Properties of Inorganic Polymers Based on Ground Waste Concrete Containing CuO and ZnO Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of CuO and ZnO Nanoparticles
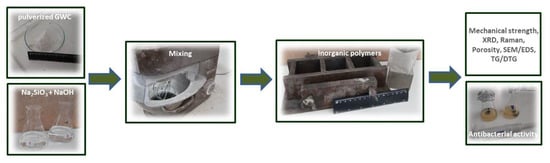
2.2. Preparation of Inorganic Polymers
2.3. Structure Analysis and Characterization
2.4. Antibacterial Activity
3. Results and Discussion
3.1. Characterization of Nanoparticles
3.2. Characterization of Inorganic Polymers from Ground Waste Concrete with Nanoparticles
3.2.1. Mechanical Strength
3.2.2. XRD Analysis
3.2.3. Raman Analysis
3.2.4. SEM/EDS Analysis
3.2.5. Pores, Water Absorption, Sorptivity
3.2.6. Hg-Porosimetry Analysis
3.2.7. Thermogravimetric (TG) Analysis
3.2.8. Antibacterial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andrew, R.M. Global CO2 emissions from cement production. Earth Syst. Sci. Data 2018, 10, 195–217. [Google Scholar] [CrossRef] [Green Version]
- Tam, V.W.Y.; Soomro, M.; Evangelista, A.C.J. A review of recycled aggregate in concrete applications (2000–2017). Constr. Build. Mater. 2018, 172, 272–292. [Google Scholar] [CrossRef]
- European Commission. A New Circular Economy Action Plan for a Cleaner and More Competitive Europe; European Commission: Brussels, Belgium, 2020. [Google Scholar]
- Scrivener, K.L.; John, V.M.; Gartner, E.M. Eco-efficient cements: Potential economically viable solutions for a low-CO2 cement-based materials industry. Cem. Concr. Res. 2018, 114, 2–26. [Google Scholar] [CrossRef]
- Eurostat. 2019a, Generation of Waste by Waste Category, Hazardousness and NACE Rev. 2 Activity [env_wasgen]. Available online: https://appsso.eurostat.ec.europa.eu/nui/show.do?dataset=env_wasgen&lang=en (accessed on 4 August 2021).
- Mavridou, S.; Kaisidou, E.; Kazdaglis, M.; Alaveras, P. Construction and demolition (C&D) waste: Potential uses and current situation in Greece and Cyprus. In Proceedings of the International Conference Industrial Waste & Wastewater Treatment & Valorisation, Crete Island, Greece, 21–23 May 2015. [Google Scholar]
- Sotelo-Piña, C.; Aguilera-González, E.N.; Martínez-Luévanos, A. Geopolymers: Past, present, and future of low carbon footprint eco-materials. In Handbook of Ecomaterials; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–21. [Google Scholar]
- Davidovits, J. False Values on CO2 Emission for Geopolymer Cement/Concrete; Technical Paper; Geopolymer Institute Library: Saint-Quentin, France, 29 June 2015; pp. 1–9. [Google Scholar]
- Maddalena, R.; Roberts, J.; Hamilton, A. Can Portland cement be replaced by low-carbon alternative materials? A study on the thermal properties and carbon emissions of innovative cements. J. Clean. Prod. 2018, 186, 933–942. [Google Scholar] [CrossRef]
- Singh, B.; Ishwarya, G.; Gupta, M.; Bhattacharyya, S. Geopolymer concrete: A review of some recent developments. Constr. Build. Mater. 2015, 85, 78–90. [Google Scholar] [CrossRef]
- Amran, Y.M.; Alyousef, R.; Alabduljabbar, H.; El-Zeadani, M. Clean production and properties of geopolymer concrete; A review. J. Clean. Prod. 2020, 251, 119679. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D.; Vlachou, A.; Bartzas, G.; Galetakis, M. Effect of synthesis parameters on the quality of construction and demolition wastes (CDW) geopolymers. Adv. Powder Technol. 2015, 26, 368–376. [Google Scholar] [CrossRef] [Green Version]
- Zaharaki, D.; Galetakis, M.; Komnitsas, K. Valorization of construction and demolition (C&D) and industrial wastes through alkali activation. Constr. Build. Mater. 2016, 121, 686–693. [Google Scholar] [CrossRef]
- Komnitsas, K.; Bartzas, G.; Karmali, V.; Petrakis, E.; Kurylak, W.; Pietek, G.; Kanasiewicz, J. Assessment of alkali activation potential of a Polish ferronickel slag. Sustainabilty 2019, 11, 1863. [Google Scholar] [CrossRef] [Green Version]
