Antibacterial Biopolymer Gel Coating on Meshes Used for Abdominal Hernia Repair Promotes Effective Wound Repair in the Presence of Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Ethics
2.2. Prosthetic Material
2.3. Antibacterial Biopolymer Gel Coatings
2.4. Scanning Electron Microscopy and UV-Vis Biopolymer Gels Characterization
2.5. Bacterial Inocula
2.6. Experimental Design
- Control (infection control): uncoated meshes without infection.
- Uncoated (coating control): uncoated meshes inoculated with S. aureus.
- CHX: meshes coated with the CHX-loaded gel inoculated with S. aureus.
- RIF: meshes coated with the RIF-loaded gel inoculated with S. aureus.
2.7. Surgical Technique
2.8. Histological Study
2.9. RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.10. Immunofluorescence Analysis of Collagens
2.11. Statistical Analysis
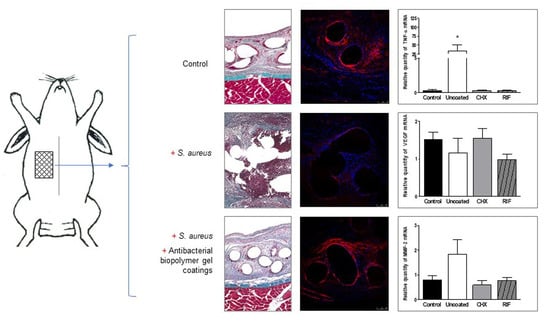
3. Results
3.1. In Vitro Characterization of Biopolymer Gels
3.2. Histological Findings
3.3. Collagens Gene Expression (qRT-PCR)
3.4. Protein Expression of Collagens
3.5. VEGF Gene Expression (qRT-PCR)
3.6. M1/M2 Macrophages (qRT-PCR)
3.7. Expression of MMPs (qRT-PCR)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Orelio, C.C.; van Hessen, C.; Sánchez-Manuel, F.J.; Aufenacker, T.J.; Scholten, R.J. Antibiotic prophylaxis for prevention of postoperative wound infection in adults undergoing open elective inguinal or femoral hernia repair. Cochrane Database Syst. Rev. 2020, 4, CD003769. [Google Scholar]
- Gavlin, A.; Kierans, A.S.; Chen, J.; Song, C.; Guniganti, P.; Mazzariol, F.S. Imaging and Treatment of Complications of Abdominal and Pelvic Mesh Repair. Radiographics 2020, 40, 432–453. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, H.B.; Weis, J.J.; Taveras, L.R.; Huerta, S. Mesh migration following abdominal hernia repair: A comprehensive review. Hernia 2019, 23, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Kao, A.M.; Arnold, M.R.; Augenstein, V.A.; Heniford, B.T. Prevention and Treatment Strategies for Mesh Infection in Abdominal Wall Reconstruction. Plast. Reconstr. Surg. 2018, 142, 149S–155S. [Google Scholar] [CrossRef] [PubMed]
- Moussi, A.; Daldoul, S.; Bourguiba, B.; Othmani, D. Zaouche, A. Gas gangrene of the abdominal wall due to late-onset enteric fistula after polyester mesh repair of an incisional hernia. Hernia 2012, 16, 215–217. [Google Scholar] [CrossRef] [PubMed]
- Clay, L.; Stark, B.; Gunnarsson, U.; Strigård, K. Full-thickness skin graft vs. synthetic mesh in the repair of giant incisional hernia: A randomized controlled multicenter study. Hernia 2018, 22, 325–332. [Google Scholar] [CrossRef] [Green Version]
- Montgomery, A.; Kallinowski, F.; Köckerling, F. Evidence for replacement of an infected synthetic by a biological mesh in abdominal wall hernia repair. Front. Surg. 2016, 2, 67. [Google Scholar] [CrossRef] [Green Version]
- Klinge, U.; Si, Z.Y.; Zheng, H.; Schumpelick, V.; Bhardwaj, R.S.; Klosterhalfen, B. Abnormal collagen I to III distribution in the skin of patients with incisional hernia. Eur. Surg. Res. 2000, 32, 43–48. [Google Scholar] [CrossRef]
- Bellon, J.M.; Bajo, A.; Ga-Honduvilla, N.; Gimeno, M.J.; Pascual, G.; Guerrero, A.; Buján, J. Fibroblasts from the transversalis fascia of young patients with direct inguinal hernias show constitutive MMP-2 overexpression. Ann. Surg. 2001, 233, 287–291. [Google Scholar] [CrossRef]
