microspheres. J. Control. Release 2004, 94, 129–141. [Google Scholar] [CrossRef]
- Determan, A.S.; Trewyn, B.G.; Lin, V.S.-Y.; Nilsen-Hamilton, M.; Narasimhan, B. Encapsulation, Stabilization, and Release of BSA-FITC from Polyanhydride Microspheres. J. Control. Release 2004, 100, 97–109. [Google Scholar] [CrossRef]
- Hong, D.-W.; Liu, T.-H.; Chu, I.-M. Encapsulation of Curcumin by Methoxy Poly(ethylene glycol-b-aromatic anhydride) Micelles. J. Appl. Polym. Sci. 2011, 122, 898–907. [Google Scholar] [CrossRef]
- Hiremath, J.G.; Rudani, C.G.; Domb, A.J.; Suthar, R.V.; Khamar, N.S. Preparation and In Vitro Characterization of Poly(Sebacic acid-co-Ricinoleic acid)-based Tamoxifen Citrate-loaded Microparticles for Breast Cancer. J. Appl. Polym. Sci. 2012, 124, 4747–4754. [Google Scholar] [CrossRef]
- Vilar, G.; Tulla-Puche, J.; Albericio, F. Polymers and Drug Delivery Systems. Curr. Drug Deliv. 2012, 9, 367–394. [Google Scholar] [CrossRef]
- Bagherifam, S.; Griffiths, G.W.; Maelandsmo, G.M.; Nystrom, B.; Hasirci, V.; Hasirci, N. Poly(Sebacic anhydride) Nanocapsules as Carriers: Effects of Preparation Parameters on Properties and Release of Doxorubicin. J. Microencapsul. 2015, 32, 166–174. [Google Scholar] [CrossRef]
- Shiehzadeh, F.; Tafaghodi, M. Dry Powder Form of Polymeric Nanoparticles for Pulmonary Drug Delivery. Curr. Pharm. Des. 2016, 22, 2549–2560. [Google Scholar] [CrossRef]
- Carothers, W.H.; Van Natta, F.J. Studies on Polymerization and Ring Formation. III. Glycol Esters of Carbonic Acid. J. Am. Chem. Soc. 1930, 52, 314–326. [Google Scholar] [CrossRef]
- Carothers, W.H.; Dorough, G.L.; Van Natta, F.J. Studies of Polymerization and Ring Formation. X. the Reversible Polymerization of Six-membered Cyclic Esters. J. Am. Chem. Soc. 1932, 54, 761–772. [Google Scholar] [CrossRef]
- Kricheldorf, H.R.; Weegen-Schulz, B. Polymers of Carbonic Acid. 13. Polymerization of Cyclotrimethylenecarbonate with Tin Tetrahalides. Polymer 1995, 36, 4997–5003. [Google Scholar] [CrossRef]
- Kricheldorf, H.R.; Weegen-Schulz, B. Polymers of Carbonic-Acid. 15. Polymerization of Cyclotrimethylene Carbonate with TiCl4 or SbCl5 as Initiator. J. Macromol. Sci. Pure Appl. Chem. 1995, A32, 1847–1862. [Google Scholar] [CrossRef]
- Albertsson, A.-C.; Eklund, M. Influence of Molecular-Structure on the Degradation Mechanism of Degradable Polymers—In-Vitro Degradation of Poly(Trimethylene carbonate), Poly(Trimethylene carbonate-co-caprolactone), and Poly(Adipic anhydride). J. Appl. Polym. Sci. 1995, 57, 87–103. [Google Scholar] [CrossRef]
- Murayama, M.; Sanda, E.; Endo, T. Anionic Ring-Opening Polymerization of a Cyclic Carbonate Having a Norbornene Structure with Amine Initiators. Macromolecules 1998, 31, 919–923. [Google Scholar] [CrossRef]
- Al-Azemi, T.F.; Bisht, K.S. Novel Functional Polycarbonate by Lipase-catalyzed Ring-opening Polymerization of 5-Methyl-5-benzyloxycarbonyl-1,3-dioxan-2-one. Macromolecules 1999, 32, 6536–6540. [Google Scholar] [CrossRef]
- Keul, H.; Höcker, H. Expected and Unexpected Reactions in Ring-opening (Co)polymerization. Macromol. Rapid Commun. 2000, 21, 869–883. [Google Scholar] [CrossRef]
- Acemoglu, M. Chemistry of Polymer Biodegradation and Implications on Parenteral Drug Delivery. Int. J. Pharm. 2004, 277, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Ree, M.; Kim, H. Acid- and Base-catalyzed Hydrolyses of Aliphatic Polycarbonates and Polyesters. Catal. Today 2006, 115, 283–287. [Google Scholar] [CrossRef]
- Rokicki, G. Aliphatic Cyclic Carbonates and Spiroorthocarbonates as Monomers. Prog. Polym. Sci. 2000, 25, 259–342. [Google Scholar] [CrossRef]
- Soga, K.; Hosoda, S.; Tazuke, Y.; Ikeda, S. Polymerization of Propylene Carbonate. Polym. Sci. Part A Polym. Chem. 1977, 15, 219–229. [Google Scholar] [CrossRef]
- Vogdanis, L.; Heitz, W. Carbon Dioxide as a Monomer, 3. The Polymerization of Ethylene Carbonate. Makromol. Chem. Rapid Commun. 1986, 7, 543–547. [Google Scholar] [CrossRef]
- Feng, J.; Su, W.; Wang, H.-F.; Huang, F.-W.; Zhang, X.-Z.; Zhuo, R.-X. Facile Fabrication of Diblock Methoxy Poly(ethylene glycol)-Poly(tetramethylene carbonate) and its Self-assembled Micelles as Drug Carriers. ACS Appl. Mater. Interfaces 2009, 1, 2729–2737. [Google Scholar] [CrossRef]
- Suriano, F.; Pratt, R.; Tan, J.P.K.; Wiradharma, N.; Nelson, A.; Yang, Y.-Y.; Dubois, P.; Hedrick, J.L. Synthesis of a Family of Amphiphilic Glycopolymers via Controlled Ring-Opening Polymerization of Functionalized Cyclic Carbonates and their Application in Drug Delivery. Biomaterials 2010, 31, 2637–2645. [Google Scholar] [CrossRef]
- Yan, G.-P.; Zong, R.-F.; Li, L.; Fu, T.; Liu, F.; Yu, X.-H. Anticancer Drug-Loaded Nanospheres Based on Biodegradable Amphiphilic Ε-Caprolactone and Carbonate Copolymers. Pharm. Res. 2010, 27, 2743–2752. [Google Scholar] [CrossRef]
- Liu, Y.; Peng, D.; Huang, K.; Liu, S.; Liu, Z. Preparation and Properties of Poly(Propylene Carbonate Maleate) Microcapsules for Controlled Release of Pazufloxacin Mesilate. J. Appl. Polym. Sci. 2011, 122, 3248–3254. [Google Scholar] [CrossRef]
- Lee, A.L.Z.; Venkataraman, S.; Sirat, S.B.M.; Gao, S.; Hedrick, J.L.; Yang, Y.Y. The Use of Cholesterol-Containing Biodegradable Block Copolymers to Exploit Hydrophobic Interactions for the Delivery of Anticancer Drugs. Biomaterials 2012, 33, 1921–1928. [Google Scholar] [CrossRef]
- Yan, L.; Wu, W.; Zhao, W.; Qi, R.; Cui, D.; Xie, Z.; Huang, Y.; Tong, T.; Jing, X. Reduction-Sensitive Core-Cross-Linked mPEG–Poly(ester-carbonate) Micelles for Glutathione-Triggered Intracellular Drug Release. Polym. Chem. 2012, 3, 2403–2412. [Google Scholar] [CrossRef]
- Jia, H.-Z.; Wang, H.-f.; Liu, C.-w.; Li, C.; Yang, J.; Xu, X.-d.; Feng, J.; Zhang, X.-Z.; Zhuo, R.-X. A pH-Sensitive Macro- and Nanohydrogel Constructed from Cationic Hydroxyl-containing Hyperbranched Polycarbonate. Soft Matter 2012, 8, 6906–6912. [Google Scholar] [CrossRef]
- Attia, A.B.E.; Yang, C.; Tan, J.P.K.; Gao, S.; Williams, D.F.; Hedrick, J.L.; Yang, Y.-Y. The Effect of Kinetic Stability on Biodistribution and Anti-Tumor Efficacy of Drug-Loaded Biodegradable Polymeric Micelles. Biomaterials 2013, 34, 3132–3140. [Google Scholar] [CrossRef]
- Hu, B.; Ke, X.-J.; Yan, G.-P.; Zhuo, R.-X.; Wu, Y.; Fan, C.-L.; Liu, Y.-J. Preparation and Properties of Polycarbonate Microspheres Containing Tetanus Toxoid Vaccine. J. Appl. Polym. Sci. 2014, 131, 40048. [Google Scholar] [CrossRef]
- Hu, B.; Du, H.-J.; Yan, G.-P.; Zhuo, R.-X.; Wu, Y.; Fan, C.-L. Magnetic Polycarbonate Microspheres for Tumor Targeted Delivery of Tumor Necrosis Factor. Drug Deliv. 2014, 21, 204–212. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.-F.; Jia, H.-Z.; Chu, Y.-F.; Feng, J.; Zhang, X.-Z.; Zhuo, R.-X. Acidity-Promoted Cellular Uptake and Drug Release Mediated by Amine-functionalized Block Polycarbonates Prepared via One-shot Ring-opening Copolymerization. Macromol. Biosci. 2014, 14, 526–536. [Google Scholar] [CrossRef]
- Wang, M.; Sun, J.; Zhai, Y.; Lian, H.; Luo, C.; Li, L.; Du, Y.; Zhang, D.; Ding, W.; Qiu, S.; et al. Enteric Polymer Based on pH-Responsive Aliphatic Polycarbonate Functionalized with Vitamin E to Facilitate Oral Delivery of Tacrolimus. Biomacromolecules 2015, 16, 1179–1190. [Google Scholar] [CrossRef]