- Soultana, A.; Valouma, A.; Bartzas, G.; Komnitsas, K. Properties of inorganic polymers produced from brick waste and metallurgical slag. Minerals 2019, 9, 551. [Google Scholar] [CrossRef] [Green Version]
- Vavouraki, A.I. Utilization of industrial waste slags to enhance ground waste concrete-based inorganic polymers. J. Sustain. Met. 2020, 6, 383–399. [Google Scholar] [CrossRef]
- Wu, Y.; Lu, B.; Bai, T.; Wang, H.; Du, F.; Zhang, Y.; Cai, L.; Jiang, C.; Wang, W. Geopolymer, green alkali activated cementitious material: Synthesis, applications and challenges. Constr. Build. Mater. 2019, 224, 930–949. [Google Scholar] [CrossRef]
- Tan, T.H.; Mo, K.H.; Ling, T.-C.; Lai, S.H. Current development of geopolymer as alternative adsorbent for heavy metal removal. Environ. Technol. Innov. 2020, 18, 100684. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and Toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Singh, N.B.; Saxena, S.K.; Kumar, M. Effect of nanomaterials on the properties of geopolymer mortars and concrete. Mater. Today Proc. 2018, 5, 9035–9040. [Google Scholar] [CrossRef]
- Rashad, A.M. Effect of nanoparticles on the properties of geopolymer materials. Mag. Concr. Res. 2019, 71, 1283–1301. [Google Scholar] [CrossRef]
- Deb, P.S.; Sarker, P.; Barbhuiya, S. Sorptivity and acid resistance of ambient-cured geopolymer mortars containing nano-silica. Cem. Concr. Compos. 2016, 72, 235–245. [Google Scholar] [CrossRef] [Green Version]
- Guzmán-Aponte, L.A.; De Gutiérrez, R.M.; Maury-Ramírez, A. Metakaolin-based geopolymer with added TiO2 particles: Physicomechanical characteristics. Coatings 2017, 7, 233. [Google Scholar] [CrossRef] [Green Version]
- Ramezanianpour, A.A.; Moeini, M.A. Mechanical and durability properties of alkali activated slag coating mortars containing nanosilica and silica fume. Constr. Build. Mater. 2018, 163, 611–621. [Google Scholar] [CrossRef]
- Zailan, S.N.; Bouaissi, A.; Mahmed, N.; Abdullah, M.M.A.B. Influence of ZnO nanoparticles on mechanical properties and photocatalytic activity of self-cleaning ZnO-based geopolymer paste. J. Inorg. Organomet. Polym. Mater. 2019, 30, 2007–2016. [Google Scholar] [CrossRef]
- Jindal, B.B.; Sharma, R. The effect of nanomaterials on properties of geopolymers derived from industrial by-products: A state-of-the-art review. Constr. Build. Mater. 2020, 252, 119028. [Google Scholar] [CrossRef]
- Xie, T.; Fang, C. Nanomaterials applied in modifications of geopolymer composites: A Review. Aust. J. Civ. Eng. 2019, 17, 32–49. [Google Scholar] [CrossRef]
- Liu, C.; Huang, X.; Wu, Y.-Y.; Deng, X.; Liu, J.; Zheng, Z.; Hui, D. Review on the research progress of cement-based and geopolymer materials modified by graphene and graphene oxide. Nanotechnol. Rev. 2020, 9, 155–169. [Google Scholar] [CrossRef] [Green Version]
- Janáky, C.; Rajeshwar, K. The role of (photo)electrochemistry in the rational design of hybrid conducting polymer/semiconductor assemblies: From fundamental concepts to practical applications. Prog. Polym. Sci. 2015, 43, 96–135. [Google Scholar] [CrossRef]
- Farhana, K.; Kadirgama, K.; Rahman, M.; Ramasamy, D.; Noor, M.; Najafi, G.; Samykano, M.; Mahamude, A. Improvement in the performance of solar collectors with nanofluids—A state-of-the-art review. Nano Struct. Nano-Objects 2019, 18, 100276. [Google Scholar] [CrossRef]
- Chang, Y.-N.; Zhang, M.; Xia, L.; Zhang, J.; Xing, G. The toxic effects and mechanisms of CuO and ZnO nanoparticles. Materials 2012, 5, 2850–2871. [Google Scholar] [CrossRef] [Green Version]
- Singh, J.; Dutta, T.; Kim, K.-H.; Rawat, M.; Samddar, P.; Kumar, P. ‘Green’ synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 2018, 16, 84. [Google Scholar] [CrossRef]
- Konsta-Gdoutos, M.S. Nanomaterials in self-consolidating concrete: A state-of-the-art review. J. Sustain. Cem. Mater. 2014, 3, 167–180. [Google Scholar] [CrossRef]
- Mendes, T.M.; Hotza, D.; Repette, W.L. Nanoparticles in cement based materials: A review. Rev. Adv. Mater. Sci. 2015, 40, 89–96. [Google Scholar]
