Metal Organic Frameworks Derived Sustainable Polyvinyl Alcohol/Starch Nanocomposite Films as Robust Materials for Packaging Applications
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Methods
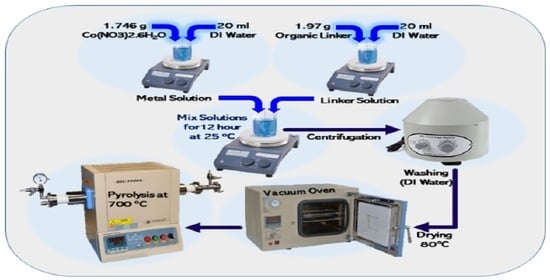
2.2. MOF’s Synthesis
2.3. Preparation of PVA-Starch-ZIF-67 Films
3. Characterization
3.1. SEM and Energy Dispersive X-ray Spectroscopy
3.2. X-ray Diffraction (XRD) Studies
3.3. Thermogravimetric Analysis (TGA)
3.4. Ultimate Tensile Testing
3.5. Fourier Transform Infrared (FTIR) Spectroscopy
3.6. Brunauer-Emmett Teller (BET) Analysis
4. Results and Discussion
4.1. Surface Morphological Analysis
4.2. Crystallinity Analysis
4.3. Thermal Stability Analysis
4.4. Surface Area Analysis
4.5. Mechanical Strength of ZIF-67 Doped PVA/Starch Films
4.6. Chemistry Analysis of PVA/Starch with ZIF-67
4.7. Interaction Between ZIF-67 and Polymer
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Mittal, A.; Garg, S.; Bajpai, S. Thermal decomposition kinetics and properties of grafted barley husk reinforced PVA/starch composite films for packaging applications. Carbohydr. Polym. 2020, 240, 116225. [Google Scholar] [CrossRef]
- Cacciotti, I.; Mori, S.; Cherubini, V.; Nanni, F. Eco-sustainable systems based on poly(lactic acid), diatomite and coffee grounds extract for food packaging. Int. J. Biol. Macromol. 2018, 112, 567–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdullah, Z.W.; Dong, Y. Biodegradable and Water Resistant Poly(vinyl) Alcohol (PVA)/Starch (ST)/Glycerol (GL)/Halloysite Nanotube (HNT) Nanocomposite Films for Sustainable Food Packaging. Front. Mater. 2019, 6, 58. [Google Scholar] [CrossRef] [Green Version]
- Mo, J.-H.; Kim, K.C.; Jang, K.-S. Well-dispersed carbon nanotube/polymer composite films and application to electromagnetic interference shielding. J. Ind. Eng. Chem. 2019, 80, 190–196. [Google Scholar] [CrossRef]
- Prabhu, T.N.; Prashantha, K. A review on present status and future challenges of starch based polymer films and their composites in food packaging applications. Polym. Compos. 2018, 39, 2499–2522. [Google Scholar] [CrossRef]
- Jiang, T.; Duan, Q.; Zhu, J.; Liu, H.; Yu, L. Starch-based biodegradable materials: Challenges and opportunities. Adv. Ind. Eng. Polym. Res. 2020, 3, 8–18. [Google Scholar] [CrossRef]
- Mustafa, P.; Niazi, M.B.K.; Jahan, Z.; Samin, G.; Hussain, A.; Ahmed, T.; Naqvi, S.R. PVA/starch/propolis/anthocyanins rosemary extract composite films as active and intelligent food packaging materials. J. Food Saf. 2019, 40, 12725. [Google Scholar] [CrossRef]
- Wang, W.; Yu, Z.; Alsammarraie, F.K.; Kong, F.; Lin, M.; Mustapha, A. Properties and antimicrobial activity of polyvinyl alcohol-modified bacterial nanocellulose packaging films incorporated with silver nanoparticles. Food Hydrocoll. 2020, 100, 105411. [Google Scholar] [CrossRef]
- Ding, L.; Li, Y.; Hu, L.; Zhang, Y.; Jiang, Y.; Mao, Z.; Xu, H.; Wang, B.; Feng, X.; Sui, X. A naked-eye detection polyvinyl alcohol/cellulose-based pH sensor for intelligent packaging. Carbohydr. Polym. 2020, 233, 115859. [Google Scholar] [CrossRef]
- Yun, Y.-H.; Yun, Y.H.; Kim, E.S.; Shim, W.G.; Yoon, S.D. Physical properties of mungbean starch/PVA bionanocomposites added nano-ZnS particles and its photocatalytic activity. J. Ind. Eng. Chem. 2018, 68, 57–68. [Google Scholar] [CrossRef]