- Yang, C.; Liu, S.Q.; Venkataraman, S.; Gao, S.J.; Ke, X.; Chia, X.T.; Hedrick, J.L.; Yang, Y.Y. Structure-Directing Star-Shaped Block Copolymers: Supramolecular Vesicles for the Delivery of Anticancer Drugs. J. Control. Release 2015, 208, 93–105. [Google Scholar] [CrossRef]
- Yu, L.; Xie, M.; Li, Z.; Lin, C.; Zheng, Z.; Zhou, L.; Su, Y.; Wang, X. Facile Construction of Near-monodisperse and Dual Responsive Polycarbonate Mixed Micelles with the Ability of pH-Induced Charge Reversal for Intracellular Delivery of Antitumor Drugs. J. Mater. Chem. B 2016, 4, 6081–6093. [Google Scholar] [CrossRef]
- Xie, M.; Yu, L.; Li, Z.; Zheng, Z.; Wang, X. Synthesis and Character of Novel Polycarbonate for Constructing Biodegradable Multi-Stimuli Responsive Delivery System. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 3583–3592. [Google Scholar] [CrossRef]
- Teo, J.Y.; Chin, W.; Ke, X.; Gao, S.; Liu, S.; Cheng, W.; Hedrick, J.L.; Yang, Y.Y. pH and Redox Dual-Responsive Biodegradable Polymeric Micelles with High Drug Loading for Effective Anticancer Drug Delivery. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Ono, R.J.; Yang, C.; Gao, S.; Tan, J.Y.M.; Hedrick, J.L.; Yang, Y.Y. Dual pH-responsive Shell-Cleavable Polycarbonate Micellar Nanoparticles for In Vivo Anticancer Drug Delivery. ACS Appl. Mater. Interfaces 2018, 10, 19355–19364. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Birnbaum, W.; Anderski, J.; Picker, M.-T.; Mulac, D.; Langer, K.; Kuckling, K. Use of Light-Degradable Aliphatic Polycarbonate Nanoparticles as Drug Carrier for Photosensitizer. Biomacromolecules 2018, 19, 4677–4690. [Google Scholar] [CrossRef]
- Li, H.; Niu, Y. Preparation of Poly(Propylene carbonate-co-ε-caprolactone) and their Applications in Drug Delivery. J. Polym. Mater. Polym. Biomater. 2018, 67, 192–198. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, N.; Qin, Z.; Wu, J.; Wang, F.; Zhang, L.; Xia, X.; Li, J.; Lu, Y. Polycarbonate-Based Core-Crosslinked Redox-Responsive Nanoparticles for Targeted Delivery of Anticancer Drug. J. Mater. Chem. B 2018, 6, 3348–3357. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Liu, X.; Hu, Z.; Hou, Z.; Guo, Z.; Chen, Z.; Hu, J.; Yang, L. Synthesis, Self-Assembly, and Drug-Release Properties of New Amphipathic Liquid Crystal Polycarbonates. Nanomaterials 2018, 8, 195. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Zhai, Y.; Ye, H.; Lv, Q.; Sun, B.; Luo, C.; Jiang, O.; Zhang, H.; Xu, Y.; Jing, Y.; et al. High Co-Loading Capacity and Stimuli-Responsive Release Based on Cascade Reaction of Self-Destructive Polymer for Improved Chemo-Photodynamic Therapy. ACS Nano 2019, 13, 7010–7023. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, X.; Chen, Z.; Hu, J.; Yang, L. New Liquid Crystal Polycarbonate Micelles for Intracellular Delivery of Anticancer Drugs. Colloids Surf. B Biointerfaces 2019, 178, 395–403. [Google Scholar] [CrossRef]
- Muller, H.-M.; Seebach, D. Poly(Hydroxyalkanoates)—A 5th Class of Physiologically Important Organic Biopolymers. Angew. Chem. Int. Ed. Eng. 1993, 32, 477–502. [Google Scholar] [CrossRef]
- Sudesh, K.; Abe, H.; Doi, Y. Synthesis, Structure and Properties of Polyhydroxyalkanoates: Biological Polyesters. Prog. Polym. Sci. 2000, 25, 1503–1555. [Google Scholar] [CrossRef]
- Steinbüchel, A.; Doi, Y. Polyesters I: Biological Systems and Biotechnological Production. In Biopolymers; Wiley-VCH: Weinheim, Germany, 2002; Volume 3a, pp. 1–472. [Google Scholar]
- Allmendinger, M.; Eberhardt, R.; Luinstra, G.A.; Rieger, B. The Cobalt-catalyzed Alternating Copolymerization of Epoxides and Carbon Monoxide: A Novel Approach to Polyesters. J. Am. Chem. Soc. 2002, 124, 5646–5647. [Google Scholar] [CrossRef]
- Allmendinger, M.; Eberhardt, R.; Luinstra, G.A.; Rieger, B. Alternating Copolymerization Reaction of Propylene Oxide and CO: Variation of Polymer Stereoregularity and Investigation into Chain Termination. Macromol. Chem. Phys. 2003, 204, 564–569. [Google Scholar] [CrossRef]
- Reichardt, R.; Rieger, B. Poly(3-hydroxybutyrate) from Carbon Monoxide. Adv. Polym. Sci. 2012, 245, 49–90. [Google Scholar] [CrossRef]
- Vert, M.; Chen, J.; Hellwich, K.-H.; Hodge, P.; Nakano, T.; Scholz, C.; Slomkowski, S.; Vohlidal, J. Nomenclature and Terminology for Linear Lactic Acid-based Polymers (IUPAC Recommendations 2019). Pure Appl. Chem. 2020, 92, 193–211. [Google Scholar] [CrossRef] [Green Version]
- Nagy, N.; Varga, Z.; Mihály, J.; Kasza, G.; Iván, B.; Kiss, É. Highly Efficient Encapsulation of Curcumin into and pH-controlled Drug Release from Poly(ε-Caprolactone) Nanoparticles Stabilized with a Novel Amphiphilic Hyperbranched Polyglycerol. eXPRESS Polym. Lett. 2020, 14, 90–101. [Google Scholar] [CrossRef]
- Mohamadpour, H.; Azadi, A.; Rostamizadeh, K.; Andalib, S.; Zanjani, M.R.S.; Hamidi, M. Preparation, Optimization, and Evaluation of Methoxy Poly(ethylene glycol)-co-poly(ε-caprolactone) Nanoparticles Loaded by Rivastigmine for Brain Delivery. ACS Chem. Neurosci. 2020, 11, 783–795. [Google Scholar] [CrossRef]
- Ragusa, J.; Gonzalez, D.; Li, S.; Noriega, S.; Skotak, M.; Larsen, G. Glucosamine/L-lactide Copolymers as Potential Carriers for the Development of a Sustained Rifampicin Release System Using Mycobacterium smegmatis as a Tuberculosis Model. Heliyon 2019, 5, e01539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuriakose, A.E.; Pandey, N.; Shan, D.; Banerjee, S.; Yang, J.; Nguyen, K.T. Characterization of Photoluminescent Polylactone-based Nanoparticles for their Applications in Cardiovascular Disease. Front. Bioeng. Biotechnol. 2019, 7, 353. [Google Scholar] [CrossRef] [Green Version]
- Shao, L.; Li, Q.; Zhao, C.; Lu, J.; Li, X.; Chen, L.; Deng, X.; Ge, G.; Wu, Y. Auto-Fluorescent Polymer Nanotheranostics for Self-monitoring of Cancer Therapy via Triple-collaborative Strategy. Biomaterials 2019, 194, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Pieper, S.; Onafuye, H.; Mulac, D.; Cinatl, J., Jr.; Wass, M.N.; Michaelis, M.; Langer, K. Incorporation of Doxorubicin in Different Polymer Nanoparticles and their Anticancer Activity. Beilstein J. Nanotechnol. 2019, 10, 2062–2072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owiti, A.O.; Pal, D.; Mitra, A. PSMA Antibody-conjugated Pentablock Copolymer Nanomicellar Formulation for Targeted Delivery to Prostate Cancer. AAPS PharmSciTech 2018, 19, 3535–3549. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Hou, Y.; Chen, H.; Liao, Z.; Chen, J.; Xu, B.B.; Kong, J. Reduction-responsive Amphiphilic Star Copolymers with Long-chain Hyperbranched Poly(ε-caprolactone) Core and Disulfide Bonds for Trigger Release of Anticancer Drugs. Eur. Polym. J. 2018, 108, 364–372. [Google Scholar] [CrossRef]
- Abyaneh, H.S.; Soleimani, A.H.; Reza Vakili, M.R.; Soudy, R.; Kaur, K.; Cuda, F.; Tavassoli, A.; Lavasanifar, A. Modulation of Hypoxia-induced Chemoresistance to Polymeric Micellar Cisplatin: The Effect of Ligand Modification of Micellar Carrier Versus Inhibition of the Mediators of Drug Resistance. Pharmaceutics 2018, 10, 196. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Li, Y.; Fu, Y.; Hou, Y.; Chen, Y.; Luo, X. The Synthesis and Co-micellization of PCL-P(HEMA/HEMA-LA) and PCL-P(HEMA/HEMA-FA) as Shell Cross-linked Drug Carriers with Target/Redox Properties. J. Biomater. Sci. Polym. Ed. 2019, 30, 276–294. [Google Scholar] [CrossRef]