- Gonzalez, A.C.; Costa, T.F.; Andrade, Z.A.; Medrado, A.R. Wound healing—A literature review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef] [Green Version]
- Pans, A.; Albert, A.; Lapière, C.M.; Nusgens, B. Biochemical study of collagen in adult groin hernias. J. Surg. Res. 2001, 95, 107–113. [Google Scholar] [CrossRef] [Green Version]
- Klinge, U.; Zheng, H.; Si, Z.; Schumpelick, V.; Bhardwaj, R.S.; Muys, L.; Klosterhalfen, B. Expression of the extracellular matrix proteins collagen I, collagen III and fibronectin and matrix metalloproteinase-1 and -13 in the skin of patients with inguinal hernia. Eur. Surg. Res. 1999, 31, 480–490. [Google Scholar] [CrossRef]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound. Repair. Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef]
- Pérez-Köhler, B.; Benito-Martínez, S.; Rodríguez, M.; García-Moreno, F.; Pascual, G.; Bellón, J.M. Experimental study on the use of a chlorhexidine-loaded carboxymethylcellulose gel as antibacterial coating for hernia repair meshes. Hernia 2019, 23, 789–800. [Google Scholar] [CrossRef]
- Pérez-Köhler, B.; Benito-Martínez, S.; García-Moreno, F.; Rodríguez, M.; Pascual, G.; Bellón, J.M. Preclinical bioassay of a novel antibacterial mesh for the repair of abdominal hernia defects. Surgery 2020, 167, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Junge, K.; Klinge, U.; Rosch, R.; Lynen, P.; Binnebösel, M.; Conze, J.; Mertens, P.R.; Schwab, R.; Schumpelick, V. Improved collagen type I/III ratio at the interface of gentamicin-supplemented polyvinylidenfluoride mesh materials. Langenbecks Arch. Surg. 2007, 392, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Klopfleisch, R. Macrophage reaction against biomaterials in the mouse model—Phenotypes, functions and markers. Acta Biomater. 2016, 43, 3–13. [Google Scholar] [CrossRef]
- Rőszer, T. Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediat. Inflamm. 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, B.N.; Badylak, S.F. Expanded applications, shifting paradigms and an improved understanding of host-biomaterial interactions. Acta Biomater. 2013, 9, 4948–4955. [Google Scholar] [CrossRef] [PubMed]
- Caley, M.P.; Martins, V.L.; O’Toole, E.A. Metalloproteinases and Wound Healing. Adv. Wound Care 2015, 4, 225–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olaso, E.; Labrador, J.P.; Wang, L.; Ikeda, K.; Eng, F.J.; Klein, R.; Lovett, D.H.; Lin, H.C.; Friedman, S.L. Discoidin domain receptor 2 regulates fibroblast proliferation and migration through the extracellular matrix in association with transcriptional activation of matrix metalloproteinase-2. J. Biol. Chem. 2002, 277, 3606–3613. [Google Scholar] [CrossRef] [Green Version]
- Pascual, G.; Rodríguez, M.; Gómez-Gil, V.; Trejo, C.; Buján, J.; Bellón, J.M. Active matrix metalloproteinase-2 upregulation in the abdominal skin of patients with direct inguinal hernia. Eur. J. Clin. Invest. 2010, 40, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Qorri, B.; Kalaydina, R.V.; Velickovic, A.; Kaplya, Y.; Decarlo, A.; Szewczuk, M.R. Agonist-Biased Signaling via Matrix Metalloproteinase-9 Promotes Extracellular Matrix Remodeling. Cells 2018, 7, 117. [Google Scholar] [CrossRef]