- Garavand, F.; Cacciotti, I.; Vahedikia, N.; Rehman, A.; Tarhan, Ö.; Akbari-Alavijeh, S.; Shaddel, R.; Rashidinejad, A.; Nejatian, M.; Jafarzadeh, S.; et al. A comprehensive review on the nanocomposites loaded with chitosan nanoparticles for food packaging. Crit. Rev. Food Sci. Nutr. 2020, 1–34. [Google Scholar] [CrossRef]
- Jamróz, E.; Kulawik, P.; Kopel, P. The Effect of Nanofillers on the Functional Properties of Biopolymer-Based Films: A Review. Polymers 2019, 11, 675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zare, Y.; Rhee, K.Y.; Park, S.J. A modeling methodology to investigate the effect of interfacial adhesion on the yield strength of MMT reinforced nanocomposites. J. Ind. Eng. Chem. 2019, 69, 331–337. [Google Scholar] [CrossRef]
- Mittal, A.; Garg, S.; Bajpai, S. Fabrication and characteristics of poly (vinyl alcohol)-starch-cellulosic material based biodegradable composite film for packaging application. Mater. Today Proc. 2020, 21, 1577–1582. [Google Scholar] [CrossRef]
- Noorbakhsh-Soltani, S.; Zerafat, M.; Sabbaghi, S.; Noorbakhsh-Soltani, S.; Zerafat, M.; Sabbaghi, S. A comparative study of gelatin and starch-based nano-composite films modified by nano-cellulose and chitosan for food packaging applications. Carbohydr. Polym. 2018, 189, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Gahlawat, P.; Sen, C.; Das, M. Effect of molecular weight of polyvinyl alcohol on properties of starch film cross-linked with glutaraldehyde. J. Agric. Eng. Food Technol. 2015, 2, 12–16. [Google Scholar]
- Abral, H.; Hartono, A.; Hafizulhaq, F.; Handayani, D.; Sugiarti, E.; Pradipta, O. Characterization of PVA/cassava starch biocomposites fabricated with and without sonication using bacterial cellulose fiber loadings. Carbohydr. Polym. 2019, 206, 593–601. [Google Scholar] [CrossRef]
- Jose, J.; Al-Harthi, M.A.; Alma’Adeed, M.A.-A.; Dakua, J.B.; De, S.K. Effect of graphene loading on thermomechanical properties of poly(vinyl alcohol)/starch blend. J. Appl. Polym. Sci. 2015, 132, 16. [Google Scholar] [CrossRef]
- Li, S.; Huo, F. Metal–organic framework composites: From fundamentals to applications. Nanoscale 2015, 7, 7482–7501. [Google Scholar] [CrossRef]
- Sharanyakanth, P.; Radhakrishnan, M. Synthesis of metal-organic frameworks (MOFs) and its application in food packaging: A critical review. Trends Food Sci. Technol. 2020, 104, 102–116. [Google Scholar] [CrossRef]
- Wu, X.; Yue, H.; Zhang, Y.; Gao, X.; Li, X.; Wang, L.; Cao, Y.; Hou, M.; An, H.; Zhang, L.; et al. Packaging and delivering enzymes by amorphous metal-organic frameworks. Nat. Commun. 2019, 10, 5165. [Google Scholar] [CrossRef]
- Şahin, F.; Topuz, B.; Kalıpçılar, H. Synthesis of ZIF-7, ZIF-8, ZIF-67 and ZIF-L from recycled mother liquors. Microporous Mesoporous Mater. 2018, 261, 259–267. [Google Scholar] [CrossRef]
- Semino, R.; Dürholt, J.P.; Schmid, R.; Maurin, G. Multiscale Modeling of the HKUST-1/Poly(vinyl alcohol) Interface: From an Atomistic to a Coarse Graining Approach. J. Phys. Chem. C 2017, 121, 21491–21496. [Google Scholar] [CrossRef]