- Kost, B.; Brzeziński, M.; Cieślak, M.; Królewska-Golińska, K.; Makowski, T.; Socka, M.; Biela, T. Stereocomplexed Micelles Based on Polylactides with β-Cyclodextrin Core as Anti-cancer Drug Carriers. Eur. Polym. J. 2019, 120, 109271. [Google Scholar] [CrossRef]
- Piao, L.; Li, Y.; Zhang, H.; Jiang, J. Stereocomplex Micelle Loaded with Paclitaxel for Enhanced Therapy of Breast Cancer in an Orthotopic Mouse Model. J. Biomater. Sci. Polym. Ed. 2019, 30, 233–246. [Google Scholar] [CrossRef]
- Lu, M.; Chen, F.; Cao, C.; Garvey, C.J.; Fletcher, N.L.; Houston, Z.H.; Lu, H.; Lord, M.S.; Thurecht, K.J.; Stenzel, M.H. Importance of Polymer Length in Fructose-based Polymeric Micelles for an Enhanced Biological Activity. Macromolecules 2019, 52, 477–486. [Google Scholar] [CrossRef]
- Jiang, J.; Li, J.; Zhou, B.; Niu, C.; Wang, W.; Wu, W.; Liang, J. Fabrication of Polymer Micelles with Zwitterionic Shell and Biodegradable Core for Reductively Responsive Release of Doxorubicin. Polymers 2019, 11, 1019. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Liu, W.; Liu, S.; Yang, R.; Pu, Y.; Zhang, W.; Wang, X.; Liu, X.; Ren, Y.; Chi, B. pH-responsive Nanomicelles of Poly(ethylene glycol)-Poly(ε-caprolactone)-Poly(L-histidine) for Targeted Drug Delivery. J. Biomater. Sci. Polym. Ed. 2020, 31, 277–292. [Google Scholar] [CrossRef] [PubMed]
- Kocabay, O.G.; Ismail, O. Preparation and Optimization of Biodegradable Self-assembled PCL-PEG-PCL Nano-sized Micelles for Drug Delivery Systems. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 328–337. [Google Scholar] [CrossRef]
- Gomes, M.L.S.; da Silva Nascimento, N.; Borsato, D.M.; Pretes, A.P.; Nadal, J.M.; Novatski, A.; Gomes, R.Z.; Fernandes, D.; Farago, P.D.; Warumby Zanin, S.M. Long-lasting Anti-platelet Activity of Cilostazol from Poly(ε-caprolactone)-Poly(ethylene glycol) Blend Nanocapsules. Mater. Sci. Eng. C 2019, 94, 694–702. [Google Scholar] [CrossRef]
- Zignani, M.; Merkli, A.; Sintzel, M.B.; Bernatchez, S.F.; Kloeti, W.; Heller, J.; Tabatabay, C.; Gurny, R. New Generation of Poly(ortho esters): Synthesis, Characterization, Kinetics, Sterilization and Biocompatibility. J. Control. Release 1997, 48, 115–129. [Google Scholar] [CrossRef]
- Heller, J.; Barr, J.; Ng, S.Y.; Abdellauoi, K.S.; Gurny, R. Poly(ortho esters): Synthesis, Characterization, Properties and Uses. Adv. Drug Deliv. Rev. 2002, 54, 1015–1039. [Google Scholar] [CrossRef]
- Haider, T.; Shyshov, O.; Suraeva, O.; Lieberwirth, I.; von Delius, M.; Wurm, F.R. Long-chain Polyorthoesters as Degradable Polyethylene Mimics. Macromolecules 2019, 52, 2411–2420. [Google Scholar] [CrossRef] [PubMed]
- Heller, J.; Barr, J. Poly(ortho esters) from Concept to Reality. Biomacromolecules 2004, 5, 1625–1632. [Google Scholar] [CrossRef]
- Nguyen, D.N.; Raghavan, S.S.; Tashima, L.M.; Lin, E.C.; Fredette, S.J.; Langer, R.S.; Wang, C. Enhancement of Poly(orthoester) Microspheres for DNA Vaccine Delivery by Blending with Poly(ethylenimine). Biomaterials 2008, 29, 2783–2793. [Google Scholar] [CrossRef] [Green Version]
- Ryan, B.; McCann, G. Novel Sub-ceiling Temperature Depolymerization–Repolymerization Reactions of Cyanoacrylate Polymers. Macromol. Rapid Commun. 1996, 17, 217–227. [Google Scholar] [CrossRef]
- Lenaerts, V.; Couvreur, P.; Christiaens-Leyh, D.; Joiris, E.; Roland, M.; Rollman, B.; Speiser, P. Degradation of Poly(isobutyl cyanoacrylate) Nanoparticles. Biomaterials 1984, 5, 65–68. [Google Scholar] [CrossRef]
- Vansnick, L.; Couvreur, P.; Christiaens-Ley, D.; Roland, M. Molecular Weights of Free and Drug-loaded Nanoparticles. Pharm. Res. 1985, 2, 36–41. [Google Scholar] [CrossRef]
- Langer, K.; Seegmuller, E.; Zimmer, A.; Kreuter, J. Characterization of Polybutylcyanoacrylate Nanoparticles: Quantification of PBCA Polymer and Dextran. Int. J. Pharm. 1994, 110, 21–27. [Google Scholar] [CrossRef]
- Vauthier, C.; Dubernet, C.; Fattal, E.; Pinto-Alphandary, H.; Couvreur, P. Poly(alkylcyanoacrylates) as Biodegradable Materials for Biomedical Applications. Adv. Drug Deliv. Rev. 2003, 55, 519–548. [Google Scholar] [CrossRef]
- Ren, F.; Chen, R.; Wang, Y.; Sun, Y.; Jiang, Y.; Li, G. Paclitaxel-Loaded Poly(N-butylcyanoacrylate) Nanoparticle Delivery System to Overcome Multidrug Resistance in Ovarian Cancer. Pharm. Res. 2011, 28, 897–906. [Google Scholar] [CrossRef]
- Alhareth, K.; Vauthier, C.; Gueutin, C.; Ponchel, G.; Moussa, F. Doxorubicin Loading and in Vitro Release from Poly(alkylcyanoacrylate) Nanoparticles Produced by Redox Radical Emulsion Polymerization. J. Appl. Polym. Sci. 2011, 119, 816–822. [Google Scholar] [CrossRef]
- Zhao, X.; Li, W.; Qiuhua Luo, Q.; Zhang, X. Enhanced Bioavailability of Orally Administered Flurbiprofen by Combined Use of Hydroxypropyl-Cyclodextrin and Poly(alkyl-cyanoacrylate) Nanoparticles. Eur. J. Drug Metab. Pharmacokinet. 2014, 39, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Sulheim, F.; Baghirov, H.; von Haartman, E.; Bøe, A.; Åslund, A.K.O.; Mørch, Y.; de Lange Davies, C. Cellular Uptake and Intracellular Degradation of Poly(alkyl cyanoacrylate) Nanoparticles. J. Nanobiotechnol. 2016, 14, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, J.S.R.; Medeiros, M.; Yamashiro-Kanashiro, E.H.; Rocha, M.C.; Cotrim, P.C.; Stephano, M.A.; Lancellotti, M.; Tavares, G.D.; Oliveira-Nascimento, L. Biodegradable Nanocarriers Coated with Polymyxin B: Evaluation of Leishmanicidal and Antibacterial Potential. PLoS Negl. Trop. Dis. 2019, 13, e0007388. [Google Scholar] [CrossRef] [Green Version]
- Vrignaud, S.; Benoit, J.-P.; Saulnier, P. Strategies for the Nanoencapsulation of Hydrophilic Molecules in Polymer-Based Nanoparticles. Biomaterials 2011, 32, 8593–8604. [Google Scholar] [CrossRef] [Green Version]
- Lai, P.; Daear, W.; Raimar Löbenberg, R.; Prenner, E.J. Overview of the Preparation of Organic Polymeric Nanoparticles for Drug Delivery Based on Gelatine, Chitosan, Poly(D,L-lactide-co-glycolic acid) and Polyalkylcyanoacrylate. Colloids Surf. B Biointerfaces 2014, 118, 154–163. [Google Scholar] [CrossRef]
- Arpicco, S.; Battaglia, L.; Brus, P.; Cavalli, R.; Chirio, D.; Dosio, F.; Gallarate, M.; Milla, P.; Peira, E.; Rocco, F.; et al. Recent Studies on the Delivery of Hydrophilic Drugs in Nanoparticulate Systems. J. Drug Deliv. Sci. Technol. 2016, 32, 298–312. [Google Scholar] [CrossRef]
- Lopes, M.A.; Abrahim, B.A.; Cabral, L.M.; Rodrigues, C.R.; Seica, R.M.F.; de Baptista Veiga, F.J.; Ribeiro, A.J. Intestinal Absorption of Insulin Nanoparticles: Contribution of M Cells. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Mura, S.; Fattal, E.; Nicolas, J. From Poly(alkyl cyanoacrylate) to Squalene as Core Material for the Design of Nanomedicines. J. Drug Target. 2019, 27, 470–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vauthier, C. A Journey Through the Emergence of Nanomedicines with Poly(alkylcyanoacrylate) Based Nanoparticles. J. Drug Target. 2019, 27, 502–524. [Google Scholar] [CrossRef]
- Jones, R.G.; Kahovec, J.; Stepto, R.; Wilks, E.S.; Hess, M.; Kitayama, T.; Metanomski, W.V. Compendium of Polymer Terminology and Nomenclature: IUPAC Recommendations 2008; The Royal Society of Chemistry: Cambridge, UK, 2008. [Google Scholar]
- Hong, S.; Choi, D.W.; Kim, H.N.; Park, C.G. Protein-Based Nanoparticles as Drug Delivery Systems. Pharmaceutics 2020, 12, 604. [Google Scholar] [CrossRef]