- Simonetti, O.; Lucarini, G.; Morroni, G.; Orlando, F.; Lazzarini, R.; Zizzi, A.; Brescini, L.; Provinciali, M.; Giacometti, A.; Offidani, A.; et al. New Evidence and Insights on Dalbavancin and Wound Healing in a Mouse Model of Skin Infection. Antimicrob. Agents Chemother. 2020, 24, e02062-19. [Google Scholar] [CrossRef] [PubMed]
- Binnebösel, M.; von Trotha, K.T.; Ricken, C.; Klink, C.D.; Junge, K.; Conze, J.; Jansen, M.; Neumann, U.P.; Lynen Jansen, P. Gentamicin supplemented polyvinylidenfluoride mesh materials enhance tissue integration due to a transcriptionally reduced MMP-2 protein expression. BMC Surg. 2012, 12, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Bellón, J.M.; Rodríguez, M.; Pérez-Köhler, B.; Pérez-López, P.; Pascual, G. The New Zealand white rabbit as a model for preclinical studies addressing tissue repair at the level of the abdominal wall. Tissue Eng. Part C 2017, 23, 863–880. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.A.; Shantier, S.W.; Mohamed, M.A.; Gadkariem, E.A.; Ismail, E.M.O. Spectrophotometric Method for the Simultaneous Determination of Isoniazid and Rifampicin in Bulk and Tablet Forms. Int. J. Pharm. Sci. Rev. Res. 2015, 32, 154–156. [Google Scholar]
- Khamar, J.C.; Patel, S.A. Q-Absorbance Ratio Spectrophotometric Method for the Simultaneous Estimation of Rifampicin and Piperine in their Combined Capsule Dosage. J. Appl. Pharm. Sci. 2012, 2, 137–141. [Google Scholar] [CrossRef] [Green Version]
- Usher, F.C.; Ochsner, J.; Tuttle, L.L., Jr. Use of Marlex mesh in the repair of incisional hernias. Am. Surg. 1958, 24, 969–974. [Google Scholar]
- Birk, D.E.; Mayne, R. Localization of collagen types I, III and V during tendon development. Changes in collagen types I and III are correlated with changes in fibril diameter. Eur. J. Cell Biol. 1997, 72, 352–361. [Google Scholar]
- Rosch, R.; Klinge, U.; Si, Z.; Junge, K.; Klosterhalfen, B.; Schumpelick, V. A role for the collagen I/III and MMP−1/−13 genes in primary inguinal hernia? BMC Med. Genet. 2002, 3, 2. [Google Scholar] [CrossRef] [Green Version]
- Rosch, R.; Junge, K.; Lynen, P.; Mertens, P.R.; Klinge, U.; Schumpelick, V. Hernia- A collagen disease? Eur. Surg. 2003, 35, 11–15. [Google Scholar] [CrossRef]
- Junge, K.; Klinge, U.; Rosch, R.; Mertens, P.R.; Kirch, J.; Klosterhalfen, B.; Lynen, P.; Schumpelick, V. Decreased collagen type I/III ratio in patients with recurring hernia after implantation of alloplastic prostheses. Langenbecks Arch Surg. 2004, 389, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Junge, K.; Rosch, R.; Klinge, U.; Krones, C.; Klosterhalfen, B.; Mertens, P.R.; Lynen, P.; Kunz, D.; Preiss, A.; Peltroche-Llacsahuanga, H.; et al. Gentamicin supplementation of polyvinylidenfluoride mesh materials for infection prophylaxis. Biomaterials 2005, 26, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Gutierrez, M.; Olivares, E.; Pascual, G.; Bellon, J.M.; San Román, J. Low-density polypropylene meshes coated with resorbable and biocompatible hydrophilic polymers as controlled release agents of antibiotics. Acta Biomater. 2013, 9, 6006–6018. [Google Scholar] [CrossRef] [PubMed]
- Guillaume, O.; Lavigne, J.P.; Lefranc, O.; Nottelet, B.; Coudane, J.; Garric, X. New antibioticeluting mesh used for soft tissue reinforcement. Acta Biomater. 2011, 7, 3390–3397. [Google Scholar] [CrossRef]