- Sharma, S.K.; Sudarshan, K.; Pujari, P.K. Unraveling the sub-nanoscopic structure at interphase in a poly(vinyl alcohol)–MOF nanocomposite, and its role in thermo-mechanical properties. Phys. Chem. Chem. Phys. 2016, 18, 25434–25442. [Google Scholar] [CrossRef]
- Lin, K.-Y.A.; Chang, H.-A. Ultra-high adsorption capacity of zeolitic imidazole framework-67 (ZIF-67) for removal of malachite green from water. Chemosphere 2015, 139, 624–631. [Google Scholar] [CrossRef]
- Ding, S.; Zhang, C.; Liu, Y.; Jiang, H.; Xing, W.; Chen, R. Pd nanoparticles supported on N-doped porous carbons derived from ZIF-67: Enhanced catalytic performance in phenol hydrogenation. J. Ind. Eng. Chem. 2017, 46, 258–265. [Google Scholar] [CrossRef]
- Du, W.; Jiang, T.; Shi, M.; Yang, Z.; Zhang, X. Structure and Properties of Starch/Poly (vinyl alcohol) Film Modificated by Different Inorganic Salts. ChemistrySelect 2019, 4, 600–607. [Google Scholar] [CrossRef]
- Pan, Y.; Li, H.; Zhang, X.-X.; Zhang, Z.; Tong, X.-S.; Jia, C.-Z.; Liu, B.; Sun, C.-Y.; Yang, L.-Y.; Chen, G.-J. Large-scale synthesis of ZIF-67 and highly efficient carbon capture using a ZIF-67/glycol-2-methylimidazole slurry. Chem. Eng. Sci. 2015, 137, 504–514. [Google Scholar] [CrossRef]
- Mittal, A.; Garg, S.; Kohli, D.; Maiti, M.; Jana, A.K.; Bajpai, S. Effect of cross linking of PVA/starch and reinforcement of modified barley husk on the properties of composite films. Carbohydr. Polym. 2016, 151, 926–938. [Google Scholar] [CrossRef]
- Zhao, J.; Wei, F.; Xu, W.; Han, X. Enhanced antibacterial performance of gelatin/chitosan film containing capsaicin loaded MOFs for food packaging. Appl. Surf. Sci. 2020, 510, 145418. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Q.; Tang, M. Preparation and properties of Starch-g-PLA/poly(vinyl alcohol) composite film. Carbohydr. Polym. 2013, 96, 384–388. [Google Scholar] [CrossRef]
- Qiang, R.; Du, Y.; Chen, D.; Ma, W.; Wang, Y.; Xu, P.; Ma, J.; Zhao, H.; Han, X. Electromagnetic functionalized Co/C composites by in situ pyrolysis of metal-organic frameworks (ZIF-67). J. Alloy. Compd. 2016, 681, 384–393. [Google Scholar] [CrossRef]
- Yap, M.H.; Fow, K.L.; Chen, G. Synthesis and applications of MOF-derived porous nanostructures. Green Energy Environ. 2017, 2, 218–245. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, X.; Wang, X.-B.; Kaneti, Y.; Chen, Y.; Liu, J.; Jiang, J.; Yamauchi, Y.; Hu, M. Spontaneous Weaving of Graphitic Carbon Networks Synthesized by Pyrolysis of ZIF-67 Crystals. Angew. Chem. Int. Ed. 2017, 56, 8435–8440. [Google Scholar] [CrossRef]
- Khan, A.; Ali, M.; Ilyas, A.; Naik, P.; Vankelecom, I.F.; Gilani, M.A.; Bilad, M.R.; Sajjad, Z.; Khan, A.L. ZIF-67 filled PDMS mixed matrix membranes for recovery of ethanol via pervaporation. Sep. Purif. Technol. 2018, 206, 50–58. [Google Scholar] [CrossRef]
- Gao, Z.; Ding, C.; Wang, J.; Ding, G.; Xue, Y.; Zhang, Y.; Zhang, K.; Liu, P.; Gao, X. Cobalt nanoparticles packaged into nitrogen-doped porous carbon derived from metal-organic framework nanocrystals for hydrogen production by hydrolysis of sodium borohydride. Int. J. Hydrogen Energy 2019, 44, 8365–8375. [Google Scholar] [CrossRef]