- Singh, I.; Luxami, V.; Paul, K. Spectroscopy and Molecular Docking Approach for Investigation on the Binding of Nocodazole to Human Serum Albumin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 235, 118289. [Google Scholar] [CrossRef] [PubMed]
- Claire, A.; Lethier, L.; Guillaume, Y.C. An Organic Monolithic Capillary Column Functionalized with Human Serum Albumin and its Application for the Nano-Chromatography Study of its Binding with Universal Cancer Peptides and its Impact on Immunogenicity. J. Liq. Chromatogr. Relat. Technol. 2020, 43, 777–783. [Google Scholar] [CrossRef]
- Callmann, C.E.; LeGuyader, C.L.M.; Burton, S.T.; Thompson, M.P.; Hennis, R.; Barback, C.; Henriksen, N.M.; Chan, W.C.; Jaremko, M.J.; Yang, J.; et al. Antitumor Activity of 1,18-Octadecanedioic Acid-Paclitaxel Complexed with Human Serum Albumin. J. Am. Chem. Soc. 2019, 141, 11765–11769. [Google Scholar] [CrossRef] [Green Version]
- Yokoyama, M.; Inoue, S.; Kataoka, K.; Yui, N.; Sakurai, Y. Preparation of Adriamycin-Conjugated Poly(ethylene glycol)-Poly(aspartic acid) Block Copolymer—A New Type of Polymeric Anticancer Agent. Makromol. Chem. Rapid Commun. 1987, 8, 431–435. [Google Scholar] [CrossRef]
- Yokoyama, M.; Miyauchi, M.; Yamada, N.; Okano, T.; Sakurai, Y.; Kataoka, K.; Inoue, S. Characterization and Anticancer Activity of the Micelle-Forming Polymeric Anticancer Drug Adriamycin-Conjugated Poly(ethylene glycol)-Poly(aspartic acid) Block Copolymer. Cancer Res. 1990, 50, 1693–1700. [Google Scholar]
- Horise, Y.; Maeda, M.; Konishi, Y.; Okamoto, J.; Ikuta, S.; Okamoto, Y.; Ishii, H.; Yoshizawa, S.; Umemura, S.; Ueyama, T.; et al. Sonodynamic Therapy With Anticancer Micelles and High-Intensity Focused Ultrasound in Treatment of Canine Cancer. Front. Pharmacol. 2019, 10, 545. [Google Scholar] [CrossRef]
- Takemae, K.; Okamoto, J.; Horise, Y.; Masamune, K.; Muragaki, Y. Function of Epirubicin-Conjugated Polymeric Micelles in Sonodynamic Therapy. Front. Pharmacol. 2019, 10, 546. [Google Scholar] [CrossRef]
- Florinas, S.; Liu, M.; Fleming, R.; Van Vlerken-Ysla, L.; Ayriss, J.; Gilbreth, R.; Dimasi, N.; Gao, C.; Wu, H.; Xu, Z.-Q.; et al. A Nanoparticle Platform To Evaluate Bioconjugation and Receptor-Mediated Cell Uptake Using Cross-Linked Polyion Complex Micelles Bearing Antibody Fragments. Biomacromolecules 2016, 17, 1818–1833. [Google Scholar] [CrossRef]
- Takashima, H.; Koga, Y.; Tsumura, R.; Yasunaga, M.; Tsuchiya, M.; Inoue, T.; Negishi, E.; Harada, M.; Yoshida, S.; Matsumura, Y. Reinforcement of Antitumor Effect of Micelles Containing Anticancer Drugs by Binding of an Anti-Tissue Factor Antibody Without Direct Cytocidal Effects. J. Control. Release 2020, 323, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.L.; Dhanya, B.S.; Rani, V.; Thakur, M.; Jeslin, J.; Kushwaha, R. Carbohydrate and Protein Based Biopolymeric Nanoparticles: Current Status and Biotechnological Applications. Int. J. Biol. Macromol. 2020, 154, 390–412. [Google Scholar] [CrossRef] [PubMed]
- Bayer, I.S. Hyaluronic Acid and Controlled Release: A Review. Molecules 2020, 25, 2649. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Maji, B.; Hari Narayana Moorthy, N.S.; Maiti, S. Xanthan Gum Derivatives: Review of Synthesis, Properties and Diverse Applications. RSC Adv. 2020, 10, 27103–27136. [Google Scholar] [CrossRef]
- Heidarian, P.; Kouzani, A.Z.; Kaynak, A.; Paulino, M.; Nasri-Nasrabadi, B.; Zolfagharian, A.; Varley, R. Dynamic Plant-Derived Polysaccharide-Based Hydrogels. Carbohydr. Polym. 2020, 231, 115743. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, X.; Wang, G. Review on Marine Carbohydrate-based Gold Nanoparticles Represented by Alginate and Chitosan for Biomedical Application. Carbohydr. Polym. 2020, 244, 116311. [Google Scholar] [CrossRef]
- Senni, K.; Pereira, J.; Gueniche, F.; Delbarre-Ladrat, C.; Sinquin, C.; Ratiskol, J.; Godeau, G.; Fischer, A.-M.; Helley, D.; Colliec-Jouault, S. Marine Polysaccharides: A Source of Bioactive Molecules for Cell Therapy and Tissue Engineering. Mar. Drugs 2011, 9, 1664–1681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gericke, M.; Schulze, P.; Heinze, T. Nanoparticles Based on Hydrophobic Polysaccharide Derivatives—Formation, Principles, Characterization Techniques, and Biomedical Applications. Macromol. Biosci. 2020, 20, 1900415. [Google Scholar] [CrossRef]
- Almalik, A.; Alradwan, I.; Kalam, M.A.; Alshamsan, A. Effect of Cryoprotection on Particle Size Stability and Preservation of Chitosan Nanoparticles with and without Hyaluronate or Alginate Coating. Saudi Pharm. J. 2017, 25, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Eliyahu, S.; Almeida, A.; Macedo, M.H.; das Neves, J.; Sarmento, B.; Bianco-Peled, H. The Effect of Freeze-drying on Mucoadhesion and Transport of Acrylated Chitosan Nanoparticles. Int. J. Pharm. 2020, 573, 118739. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Marchetti, R.; Aman, A.; Silipo, A.; Ul Qader, S.A.; Molinaro, A. Enzymatic and Acidic Degradation of High Molecular Weight Dextran into Low Molecular Weight and its Characterizations Using Novel Diffusion-ordered NMR Spectroscopy. Int. J. Biol. Macromol. 2017, 103, 744–750. [Google Scholar] [CrossRef]
- Beaubier, S.; Framboisier, X.; Ioannou, I.; Galet, O.; Kapel, R. Simultaneous Quantification of the Degree of Hydrolysis, Protein Conversion Rate and Mean Molar Weight of Peptides Released in the Course of Enzymatic Proteolysis. J. Chromatogr. B 2019, 1105, 1–9. [Google Scholar] [CrossRef]
- Del Castillo-Santaella, T.; Maldonado-Valderrama, J.; Molina-Bolivar, J.A.; Galisteo-Gonzalez, F. Effect of Cross-Linker Glutaraldehyde on Gastric Digestion of Emulsified Albumin. Colloids Surf. B Biointerfaces 2016, 145, 899–905. [Google Scholar] [CrossRef]
- Varkhede, N.; Bommana, R.; Schöneich, C.; Forrest, M.L. Proteolysis and Oxidation of Therapeutic Proteins After Intradermal or Subcutaneous Administration. J. Pharm. Sci. 2020, 109, 191–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barakat, I.; Dubois, P.; Jérôme, R.; Teyssié, P.; Mazurek, M. Polymerization of Glycolide Promoted by ω-Al-alkoxide Poly(ε-caprolactone) Macro-Initiators and Formation of Stable Colloidal Dispersions. Macromol. Symp. 1994, 88, 227–244. [Google Scholar] [CrossRef]
- Sosnowski, S.; Gadzinowski, M.; Slomkowski, S.; Penczek, S. Synthesis of Bioerodible Poly(ε-caprolactone) Latexes and Poly(D, L-lactide) Microspheres by Ring-Opening Polymerization. J. Bioact. Compat. Polym. 1994, 9, 345–366. [Google Scholar] [CrossRef]
- Slomkowski, S.; Sosnowski, S.; Gadzinowski, M. Synthesis and Properties of Bioerodible Latexes and Microspheres. Polym. Prepr. 1996, 37, 135. [Google Scholar]
- Gadzinowski, M.; Slomkowski, S.; Elaïssari, A.; Pichot, C. Phase Transfer and Characterization of Poly(ε-caprolactone) and Poly(L-lactide) Microspheres. J. Biomater. Sci. Polym. Ed. 2000, 11, 459–480. [Google Scholar] [CrossRef]
- Lassalle, V.; Ferreira, M.L. PLA Nano- and Microparticles for Drug Delivery: An Overview of the Methods of Preparation. Macromol. Biosci. 2007, 7, 767–783. [Google Scholar] [CrossRef]
- Allen, S.D.; Bobbala, S.; Karabin, N.B.; Scott, E.A. on the Advancement of Polymeric Bicontinuous Nanospheres Toward Biomedical Applications. Nanoscale Horiz. 2019, 4, 258–272. [Google Scholar] [CrossRef] [PubMed]
