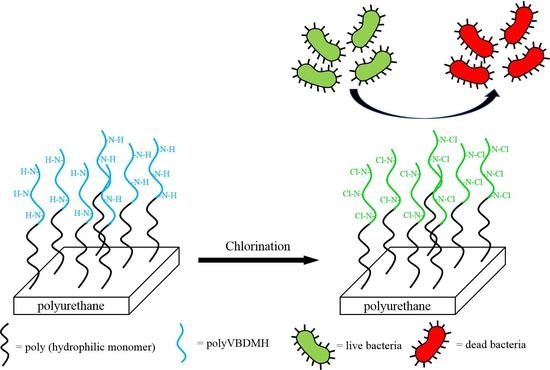
Surface Modification of Polyurethane Membrane with Various Hydrophilic Monomers and N-Halamine: Surface Characterization and Antimicrobial Properties Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of 3-(4’-Vinylbenzyl)-5,5-dimethylhydantoin (VBDMH)
2.3. Surface Modification of PU Membranes
2.3.1. Preparation of PU Membranes
2.3.2. Preparation of Initiator Immobilized PU Membranes (PU-Br)
2.3.3. Surface Grafting Polymeric Chains with Surface-Initiated Atom Transfer Radical Polymerization (SI-ATRP)
2.4. Chlorination and Titration
2.5. Characterization Methods
2.6. Antimicrobial Activity Testing
3. Results and Discussion
3.1. VBDMH Characterization
3.2. Surface Characterization of Different Modified PU Substrates
3.2.1. Surface Hydrophilicity
3.2.2. ATR-FTIR Analysis
3.2.3. XPS Analysis
3.3. Chlorination of Different VBDMH Modified PU Substrates
3.4. Antimicrobial Activity of Different Chlorinated VBDMH Modified PU Substrates
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Page, K.; Wilson, M.; Parkin, I.P. Antimicrobial surfaces and their potential in reducing the role of the inanimate environment in the incidence of hospital-acquired infections. J. Mater. Chem. 2009, 19, 3819–3831. [Google Scholar] [CrossRef]
- Alfei, S.; Schito, A.M. From Nanobiotechnology, Positively Charged Biomimetic Dendrimers as Novel Antibacterial Agents: A Review. Nanomaterials 2020, 10, 2022. [Google Scholar] [CrossRef] [PubMed]
- Schito, A.M.; Alfei, S. Antibacterial Activity of Non-Cytotoxic, Amino Acid-Modified Polycationic Dendrimers against Pseudomonas aeruginosa and Other Non-Fermenting Gram-Negative Bacteria. Polymers 2020, 12, 1818. [Google Scholar] [CrossRef] [PubMed]
- Schito, A.M.; Schito, G.C.; Alfei, S. Synthesis and Antibacterial Activity of Cationic Amino Acid-Conjugated Dendrimers Loaded with a Mixture of Two Triterpenoid Acids. Polymers 2021, 13, 521. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Cao, Z.; Porteous, N.; Sun, Y. Amine, Melamine, and Amide N-Halamines as Antimicrobial Additives for Polymers. Ind. Eng. Chem. Res. 2010, 49, 11206–11213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Cao, Z.; Porteous, N.; Sun, Y. An N-halamine-based rechargeable antimicrobial and biofilm controlling polyurethane. Acta Biomater. 2012, 8, 1498–1506. [Google Scholar] [CrossRef] [Green Version]
- Cerkez, I.; Kocer, H.B.; Worley, S.D.; Broughton, R.M.; Huang, T.S. N-halamine copolymers for biocidal coatings. React. Funct. Polym. 2012, 72, 673–679. [Google Scholar] [CrossRef]
- Kocer, H.B.; Worley, S.D.; Broughton, R.M.; Huang, T.S. A novel N-halamine acrylamide monomer and its copolymers for antimicrobial coatings. React. Funct. Polym. 2011, 71, 561–568. [Google Scholar] [CrossRef]
- Ren, X.; Kou, L.; Kocer, H.B.; Zhu, C.; Worley, S.D.; Broughton, R.M.; Huang, T.S. Antimicrobial coating of an N-halamine biocidal monomer on cotton fibers via admicellar polymerization. Colloids Surf. A. 2008, 317, 711–716. [Google Scholar] [CrossRef]
- Dong, A.; Lan, S.; Huang, J.; Wang, T.; Zhao, T.; Wang, W.; Xiao, L.; Zheng, X.; Liu, F.; Gao, G.; et al. Preparation of magnetically separable N-halamine nanocomposites for the improved antibacterial application. J. Colloid Interface Sci. 2011, 364, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Yang, Y.; Xiao, S.; Zhang, L.; Huang, L.; Chen, F.; Fan, P.; Zhong, M.; Tan, J.; Yang, J. Mixed polymer brushes with integrated antibacterial and antifouling properties. Prog. Org. Coat. 2019, 130, 75–82. [Google Scholar] [CrossRef]