- Zhao, J.; Li, H.; Li, C.; Zhang, Q.; Sun, J.; Wang, X.; Guo, J.; Xie, L.; Xie, J.; He, B.; et al. MOF for template-directed growth of well-oriented nanowire hybrid arrays on carbon nanotube fibers for wearable electronics integrated with triboelectric nanogenerators. Nano Energy 2018, 45, 420–431. [Google Scholar] [CrossRef]
- Li, Y.; Yan, X.; Hu, X.; Feng, R.; Zhou, M. Trace pyrolyzed ZIF-67 loaded activated carbon pellets for enhanced adsorption and catalytic degradation of Rhodamine B in water. Chem. Eng. J. 2019, 375, 122003. [Google Scholar] [CrossRef]
- Alshameri, A.; He, H.; Zhu, J.; Xi, Y.; Zhu, R.; Ma, L.; Tao, Q. Adsorption of ammonium by different natural clay minerals: Characterization, kinetics and adsorption isotherms. Appl. Clay Sci. 2018, 159, 83–93. [Google Scholar] [CrossRef]
- Li, B.; Igawa, K.; Chai, J.; Chen, Y.; Wang, Y.; Fam, D.W.; Tham, N.N.; An, T.; Konno, T.; Sng, A.; et al. String of pyrolyzed ZIF-67 particles on carbon fibers for high-performance electrocatalysis. Energy Storage Mater. 2020, 25, 137–144. [Google Scholar] [CrossRef]
- Suganthi, S.; Vignesh, S.; Sundar, J.K.; Raj, V. Fabrication of PVA polymer films with improved antibacterial activity by fine-tuning via organic acids for food packaging applications. Appl. Water Sci. 2020, 10, 100. [Google Scholar] [CrossRef] [Green Version]
- Vahedikia, N.; Garavand, F.; Tajeddin, B.; Cacciotti, I.; Jafari, S.M.; Omidi, T.; Zahedi, Z. Biodegradable zein film composites reinforced with chitosan nanoparticles and cinnamon essential oil: Physical, mechanical, structural and antimicrobial attributes. Colloids Surf. B Biointerfaces 2019, 177, 25–32. [Google Scholar] [CrossRef]
- Abdullah, Z.W.; Dong, Y. Preparation and characterisation of poly (vinyl) alcohol (PVA)/starch (ST)/halloysite nanotube (HNT) nanocomposite films as renewable materials. J. Mater. Sci. 2018, 53, 3455–3469. [Google Scholar] [CrossRef] [Green Version]
- Khajavian, M.; Salehi, E.; Vatanpour, V. Chitosan/polyvinyl alcohol thin membrane adsorbents modified with zeolitic imidazolate framework (ZIF-8) nanostructures: Batch adsorption and optimization. Sep. Purif. Technol. 2020, 241, 116759. [Google Scholar] [CrossRef]
- Qian, J.; Sun, F.; Qin, L. Hydrothermal synthesis of zeolitic imidazolate framework-67 (ZIF-67) nanocrystals. Mater. Lett. 2012, 82, 220–223. [Google Scholar] [CrossRef]
- Aleksandrov, H.A.; Neyman, K.M.; Hadjiivanov, K.I.; Vayssilov, G.N. Can the state of platinum species be unambiguously determined by the stretching frequency of an adsorbed CO probe molecule? Phys. Chem. Chem. Phys. 2016, 18, 22108–22121. [Google Scholar] [CrossRef] [Green Version]
- Javadian, H.; Ghorbani, F.; Tayebi, H.-A.; Asl, S.M.H. Study of the adsorption of Cd (II) from aqueous solution using zeolite-based geopolymer, synthesized from coal fly ash; kinetic, isotherm and thermodynamic studies. Arab. J. Chem. 2015, 8, 837–849. [Google Scholar] [CrossRef] [Green Version]
- Muigai, H.H.; Bordoloi, U.; Hussain, R.; Ravi, K.; Moholkar, V.S.; Kalita, P. A comparative study on synthesis and characterization of biochars derived from lignocellulosic biomass for their candidacy in agronomy and energy applications. Int. J. Energy Res. 2021, 45, 4765–4781. [Google Scholar] [CrossRef]