- Bobbala, S.; Allen, S.D.; Scott, E.A. Flash Nanoprecipitation Permits Versatile Assembly and Loading of Polymeric Bicontinuous Cubic Nanospheres. Nanoscale 2018, 10, 5078–5088. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, B.E.; De Visser, J.F.; Portale, G.; Hermida-Merino, D.; Friedrich, H.; Bomans, P.H.H.; Bras, W.; Monaghan, O.R.; Holder, S.J.; Sommerdijk, N.A.J.M. The Evolution of Bicontinuous Polymeric Nanospheres in Aqueous Solution. Soft Matter 2016, 12, 4113–4122. [Google Scholar] [CrossRef] [Green Version]
- Jain, R.A. The Manufacturing Techniques of Various Drug Loaded Biodegradable Poly(lactide-co-glycolide) (PLGA) Devices. Biomaterials 2000, 21, 2475–2490. [Google Scholar] [CrossRef]
- Prosapio, V.; De Marco, J.; Reverchon, E. Supercritical Antisolvent Coprecipitation Mechanisms. J. Supercrit. Fluids 2018, 138, 247–258. [Google Scholar] [CrossRef]
- Trucillo, P.; Campardelli, R.; Scognamiglio, M.; Reverchon, E. Control of Liposomes Diameter at Micrometric and Nanometric Level Using a Supercritical Assisted Technique. J. CO2 Util. 2019, 32, 119–127. [Google Scholar] [CrossRef]
- La, Y.; An, T.H.; Shin, T.J.; Park, C.; Kim, K.T. A Morphological Transition of Inverse Mesophases of a Branched-Linear Block Copolymer Guided by Using Cosolvents. Angew. Chem. Int. Ed. 2015, 54, 10483–10487. [Google Scholar] [CrossRef]
- Cho, A.; La, Y.; Shin, T.J.; Park, C.; Kim, K.T. Structural Requirements of Block Copolymers for Self-Assembly into Inverse Bicontinuous Cubic Mesophases in Solution. Macromolecules 2016, 49, 4510–4519. [Google Scholar] [CrossRef]
- Lin, Z.; Liu, S.; Mao, W.; Tian, H.; Wang, N.; Zhang, N.; Tian, F.; Han, L.; Feng, X.; Mai, Y. Tunable Self-assembly of Diblock Copolymers into Colloidal Particles with Triply Periodic Minimal Surfaces. Angew. Chem. Int. Ed. 2017, 56, 7135–7140. [Google Scholar] [CrossRef]
- Ku, K.H.; Shin, J.M.; Yun, H.; Yi, G.-R.; Jang, S.G.; Kim, B.J. Multidimensional Design of Anisotropic Polymer Particles from Solvent-evaporative Emulsion. Adv. Funct. Mater. 2018, 28, 1802961. [Google Scholar] [CrossRef]
- Pichot, C. Surface-Functionalized Latexes for Biotechnological Applications. Curr. Opin. Colloid Interface Sci. 2004, 9, 213–221. [Google Scholar] [CrossRef]
- Kang, Y.; Pitto-Barry, A.; Rolph, M.S.; Hua, Z.; Hands-Portman, I.; Kirby, N.; O’Reilly, R.K. Use of Complementary Nucleobase-containing Synthetic Polymers to Prepare Complex Self-assembled Morphologies in Water. Polym. Chem. 2016, 7, 2836–2846. [Google Scholar] [CrossRef] [Green Version]
- Gaitzsch, J.; Chudasama, V.; Morecroft, E.; Messager, L.; Battaglia, G. Synthesis of an Amphiphilic Miktoarm Star Terpolymer for Self-assembly into Patchy Polymersomes. ACS Macro Lett. 2016, 5, 351–354. [Google Scholar] [CrossRef]
- Socka, M.; Brzezinski, M.; Michalski, A.; Kacprzak, A.; Makowski, T.; Duda, A. Self-assembly of Triblock Copolymers from Cyclic Esters as a Tool for Tuning their Particle Morphology. Langmuir 2018, 34, 3701–3710. [Google Scholar] [CrossRef]
- Nagasaki, Y.; Okada, T.; Scholz, C.; Iijima, M.; Kato, M.; Kataoka, K. The Reactive Polymeric Micelle Based on an Aldehyde-Ended Poly(ethylene glycol)/Poly(lactide) Block Copolymer. Macromolecules 1998, 31, 1473–1479. [Google Scholar] [CrossRef]
- Le Fer, G.L.; Le Coeur, C.; Guigner, J.-M.; Amiel, C.; Volet, G. Amphiphilic Diblock and Triblock Copolymers Based on Poly(2-Methyl-2-oxazoline) and Poly(D,L-lactide): Synthesis, Physicochemical Characterizations and Self-assembly Properties. Polymer 2019, 171, 149–160. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, C.Y.; Wu, J.; Zhou, H.; Bai, R.; Shen, Z.; Deng, F.; Liu, Y.; Liu, J. PEG-detachable Polymeric Micelles Self-assembled from Amphiphilic Copolymers for Tumor-Acidity-Triggered Drug Delivery and Controlled Release. ACS Appl. Mater. Interfaces 2019, 11, 5701–5713. [Google Scholar] [CrossRef]
- Staubli, A.; Mathiowitz, E.; Lucarelli, M.; Langer, R. Characterization of Hydrolytically Degradable Amino Acid Containing Poly(anhydride-co-imides). Macromolecules 1991, 24, 2283–2290. [Google Scholar] [CrossRef]
- Ray, P.; Ferraro, M.; Haag, R.; Quadir, M. Dendritic Polyglycerol-derived Nano-Architectures as Delivery Platforms of Gemcitabine for Pancreatic Cancer. Macromol. Biosci. 2019, 19, 1900073. [Google Scholar] [CrossRef]
- Yi, X.; Zhao, D.; Zhang, Q.; Xu, J.; Yuan, G.; Zhuo, R.; Li, F. A Co-delivery System Based on a Reduction-Sensitive Polymeric Prodrug Capable of Loading Hydrophilic and Hydrophobic Drugs for Combination Chemotherapy. Polym. Chem. 2016, 7, 5966–5977. [Google Scholar] [CrossRef]
- Tsai, F.-T.; Wang, Y.; Darensbourg, D.J. Environmentally Benign CO2-Based Copolymers: Degradable Polycarbonates Derived from Dihydroxybutyric Acid and their Platinum–Polymer Conjugates. J. Am. Chem. Soc. 2016, 138, 4626–4633. [Google Scholar] [CrossRef]
- Stevens, D.M.; Rahalkar, A.; Spears, B.; Gilmore, K.; Douglas, E.; Muthukumar, M.; Harth, E. Semibranched Polyglycidols as “Fillers” in Polycarbonate Hydrogels to Tune Hydrophobic Drug Release. Polym. Chem. 2015, 6, 1096–1102. [Google Scholar] [CrossRef]
- Wang, Y.; Ke, X.; Voo, Z.X.; Yap, S.S.L.; Yang, C.; Gao, S.; Liu, S.; Venkataraman, S.; Obuobi, S.A.O.; Khara, J.S.; et al. Biodegradable Functional Polycarbonate Micelles for Controlled Release of Amphotericin B. Acta Biomater. 2016, 46, 211–220. [Google Scholar] [CrossRef] [Green Version]
- Ke, X.; Coady, D.J.; Yang, C.; Engler, A.C.; Hedrick, J.L.; Yang, Y.Y. pH-Sensitive Polycarbonate Micelles for Enhanced Intracellular Release of Anticancer Drugs: A Strategy to Circumvent Multidrug Resistance. Polym. Chem. 2014, 5, 2621–2628. [Google Scholar] [CrossRef]
- Peng, T.; Su, J.; Cheng, S.-X.; Zhuo, R.-X. Poly-α,β-(N-(2-hydroxyethyl)-L-aspartamide)-g-poly (1,3-trimethylene carbonate) Amphiphilic Graft Co-Polymer as a Potential Drug Carrier. J. Biomater. Sci. Polym. Ed. 2006, 17, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Singh, S.K.; Arya, S.K.; Kundu, S.C.; Kapoor, S. Protein Nanoparticles: Promising Platforms for Drug Delivery Applications. ACS Biomater. Sci. Eng. 2018, 4, 3939–3961. [Google Scholar] [CrossRef]
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J.; Lu, H.; Richmond, J.; Kaplan, D.L. Silk-Based Biomaterials. Biomaterials 2003, 24, 401–416. [Google Scholar] [CrossRef] [Green Version]
- Mandal, B.B.; Kundu, S.C. Self-assembled Silk Sericin/Poloxamer Nanoparticles as Nanocarriers of Hydrophobic and Hydrophilic Drugs for Targeted Delivery. Nanotechnology 2009, 20, 355101. [Google Scholar] [CrossRef] [PubMed]
- Lomis, N.; Westfall, S.; Farahdel, L.; Malhotra, M.; Shum-Tim, D.; Prakash, S. Human Serum Albumin Nanoparticles for Use in Cancer Drug Delivery: Process Optimization and In Vitro Characterization. Nanomaterials 2016, 6, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arangoa, M.A.; Ponchel, G.; Orecchioni, A.M.; Renedo, M.J.; Duchene, D.; Irache, J.M. Bioadhesive Potential of Gliadin Nanoparticulate Systems. Eur. J. Pharm. Sci. 2000, 11, 333–341. [Google Scholar] [CrossRef]
- Bayrak, A.; Pruger, P.; Stock, U.A.; Seifert, M. Absence of Immune Responses with Xenogeneic Collagen and Elastin. Tissue Eng. Part A 2013, 19, 1592–1600. [Google Scholar] [CrossRef] [Green Version]
- Kowalczyk, T.; Hnatuszko-Konka, K.; Gerszberg, A.; Kononowicz, A.K. Elastin-like Polypeptides as a Promising Family of Genetically-engineered Protein Based Polymers. World J. Microbiol. Biotechnol. 2014, 30, 2141–2152. [Google Scholar] [CrossRef] [Green Version]