- Webb, A.H.; Gao, B.T.; Goldsmith, Z.K.; Irvine, A.S.; Saleh, N.; Lee, R.P.; Lendermon, J.B.; Bheemreddy, R.; Zhang, Q.; Brennan, R.; et al. Inhibition of MMP-2 and MMP-9 decreases cellular migration, and angiogenesis in in vitro models of retinoblastoma. BMC Cancer 2017, 17, 434. [Google Scholar] [CrossRef]
- Ardi, V.C.; Van den Steen, P.E.; Opdenakker, G.; Schweighofer, B.; Deryugina, E.I.; Quigley, J.P. Neutrophil MMP-9 proenzyme, unencumbered by TIMP-1, undergoes efficient activation in vivo and catalytically induces angiogenesis via a basic fibroblast growth factor (FGF-2)/FGFR-2 pathway. J. Biol. Chem. 2009, 284, 25854–25866. [Google Scholar] [CrossRef] [Green Version]
- Simonetti, O.; Cirioni, O.; Ghiselli, R.; Goteri, G.; Orlando, F.; Monfregola, L.; De Luca, S.; Zizzi, A.; Silvestri, C.; Veglia, G.; et al. Antimicrobial properties of distinctin in an experimental model of MRSA-infected wounds. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 3047–3055. [Google Scholar] [CrossRef]
- Mantovani, A.; Biswas, S.K.; Galdiero, M.R.; Sica, A.; Locati, M. Macrophage plasticity and polarization in tissue repair and remodelling. J. Pathol. 2013, 229, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.; Taylor, P.R. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 2005, 5, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Madsen, D.H.; Leonard, D.; Masedunskas, A.; Moyer, A.; Jürgensen, H.J.; Peters, D.E.; Amornphimoltham, P.; Selvaraj, A.; Yamada, S.S.; Brenner, D.A.; et al. M2-like macrophages are responsible for collagen degradation through a mannose receptor-mediated pathway. J. Cell Biol. 2013, 202, 951–966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thurlow, L.R.; Hanke, M.L.; Fritz, T.; Angle, A.; Aldrich, A.; Williams, S.H.; Engebretsen, I.L.; Bayles, K.W.; Horswill, A.R.; Kielian, T. Staphylococcus aureus biofilms prevent macrophage phagocytosis and attenuate inflammation in vivo. J. Immunol. 2011, 186, 6585–6596. [Google Scholar] [CrossRef] [PubMed] [Green Version]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benito-Martínez, S.; Pérez-Köhler, B.; Rodríguez, M.; García-Moreno, F.; Gómez-Gil, V.; Pascual, G.; Bellón, J.M. Antibacterial Biopolymer Gel Coating on Meshes Used for Abdominal Hernia Repair Promotes Effective Wound Repair in the Presence of Infection. Polymers 2021, 13, 2371. https://doi.org/10.3390/polym13142371
Benito-Martínez S, Pérez-Köhler B, Rodríguez M, García-Moreno F, Gómez-Gil V, Pascual G, Bellón JM. Antibacterial Biopolymer Gel Coating on Meshes Used for Abdominal Hernia Repair Promotes Effective Wound Repair in the Presence of Infection. Polymers. 2021; 13(14):2371. https://doi.org/10.3390/polym13142371
Chicago/Turabian StyleBenito-Martínez, Selma, Bárbara Pérez-Köhler, Marta Rodríguez, Francisca García-Moreno, Verónica Gómez-Gil, Gemma Pascual, and Juan Manuel Bellón. 2021. "Antibacterial Biopolymer Gel Coating on Meshes Used for Abdominal Hernia Repair Promotes Effective Wound Repair in the Presence of Infection" Polymers 13, no. 14: 2371. https://doi.org/10.3390/polym13142371
APA StyleBenito-Martínez, S., Pérez-Köhler, B., Rodríguez, M., García-Moreno, F., Gómez-Gil, V., Pascual, G., & Bellón, J. M. (2021). Antibacterial Biopolymer Gel Coating on Meshes Used for Abdominal Hernia Repair Promotes Effective Wound Repair in the Presence of Infection. Polymers, 13(14), 2371. https://doi.org/10.3390/polym13142371