- Choi, J.; Moon, D.S.; Ryu, S.G.; Lee, B.; Ying, W.B.; Lee, K.J. N-chloro hydantoin functionalized polyurethane fibers toward protective cloth against chemical warfare agents. Polymer 2018, 138, 146–155. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Z.; Gao, Y.; Gao, W.; Hou, Z.; Zhu, Y. Facile Method for Surface-Grafted Chitooligosaccharide on Medical Segmented Poly(ester-urethane) Film to Improve Surface Biocompatibility. Membranes 2021, 11, 37. [Google Scholar] [CrossRef]
- Liu, X.; Yang, B.; Hou, Z.; Zhang, N.; Gao, Y. A mild method for surface-grafting MPC onto poly(ester-urethane) based on aliphatic diurethane diisocyanate with high grafting efficiency. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109952. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Gao, Y.; Zhao, J.; Yuan, L.; Li, C.; Liu, Z.; Hou, Z. A Mild Method for Surface-Grafting PEG Onto Segmented Poly(Ester-Urethane) Film with High Grafting Density for Biomedical Purpose. Polymers 2018, 10, 1125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiu, K.; Wen, J.; Liu, J.; He, C.; Sun, Y. Controlling the Structure and Antimicrobial Function of N-Halamine-Based Polyurethane Semi-interpenetrating Polymer Networks. Ind. Eng. Chem. Res. 2017, 56, 12032–12037. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, G. Durable and refreshable polymeric N-halamine biocides containing 3-(4′-vinylbenzyl)-5, 5-dimethylhydantoin. J. Polym. Sci. Part. A Polym. Chem. 2001, 39, 3348–3355. [Google Scholar] [CrossRef]
- Khan, M.; Yang, J.; Shi, C.; Lv, J.; Feng, Y.; Zhang, W. Surface tailoring for selective endothelialization and platelet inhibition via a combination of SI-ATRP and click chemistry using Cys-Ala-Gly-peptide. Acta Biomater. 2015, 20, 69–81. [Google Scholar] [CrossRef]
- Yuan, W.; Feng, Y.; Wang, H.; Yang, D.; An, B.; Zhang, W.; Khan, M.; Guo, J. Hemocompatible surface of electrospun nanofibrous scaffolds by ATRP modification. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 3644–3651. [Google Scholar] [CrossRef]
- Surman, F.; Riedel, T.; Bruns, M.; Kostina, N.Y.; Sedlakova, Z.; Rodriguez-Emmenegger, C. Polymer brushes interfacing blood as a route toward high performance blood contacting devices. Macromol. Biosci. 2015, 15, 636–646. [Google Scholar] [CrossRef]
- Dai, J.; Dong, Y.; Yu, C.; Liu, Y.; Teng, X. A novel Nafion-g-PSBMA membrane prepared by grafting zwitterionic SBMA onto Nafion via SI-ATRP for vanadium redox flow battery application. J. Membr. Sci. 2018, 554, 324–330. [Google Scholar] [CrossRef]
- Qiao, M.; Ren, T.; Huang, T.-S.; Weese, J.; Liu, Y.; Ren, X.; Farag, R. N-Halamine modified thermoplastic polyurethane with rechargeable antimicrobial function for food contact surface. RSC Adv. 2017, 7, 1233–1240. [Google Scholar] [CrossRef] [Green Version]
- Chien, H.W.; Chen, Y.Y.; Chen, Y.L.; Cheng, C.H.; Lin, J.C. Studies of PET nonwovens modified by novel antimicrobials configured with both N-halamine and dual quaternary ammonium with different alkyl chain length. RSC Adv. 2019, 9, 7257–7265. [Google Scholar] [CrossRef] [Green Version]
- Antimicrobial Products-Test for Antimicrobial Activity and Efficacy; Japanese Industrial Standard: Tokyo, Japan, 2000; JIS Z 2801.