- Garland, S.M.; Hernandez-Avila, M.; Wheeler, C.M.; Perez, G.; Harper, D.M.; Leodolter, S.; Tang, G.W.K.; Ferris, D.G.; Steben, M.; Bryan, J.; et al. Quadrivalent Vaccine Against Human Papillomavirus to Prevent Anogenital Diseases. N. Engl. J. Med. 2007, 356, 1928–1943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Cui, F.; Cun, D.; Tao, A.; Shi, K.; Lin, W. Preparation, Characterization and Biodistribution of the Lactone Form of 10-Hydroxycamptothecin (HCPT)-loaded Bovine Serum Albumin (BSA) Nanoparticles. Int. J. Pharm. 2007, 340, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.; Coester, C.; Kreuter, J.; Langer, K. Desolvation Process and Surface Characterisation of Protein Nanoparticles. Int. J. Pharm. 2000, 194, 91–102. [Google Scholar] [CrossRef]
- Fan, Y.F.; Wang, Y.N.; Fan, Y.G.; Ma, J.B. Preparation of Insulin Nanoparticles and their Encapsulation with Biodegradable Polyelectrolytes via the Layer-By-Layer Adsorption. Int. J. Pharm. 2006, 324, 158–167. [Google Scholar] [CrossRef]
- Jaworek, A. Micro- and Nanoparticle Production by Electrospraying. Powder Technol. 2007, 176, 18–35. [Google Scholar] [CrossRef]
- Islam, N.; Ferro, V. Recent Advances in Chitosan-Based Nanoparticulate Pulmonary Drug Delivery. Nanoscale 2016, 8, 14341–14358. [Google Scholar] [CrossRef] [Green Version]
- Muhsin, M.D.A.; George, G.; Beagley, K.; Ferro, V.; Armitage, C.; Islam, N. Synthesis and Toxicological Evaluation of a Chitosan-L-leucine Conjugate for Pulmonary Drug Delivery Applications. Biomacromolecules 2014, 15, 3596–3607. [Google Scholar] [CrossRef]
- Choi, M.; Cho, M.; Han, B.S.; Hong, J.; Jeong, J.; Park, S.; Cho, M.-H.; Kim, K.; Cho, W.-S. Chitosan Nanoparticles Show Rapid Extrapulmonary Tissue Distribution and Excretion with Mild Pulmonary Inflammation to Mice. Toxicol. Lett. 2010, 199, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Merchant, Z.; Taylor, K.M.G.; Stapleton, P.; Razak, S.A.; Kunda, N.; Alfagih, I.; Sheikh, K.; Saleem, I.Y.; Somavarapu, S. Engineering Hydrophobically Modified Chitosan for Enhancing the Dispersion of Respirable Microparticles of Levofloxacin. Eur. J. Pharm. Biopharm. 2014, 88, 816–829. [Google Scholar] [CrossRef] [Green Version]
- Makhlof, A.; Werle, M.; Tozuka, Y.; Takeuchi, H. Nanoparticles of Glycol Chitosan and its Thiolated Derivative Significantly Improved the Pulmonary Delivery of Calcitonin. Int. J. Pharm. 2010, 397, 92–95. [Google Scholar] [CrossRef]
- Jin, H.; Xu, C.X.; Kim, H.W.; Chung, Y.S.; Shin, J.Y.; Chang, S.H.; Park, S.J.; Lee, E.S.; Hwang, S.K.; Kwon, J.T.; et al. Urocanic Acid-modified Chitosan-mediated PTEN Delivery via Aerosol Suppressed Lung Tumorigenesis in K-rasLA1 Mice. Cancer Gene Ther. 2008, 15, 275–283. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Zhai, X.; Ma, C.; Sun, P.; Fu, Z.; Liu, W.; Xu, J. An Inhalable β2-adrenoceptor Ligand-directed Guanidinylated Chitosan Carrier for Targeted Delivery of siRNA to Lung. J. Control. Release 2012, 162, 28–36. [Google Scholar] [CrossRef]
- Fischer, N.O.; Blanchette, C.D.; Segelke, B.W.; Corzett, M.; Chromy, B.A.; Kuhn, E.A.; Bench, G.; Hoeprich, P.D. Isolation, Characterization, and Stability of Discretely-sized Nanolipoprotein Particles Assembled with Apolipophorin-III. PLoS ONE 2010, 5, e11643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nisha, C.K.; Manorama, S.V.; Ganguli, M.; Maiti, S.; Kizhakkedathu, J.N. Complexes of Poly(ethylene glycol)-based Cationic Random Copolymer and Calf Thymus DNA: A Complete Biophysical Characterization. Langmuir 2004, 20, 2386–2396. [Google Scholar] [CrossRef]
- Van de Manakker, F.; Vermonden, T.; van Nostrum, C.F.; Hennink, W.E. Cyclodextrin-Based Polymeric Materials: Synthesis, Properties, and Pharmaceutical/Biomedical Applications. Biomacromolecules 2009, 10, 3157–3175. [Google Scholar] [CrossRef]
- Yao, X.; Huang, P.; Nie, Z. Cyclodextrin-Based Polymer Materials: From Controlled Synthesis to Applications. Prog. Polym. Sci. 2019, 93, 1–35. [Google Scholar] [CrossRef]
- Prochowicz, D.; Kornowicz, A.; Lewinski, J. Interactions of Native Cyclodextrins with Metal Ions and Inorganic Nanoparticles: Fertile Landscape for Chemistry and Materials Science. Chem. Rev. 2017, 117, 13461–13501. [Google Scholar] [CrossRef]
- Loh, X.J. Supramolecular Host-Guest Polymeric Materials for Biomedical Applications. Mater. Horiz. 2014, 1, 185–195. [Google Scholar] [CrossRef]
- Bukchin, A.; Kuplennik, N.; Carcaboso, Á.M.; Sosnik, A. Effect of Growing Glycosylation Extents on the Self-Assembly and Active Targeting in Vitro of Branched Poly(ethylene oxide)-Poly(propylene oxide) Block Copolymers. Appl. Mater. Today 2018, 11, 57–69. [Google Scholar] [CrossRef]
- Willersinn, J.; Schmidt, B.V.K.J. Pure Hydrophilic Block Copolymer Vesicles with Redox- and pH-Cleavable Crosslinks. Polym. Chem. 2018, 9, 1626–1637. [Google Scholar] [CrossRef] [Green Version]
- Kumar, J.N.; Wu, Y.-L.; Loh, X.J.; Ho, N.Y.; Aik, S.X.; Pang, V.Y. the Effective Treatment of Multi-Drug Resistant Tumors with Self-Assembling Alginate Copolymers. Polym. Chem. 2019, 10, 278–286. [Google Scholar] [CrossRef]
- Liu, B.W.; Zhou, H.; Zhou, S.T.; Zhang, H.J.; Feng, A.C.; Jian, C.M.; Hu, J.; Gao, W.P.; Yuan, J.Y. Synthesis and Self-Assembly of CO2-Temperature Dual Stimuli-Responsive Triblock Copolymers. Macromolecules 2014, 47, 2938–2946. [Google Scholar] [CrossRef]
- Feng, A.C.; Yan, Q.; Zhang, H.J.; Peng, L.; Yuan, J.Y. Electrochemical Redox Responsive Polymeric Micelles Formed from Amphiphilic Supramolecular Brushes. Chem. Commun. 2014, 50, 4740–4742. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yan, C.; Chen, Z.; Liu, J.; Song, H.; Wang, W.; Liu, J.; Yang, N.; Zhao, Y.; Chen, L. Dual-targeting Nanoparticles with Core-Crosslinked and pH/Redox Bioresponsive Properties for Enhanced Intracellular Drug Delivery. J. Colloid Interface Sci. 2019, 540, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Canosa, J.B.; Medintz, I.L.; Farrell, D.; Mattoussi, H.; Dawson, P.E. Rapid Covalent Ligation of Fluorescent Peptides to Water Solubilized Quantum Dots. J. Am. Chem. Soc. 2010, 132, 10027–10033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Connal, L.A.; Kinnane, C.R.; Zelikin, A.N.; Caruso, F. Stabilization and Functionalization of Polymer Multilayers and Capsules via Thiol-ene Click Chemistry. Chem. Mater. 2009, 21, 576–578. [Google Scholar] [CrossRef]
- Ma, H.; Jiang, L.; Hajizadeh, S.; Gong, H.; Lu, B.; Ye, L. Nanoparticle-supported Polymer Brushes for Temperature-regulated Glycoprotein Separation: Investigation of Structure–Function Relationship. J. Mater. Chem. B 2018, 6, 3770–3781. [Google Scholar] [CrossRef] [Green Version]
- Bagheri, A.; Sadrearhami, Z.; Adnan, N.N.M.; Boyer, C.; Lim, M. Surface Functionalization of Upconversion Nanoparticles Using Visible Light-mediated Polymerization. Polymer 2018, 151, 6–14. [Google Scholar] [CrossRef]
- Finetti, C.; Sola, L.; Pezzullo, M.; Prosperi, D.; Colombo, M.; Riva, B.; Avvakumova, S.; Morasso, C.; Picciolini, S.; Chiari, M. Click Chemistry Immobilization of Antibodies on Polymer Coated Gold Nanoparticles. Langmuir 2016, 32, 7435–7441. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, M.; Karimian, R.; Mostafidi, E.; Noruzi, E.B.; Taghizadeh, S.; Shokouhid, B.; Kafil, H.S. Highly Branched Amine-Functionalized P-sulfonatocalix[4]arene Decorated with Human Plasma Proteins as a Smart, Targeted, and Stealthy Nano-Vehicle for the Combination Chemotherapy of MCF7 Cells. New J. Chem. 2018, 42, 13010–13024. [Google Scholar] [CrossRef]