- Lee, S.; Lei, Y.; Wang, D.; Li, C.; Cheng, J.; Wang, J.; Meng, W.; Liu, M. The Study of Zeolitic Imidazolate Framework (ZIF-8) Doped Polyvinyl Alcohol/Starch/Methyl Cellulose Blend Film. Polymers 2019, 11, 1986. [Google Scholar] [CrossRef] [Green Version]
- Qian, L.; Lei, D.; Duan, X.; Zhang, S.; Song, W.; Hou, C.; Tang, R. Design and preparation of metal-organic framework papers with enhanced mechanical properties and good antibacterial capacity. Carbohydr. Polym. 2018, 192, 44–51. [Google Scholar] [CrossRef]
- Râpă, M.; Grosu, E.; Stoica, P.; Andreica, M.; Hetvary, M. Journal of Environmental Research and Protection Polyvinyl alcohol and starch blends: Properties and biodegradation behavior. J. Environ. Res. Prot. 2014, 11, 34–42. [Google Scholar]
- Chisenga, S.M.; Tolesa, G.N.; Workneh, T.S. Biodegradable Food Packaging Materials and Prospects of the Fourth Industrial Revolution for Tomato Fruit and Product Handling. Int. J. Food Sci. 2020, 2020, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, E.M.; Tan, J.-C. Dynamic molecular interactions between polyurethane and ZIF-8 in a polymer-MOF nanocomposite: Microstructural, thermo-mechanical and viscoelastic effects. Polymer 2016, 97, 31–43. [Google Scholar] [CrossRef]










| Amount of ZIF-67 (wt.%) | Amount of ZIF-67 (g) | Amount of PVA (g) | Amount of Starch (g) |
|---|---|---|---|
| Blank | 0.00 | 0.94 | 0.56 |
| 2 | 0.03 | 0.92 | 0.55 |
| 4 | 0.06 | 0.90 | 0.54 |
| 6 | 0.09 | 0.88 | 0.53 |
| 8 | 0.12 | 0.86 | 0.52 |
| 10 | 0.15 | 0.84 | 0.51 |
| Additive Concentration | Cobalt Concentration before Pyrolysis | Cobalt Concentration after Pyrolysis |
|---|---|---|
| (wt.%) | (wt.%) | (wt.%) |
| Blank | 0 | 0 |
| ZIF-67 | 22.92 | 16.47 |
| 2 | 0.11 | 0.01 |
| 4 | 0.18 | 0.03 |
| 6 | 0.22 | 0.05 |
| 8 | 0.25 | 0.07 |
| 10 | 0.26 | 0.10 |
| Sample | SBET (m²/g) | Vmic (cm3/g) | Vmeso (cm3/g) | Vtotal (cm3/g) | Langmuir Surface Area (m2/g) |
|---|---|---|---|---|---|
| ZIF-67 | 200.4 | 0.15 | 0.31 | 0.40 | 346.3 |
| Pyrolyzed ZIF-67 | 246 | 0.12 | 0.30 | 0.42 | 469.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, N.A.; Niazi, M.B.K.; Sher, F.; Jahan, Z.; Noor, T.; Azhar, O.; Rashid, T.; Iqbal, N. Metal Organic Frameworks Derived Sustainable Polyvinyl Alcohol/Starch Nanocomposite Films as Robust Materials for Packaging Applications. Polymers 2021, 13, 2307. https://doi.org/10.3390/polym13142307
Khan NA, Niazi MBK, Sher F, Jahan Z, Noor T, Azhar O, Rashid T, Iqbal N. Metal Organic Frameworks Derived Sustainable Polyvinyl Alcohol/Starch Nanocomposite Films as Robust Materials for Packaging Applications. Polymers. 2021; 13(14):2307. https://doi.org/10.3390/polym13142307
Chicago/Turabian StyleKhan, Naveed Ahmed, Muhammad Bilal Khan Niazi, Farooq Sher, Zaib Jahan, Tayyaba Noor, Ofaira Azhar, Tazien Rashid, and Naseem Iqbal. 2021. "Metal Organic Frameworks Derived Sustainable Polyvinyl Alcohol/Starch Nanocomposite Films as Robust Materials for Packaging Applications" Polymers 13, no. 14: 2307. https://doi.org/10.3390/polym13142307
APA StyleKhan, N. A., Niazi, M. B. K., Sher, F., Jahan, Z., Noor, T., Azhar, O., Rashid, T., & Iqbal, N. (2021). Metal Organic Frameworks Derived Sustainable Polyvinyl Alcohol/Starch Nanocomposite Films as Robust Materials for Packaging Applications. Polymers, 13(14), 2307. https://doi.org/10.3390/polym13142307