- Mi, L.; Jiang, S. Integrated antimicrobial and nonfouling zwitterionic polymers. Angew. Chem. Int. Ed. Engl. 2014, 53, 1746–1754. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Xu, J.; Teng, J.; Jia, Q.; Wang, X. Facile preparation of medical segmented poly(ester-urethane) containing uniformly sized hard segments and phosphorylcholine groups for improved hemocompatibility. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 109, 110571. [Google Scholar] [CrossRef]
- Beamson, G. High Resolution XPS of Organic Polymers: The Scienta ESCA300 Database. J. Chem. Educ. 1993, 70. [Google Scholar] [CrossRef]
- Hirsch, U.; Ruehl, M.; Teuscher, N.; Heilmann, A. Antifouling coatings via plasma polymerization and atom transfer radical polymerization on thin film composite membranes for reverse osmosis. Appl. Surf. Sci. 2018, 436, 207–216. [Google Scholar] [CrossRef]










| Atomic Percentage % | ||||||
|---|---|---|---|---|---|---|
| C1s | O1s | N1s | Br3d | S2p | O/C | |
| Pristine PU | 71.3 | 26.5 | 2.2 | / | / | 0.37 |
| PU-OH | 66.5 | 31.1 | 2.4 | / | / | 0.47 |
| PU-Br | 64.5 | 32.0 | 2.8 | 0.7 | / | 0.50 |
| polyPEGMA | 68.2 | 31.1 | 0.6 | / | / | 0.46 |
| polyPEGMA/polyVBDMH | 72.0 | 24.5 | 3.5 | / | / | 0.34 |
| polyHEMA | 66.7 | 32.3 | 0.9 | / | / | 0.48 |
| polyHEMA/polyVBDMH | 73.8 | 21.9 | 4.3 | / | / | 0.30 |
| polySBMA | 67.3 | 27.8 | 2.7 | 0.7 | 1.5 | 0.41 |
| polySBMA/polyVBDMH | 73.1 | 23.4 | 3.0 | 0.25 | 0.25 | 0.32 |
| polyVBDMH | 75.3 | 20.1 | 4.6 | / | / | 0.27 |
| Bond Percentage % | ||||
|---|---|---|---|---|
| C–C 285.0 eV | C–O–C/C–O/C–N 286.5 eV | –O– (C=O) 288.9 eV | –N– (C=O) –O 289.2 eV | |
| Pristine PU | 69.4 | 16.8 | 6.5 | 7.2 |
| PU-OH | 62.8 | 21.8 | 6.5 | 8.9 |
| PU-Br | 61.6 | 28.6 | 6.7 | 3.0 |
| polyPEGMA | 37.4 | 55.8 | 6.3 | 0.6 |
| polyPEGMA/polyVBDMH | 54.7 | 35.2 | / | 10.1 |
| polyHEMA | 64.7 | 21.9 | 8.4 | 5.0 |
| polyHEMA/polyVBDMH | 65.8 | 20.8 | 3.0 | 10.4 |
| polySBMA | 63.3 | 26.2 | 7.5 | 3.0 |
| polySBMA/polyVBDMH | 60.7 | 25.9 | 3.1 | 10.3 |
| polyVBDMH | 63.9 | 21.9 | / | 14.3 |
| Specimen | Bacteria Log Reduction | |
|---|---|---|
| S. aureus | E. coli | |
| (ATCC 21351) | (ATCC 23501) | |
| polyPEGMA/polyVBDMH | 2.44 ± 0.13 | 2.56 ± 0.00 |
| polyHEMA/polyVBDMH | 2.02 ± 0.17 | 2.02 ± 0.16 |
| polySBMA/polyVBDMH | 2.24 ± 0.20 | 2.40 ± 0.21 |
| polyVBDMH | 1.66 ± 0.08 | 1.63 ± 0.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, C.-H.; Liu, H.-C.; Lin, J.-C. Surface Modification of Polyurethane Membrane with Various Hydrophilic Monomers and N-Halamine: Surface Characterization and Antimicrobial Properties Evaluation. Polymers 2021, 13, 2321. https://doi.org/10.3390/polym13142321
Cheng C-H, Liu H-C, Lin J-C. Surface Modification of Polyurethane Membrane with Various Hydrophilic Monomers and N-Halamine: Surface Characterization and Antimicrobial Properties Evaluation. Polymers. 2021; 13(14):2321. https://doi.org/10.3390/polym13142321
Chicago/Turabian StyleCheng, Chi-Hui, Han-Cheng Liu, and Jui-Che Lin. 2021. "Surface Modification of Polyurethane Membrane with Various Hydrophilic Monomers and N-Halamine: Surface Characterization and Antimicrobial Properties Evaluation" Polymers 13, no. 14: 2321. https://doi.org/10.3390/polym13142321
APA StyleCheng, C. -H., Liu, H. -C., & Lin, J. -C. (2021). Surface Modification of Polyurethane Membrane with Various Hydrophilic Monomers and N-Halamine: Surface Characterization and Antimicrobial Properties Evaluation. Polymers, 13(14), 2321. https://doi.org/10.3390/polym13142321