- De Moraes Profirio, D.; Pessine, F.B.T. Formulation of Functionalized PLGA Nanoparticles with Folic Acid Conjugated Chitosan for Carboplatin Encapsulation. Eur. Polym. J. 2018, 108, 311–321. [Google Scholar] [CrossRef]
- Liu, R.; Zhao, J.; Han, Q.; Hu, X.; Wang, D.; Zhang, X.; Yang, P. One-step Assembly of a Biomimetic Biopolymer Coating for Particle Surface Engineering. Adv. Mater. 2018, 30, 1802851. [Google Scholar] [CrossRef]
- Zlitni, A.; Janzen, N.; Foster, F.S.; Valliant, J.F. Catching Bubbles: Targeting Ultrasound Microbubbles Using Bioorthogonal Inverse-Electron-Demand Diels–Alder Reactions. Angew. Chem. Int. Ed. 2014, 53, 6459–6463. [Google Scholar] [CrossRef]
- Zhang, Q.; Nurumbetov, G.; Simula, A.; Zhu, C.; Li, M.; Wilson, P.; Kempe, K.; Yang, B.; Taob, L.; Haddleton, D.M. Synthesis of Well-Defined Catechol Polymers for Surface Functionalization of Magnetic Nanoparticles. Polym. Chem. 2016, 7, 7002–7010. [Google Scholar] [CrossRef] [Green Version]
- Khlebtsov, B.N.; Burov, A.M.; Pylaev, T.E.; Khlebtsov, N.G. Polydopamine-coated Au Nanorods for Targeted Fluorescent Cell Imaging and Photothermal Therapy. Beilstein J. Nanotechnol. 2019, 10, 794–803. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and its Derivative Materials: Synthesis and Promising Applications in Energy, Environmental, and Biomedical Fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef]
- Cao, J.; Choi, J.-S.; Oshi, M.A.; Lee, J.; Hasan, N.; Kim, J.; Yoo, J.-W. Development of PLGA Micro- and Nanorods with High Capacity of Surface Ligand Conjugation for Enhanced Targeted Delivery. Asian J. Pharm. Sci. 2019, 14, 86–94. [Google Scholar] [CrossRef]
- Hao, J.; Huang, L.L.; Zhang, R.; Wang, H.Z.; Xie, H.Y. A Mild and Reliable Method to Label Enveloped Virus with Quantum Dots by Copper-Free Click Chemistry. Anal. Chem. 2012, 84, 8364–8370. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, T.; Liu, X.; Ouyang, H.; Ren, L. Recent Trends in Click Chemistry as a Promising Technology for Virus-related Research. Virus Res. 2018, 256, 21–28. [Google Scholar] [CrossRef]
- Basiruddin, S.K.; Maity, A.R.; Jana, N.R. Glucose/Galactose/Dextran-functionalized Quantum Dots, Iron Oxide and Doped Semiconductor Nanoparticles with < 100 nm Hydrodynamic Diameter. RSC Adv. 2012, 2, 11915–11921. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Abdullah, F.; Mukherjee, A. Fabrication and Fluorescent Labeling of Guar Gum Nanoparticles in a Surfactant Free Aqueous Environment. Mater. Sci. Eng. C 2015, 46, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Kadam, R.; Zilli, M.; Maas, M.; Rezwan, K. Nanoscale Janus Particles with Dual Protein Functionalization. Part. Part. Syst. Charact. 2018, 35, 1700332. [Google Scholar] [CrossRef]
- Clarke, K.C.; Lyon, L.A. Microgel Surface Modification with Self-assembling Peptides. Macromolecules 2016, 49, 5366–5373. [Google Scholar] [CrossRef]
- Hong, V.; Presolski, S.I.; Ma, C.; Finn, M.G. Analysis and Optimization of Copper-Catalyzed Azide–Alkyne Cycloaddition for Bioconjugation. Angew. Chem. Int. Ed. 2009, 48, 9879–9883. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.; Horak, D.; An, Z.; Plichta, Z. Raft Polymerization of N,N-dimethylacrylamide from Magnetic Poly(2-hydroxyethyl methacrylate) Microspheres to Suppress Nonspecific Protein Adsorption. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 1036–1043. [Google Scholar] [CrossRef]
- Gindy, M.E.; Ji, S.; Hoye, T.R.; Panagiotopoulos, A.Z.; Prud’homme, R.K. Preparation of Poly(ethylene glycol) Protected Nanoparticles with Variable Bioconjugate Ligand Density. Biomacromolecules 2008, 9, 2705–2711. [Google Scholar] [CrossRef]
- Ji, S.; Zhu, Z.; Hoye, T.R.; Macosko, C.W. Maleimide Functionalized Poly(ε-caprolactone)-Block-Poly (ethylene glycol) (PCL-PEG-MAL): Synthesis, Nanoparticle Formation, and Thiol Conjugation. Macromol. Chem. Phys. 2009, 210, 823–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Zhou, H.; Yang, L.; Du, G.; Pai-Panandiker, A.S.; Huang, X.; Yan, B. Enhancement of Cell Recognition in Vitro by Dual-Ligand Cancer Targeting Gold Nanoparticles. Biomaterials 2011, 32, 2540–2545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, S.; Choi, J.-S.; Garcia, M.A.; Xing, Y.; Chen, K.-J.; Chen, Y.-M.; Jiang, Z.K.; Ro, T.; Wu, L.; Stout, D.B.; et al. Pretargeted Positron Emission Tomography Imaging That Employs Supramolecular Nanoparticles with in Vivo Bioorthogonal Chemistry. ACS Nano 2016, 10, 1417–1424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.B.; Kim, H.L.; Jeong, H.J.; Lim, S.T.; Sohn, M.H.; Kim, D.W. Mesoporous Silica Nanoparticle Pretargeting for PET Imaging Based on a Rapid Bioorthogonal Reaction in a Living Body. Angew. Chem. Int. Ed. 2013, 52, 10549–10552. [Google Scholar] [CrossRef] [PubMed]
- Hooks, M.A.; Wade, C.S.; Millikan, W.J. Muromonab CD-3: A Review of its Pharmacology, Pharmacokinetics, and Clinical Use in Transplantation. Pharmacotherapy 1991, 11, 26–37. [Google Scholar] [CrossRef]
- Tiller, K.E.; Tessier, P.M. Advances in Antibody Design. Annu. Rev. Biomed. Eng. 2015, 17, 191–216. [Google Scholar] [CrossRef] [Green Version]
- Jung, Y.; Jeong, J.Y.; Chung, B.H. Recent Advances in Immobilization Methods of Antibodies on Solid Supports. Analyst 2008, 133, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Norret, M.; House, M.J.; Galabura, Y.; Bradshaw, M.; Ho, D.W.; Woodward, R.C.; Pierre, T.G.S.; Luzinov, I.; Smith, N.M.; et al. Dose-Dependent Therapeutic Distinction Between Active and Passive Targeting Revealed Using Transferrin-Coated PGMA Nanoparticles. Small 2016, 12, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.P.; Prado, A.R.; Keijok, W.J.; Antunes, P.W.P.; Yapuchura, E.R.; Guimarães, M.C.C. Impact of Conjugation Strategies for Targeting of Antibodies in Gold Nanoparticles for Ultrasensitive Detection of 17β-Estradiol. Sci. Rep. 2019, 9, 13859. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Wan, J.; Schaefer, C.G.; Zhang, Z.; Tan, J.; Guo, J.; Wu, L.; Wang, C. Specific On-Site Assembly of Multifunctional Magnetic Nanocargos Based on Highly Efficient and Parallelized Bioconjugation: Toward Personalized Cancer Targeting Therapy. ACS Biomater. Sci. Eng. 2017, 3, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Mukai, H.; Kogawa, T.; Matsubara, N.; Naito, Y.; Sasaki, M.; Hosono, A. A First-in-Human Phase 1 Study of Epirubicin-Conjugated Polymer Micelles (K-912/NC-6300) in Patients with Advanced Or Recurrent Solid Tumors. Investig. New Drugs 2017, 35, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, J.; Kataoka, K. Chemo-physical Strategies to Advance the in Vivo Functionality of Targeted Nanomedicine: The Next Generation. J. Am. Chem. Soc. 2021, 143, 538–559. [Google Scholar] [CrossRef]
- Li, J.; Dirisala, A.; Ge, Z.; Wang, Y.; Yin, W.; Ke, W.; Toh, K.; Xie, J.; Matsumoto, Y.; Anraku, Y.; et al. Therapeutic Vesicular Nanoreactors with Tumor-Specific Activation and Self-Destruction for Synergistic Tumor Ablation. Angew. Chem. Int. Ed. 2017, 56, 14025–14030. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Y.; Wang, Y.; Ke, W.; Chen, W.; Wang, W.; Ge, Z. Polymer Prodrug-Based Nanoreactors Activated by Tumor Acidity for Orchestrated Oxidation/Chemotherapy. Nano Lett. 2017, 17, 6983–6990. [Google Scholar] [CrossRef]
- Li, J.; Anraku, Y.; Kataoka, K. Self-Boosting Catalytic Nanoreactors Integrated with Triggerable Crosslinking Membrane Networks for Initiation of Immunogenic Cell Death by Pyroptosis. Angew. Chem. Int. Ed. 2020, 59, 13526–13530. [Google Scholar] [CrossRef] [PubMed]



































| No. | Chemical Composition | Functional Chemical Group(s) | Reference |
|---|---|---|---|
| Copolymers obtained exclusively via synthetic routes | |||
| 1 | Poly(oligoethylene glycol) methyl ether methacrylate-co-poly(propyl methacrylate) | –OH, –COOH | [167] |
| 2 | Poly(diisopropylaminoethyl methacrylate)- poly(ethylene glycol)-poly(methacrylphosphoryl choline) | –OH, –PO42−, –N(CH3)3 | [168] |
| 3 | Poly(d,l-lactides) and copolymers with PEO or poly(2-methyl-2-oxazoline) | –OH, –COOH (after hydrolysis) | [169,170,171] |
| 4 | Poly(ethylene glycol) methyl ether-Dlabile-poly(β-amino ester)-Dlabile-poly(ethylene glycol) methyl ether | without reactive functions | [172] |
| 5 | Poly(anhydride-co-imides): poly(trimellitic anhydride-glycine/sebacic acid); poly(sebacic anhydride); poly(sebacic anhydride) and poly(1,6-bis-p-carboxyphenoxy)hexane | without reactive functions | [39,40,173] |
| 6 | Polyglycerol-co-polycaprolactone | –OH | [174] |
| 7 | Poly(tetraethylene glycolyl poly(trimethylene carbonate) grafted poly(2-nitrobenzyl methacrylate) linked by disulfide bond)-co-(5-methyl-5-propargyloxycarbonyl-1,3-dioxan-2-one); poly(ethylene glycol)-b-poly(5-methyl-5-propargyl-1,3-dioxan-2-one) |  | [73,175] |
| 8 | Poly(methyl-benzyloxycarbonyl) carbonate; Poly(ethylene glycol)-b-polycarbonate with benzyloxycarbonyl group; poly(ethylene glycol)-poly(2-methyl-2-benzyloxycarbonyl-propylene carbonate) |  | [52,70,80,81] |
| 9 | Poly(ethylene glycol)-poly(2-methyl-2-benzyloxy-methylene carbonate); |  | [79] |
| 10 | Poly(ethylene glycol)-poly(2-methyl-2-carbonyl-oxy-methylene alkyne carbonate); |  | [73,78] |
| 11 | Poly(trimetylene carbonate) with 4,5-dimethoxy-2-nitrobenzyl group |  | [76] |
| 12 | Poly(ethylene glycol)-b-polycarbonate with catechol bearing moiety |  | [75] |
| 13 | Poly(ethylene glycol)-b-polycarbonate with benzyloxy-p-chloromethyl group in each repeating unit |  | [74] |
| 14 | Poly(3,4-dihydroxybutyric acid carbonate) | –COOH | [176] |
| 15 | Poly(ethylene glycol)-b-poly(5-allyloxycarbonyl-trimethylene carbonate) |  | [72,177] |
| 16 | Poly(ethylene glycol)-b-poly(4-(hydroxymethyl) phenylboronic acid pinacol ester carbonate) |  | [178] |
| 17 | Poly(trimethylene carbonate) triol functionalized vinyl sulfone |  | [71] |
| 18 | Poly(ethylene glycol)-b-poly(trimetylene-3-hydroxypropoxybenzaldehyde) |  | [179] |
| 19 | Poly(ethylene glycol)-b-polycarbonate functionalized urea |  | [66] |
| 20 | Poly(ethylene glycol)-b-2-(2,4- dinitrophenylthio)ethyl-2-oxo-1,3-dioxane-5-carbonate |  | [64] |
| 21 | Poly(ethylene glycol)-b-cholesteryl 2-(2-oxo-5-carboxyloyloxy)ethyl polycarbonate |  | [63] |
| 22 | Polycarbonate ester-co-poly(ε-caprolactone-co-9-phenyl-2,4,8,10- tetraoxaspiro-[5,5]undecane-3-one) containing hydroxyl groups |  –OH | [61] |
| 23 | Polycarbonate bearing carbohydrate function |  R-diacetonide sugar R-diacetonide sugar | [60] |
| 24 | Poly-α,β-(N-(2-hydroxyethyl)-l-aspartamide)-g-poly(1,3-trimethylene carbonate) | –(CH2)2OH | [180] |
| Natural polymers and copolymers (and/or natural polymers conjugated with synthetic polymers) | |||
| 25 | Proteins and proteins linked with oligosaccharides | –COOH, –NH2, –OH | [181,182,183,184,185,186,187,188,189,190,191,192] |
| 26 | Functionalized chitosan-substitution of amine group of chitosan’ monomer unit in oligosaccharide chain in position R1: leucine conjugated chitosan; (5β-cholanic acid) glycol chitosan; octanoyl functionalized chitosan; thioglycolic acid conjugated chitosan; urocanic acid functionalized amine group of chitosan; position R1—salbutamol group; position R2—guanidine group |  (a)  R2 = H (b)  R2 = CH2CH2OH (c)  R2 = H or CO(CH2)6CH3 (d)  R2 = CH2CH2OH (e)  R2 = H (f)  R2 = R2 =  | [193,194,195,196,197,198,199] |
| 27 | Apolipoproteins e.g., 1,2-dimyristoyl-sn-glycero-3-phosphocholine | –NH2, –OH, –PO42−, –N(CH3)3 | [200] |
| 28 | Nucleic acids with synthetic polymers | –OH, –NH2, –PO42− | [201] |
| 29 | Oligosaccharides: dextran, cyclodextrins | –OH | [202,203,204,205] |
| 30 | Synthetic polymers copolymerized with oligosaccharides: Simple sugars conjugated with PEO-PPO; Pullulan-b-poly(N-vinylpyrrolidone); Alginate-g-poly(oligoethylene glycol methacrylate); PDMAEMA-βCDs; Poly(ethylene glycol)-bpoly(glycidyl methacrylate) with βCD tags; Folic acid-poly(6-O-methacryloyl-d-galactopyranose)-b-poly(2-diisopropylamino)ethyl methacrylate-co-pyridyl disulfide methylacrylate; | (a) –OH (b) –OH (c) –OH, –COO- (d) –OH, –N(CH3)3+Cl− (e) –ethylene oxide, –OH (f) –OH, –NH2, –COOH | [206,207,208,209,210,211] |
| 31 | Dihydrolipoic acid-poly(ethylene glycol) shell QDs ended 4-formyl benzoyl group | –CHO | [212] |
| Type (Material) of Particle | Attached Ligand | Target Cells, Tissue, Tumor, Factor in the Body, Disease, etc. | Reference |
|---|---|---|---|
| PEGylated silica mesoporous nanoparticles with Dibenzocyclooctyne (DBCO) | [(18F)]fluoro pentaethylene glycolic azide | Solid tumor | [238] |
| Supramolecular nanoparticles composed of poly(ethylene imine) | Trans-cyclooctene (TCO) | Solid tumor | [237] |
| Liposomes | Muromonab-CD3 (monoclonal antibody) | Autoimmune disorder | [239] |
| Fab fragment of antibody | [240,241] | ||
| Poly(glycidol methacrylate) particles loaded with Docetaxel | Transferrin | Membrane bound transferrin receptors on prostate cancer | [242] |
| Liposomes | Internalizing RGD (arginine-glycine-aspartate) motif | α√β3 integrin receptor on angiogenic endothelial cells | [241] |
| Gold nanoparticles with carboxyl ended linker | Anti-17β-estradiol IgG antibodies | 17β-estradiol | [243] |
| Gold nanoparticles with dual functionalities | Glucose and folic acid | Folate receptor/epidermal growth factor receptor on cancer cells | [236] |
| Functionalized microbubbles | Tetrazine | Endothelial growth factor intravascular VEGFR2 receptors and introduced bound antibodies (TCO-anti-VEGFR2) | [220] |
| Magnetic supraparticles core and poly-(methylacrylic acid-co-N,N-bis(acryloyl) cystamine) shell nanoparticles with streptavidin | Biotin labeled multiple targeting ligands | Folate and integrin receptors of HeLa cells | [244] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basinska, T.; Gadzinowski, M.; Mickiewicz, D.; Slomkowski, S. Functionalized Particles Designed for Targeted Delivery. Polymers 2021, 13, 2022. https://doi.org/10.3390/polym13122022
Basinska T, Gadzinowski M, Mickiewicz D, Slomkowski S. Functionalized Particles Designed for Targeted Delivery. Polymers. 2021; 13(12):2022. https://doi.org/10.3390/polym13122022
Chicago/Turabian StyleBasinska, Teresa, Mariusz Gadzinowski, Damian Mickiewicz, and Stanislaw Slomkowski. 2021. "Functionalized Particles Designed for Targeted Delivery" Polymers 13, no. 12: 2022. https://doi.org/10.3390/polym13122022
APA StyleBasinska, T., Gadzinowski, M., Mickiewicz, D., & Slomkowski, S. (2021). Functionalized Particles Designed for Targeted Delivery. Polymers, 13(12), 2022. https://doi.org/10.3390/polym13122022