- Du, S.; Wu, J.; Alshareedah, O.; Shi, X. Nanotechnology in cement-based materials: A review of durability, modeling, and advanced characterization. Nanomaterials 2019, 9, 1213. [Google Scholar] [CrossRef] [Green Version]
- Rashad, A.M. Effects of ZnO2, ZrO2, Cu2O3, CuO, CaCO3, SF, FA, cement and geothermal silica waste nanoparticles on properties of cementitious materials—A short guide for Civil Engineer. Constr. Build. Mater. 2013, 48, 1120–1133. [Google Scholar] [CrossRef]
- Shafeek, A.M.; Khedr, M.H.; El-Dek, S.I.; Shehata, N. Influence of ZnO nanoparticle ratio and size on mechanical properties and whiteness of White Portland Cement. Appl. Nanosci. 2020, 10, 3603–3615. [Google Scholar] [CrossRef]
- Sikora, P.; Augustyniak, A.; Cendrowski, K.; Nawrotek, P.; Mijowska, E. Antimicrobial activity of Al2O3, CuO, Fe3O4, and ZnO nanoparticles in scope of their further application in cement-based building materials. Nanomaterials 2018, 8, 212. [Google Scholar] [CrossRef] [Green Version]
- Noeiaghaei, T.; Mukherjee, A.; Dhami, N.K.; Chae, S. Biogenic deterioration of concrete and its mitigation technologies. Constr. Build. Mater. 2017, 149, 575–586. [Google Scholar] [CrossRef]
- Adak, D.; Sarkar, M.; Maiti, M.; Tamang, A.; Mandal, S.; Chattopadhyay, B. Anti-microbial efficiency of nano silver–silica modified geopolymer mortar for eco-friendly green construction technology. RSC Adv. 2015, 5, 64037–64045. [Google Scholar] [CrossRef]
- Armayani, M.; Pratama, M.A. The properties of nano silver (Ag)-geopolymer as antibacterial composite for functional surface materials. MATEC Web Conf. 2017, 97, 1010. [Google Scholar] [CrossRef] [Green Version]
- Cerna, K.D.; Janairo, J.I.; Promentilla, M.A. Development of nanosilver-coated geopolymer beads (AgGP) from fly ash and baluko shells for antimicrobial applications. MATEC Web Conf. 2019, 268, 05003. [Google Scholar] [CrossRef]
- Qadry, A.N.; Kharisma, N.A. The properties of geopolymer-cuo nanoparticles as a functional composite. Mater. Sci. Forum 2019, 967, 281–285. [Google Scholar] [CrossRef]
- Zidi, Z.; Ltifi, M.; Ben Ayadi, Z.; El Mir, L.; Nóvoa, X. Effect of nano-ZnO on mechanical and thermal properties of geopolymer. J. Asian Ceram. Soc. 2020, 8, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Nur, Q.A.; Sari, N.U. Development of geopolymers composite based on metakaolin-nano ZnO for antibacterial application. IOP Conf. Ser. Mater. Sci. Eng. 2017, 180, 12289. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez, R.; Villaquirán-Caicedo, M.; Ramírez-Benavides, S.; Astudillo, M.; Mejía, D. Evaluation of the antibacterial activity of a geopolymer mortar based on metakaolin supplemented with TiO2 and CuO particles using glass waste as fine aggregate. Coatings 2020, 10, 157. [Google Scholar] [CrossRef] [Green Version]
- Nawaz, M.; Heitor, A.; Sivakumar, M. Geopolymers in construction—recent developments. Constr. Build. Mater. 2020, 260, 120472. [Google Scholar] [CrossRef]
- Shehata, N.; Sayed, E.T.; Abdelkareem, M.A. Recent progress in environmentally friendly geopolymers: A review. Sci. Total. Environ. 2021, 762, 143166. [Google Scholar] [CrossRef]
- Carbone, M.; Briancesco, R.; Bonadonna, L. Antimicrobial power of Cu/Zn mixed oxide nanoparticles to Escherichia coli. Environ. Nanotechnol. Monit. Manag. 2017, 7, 97–102. [Google Scholar] [CrossRef]
- Cullity, B.D.; Stock, S.R. The Elements of X-ray Diffraction, 3rd ed.; Wesley, Pearson New International Press: Edinburgh, UK, 2002. [Google Scholar]
- Jabbar, S.M. Synthesis of CuO nano structure via sol-gel and precipitation chemical methods. Al-Khwarizmi Eng. J. 2016, 12, 126–131. [Google Scholar] [CrossRef]
- Dörner, L.; Cancellieri, C.; Rheingans, B.; Walter, M.; Kägi, R.; Schmutz, P.; Kovalenko, M.V.; Jeurgens, L.P.H. Cost-effective sol-gel synthesis of porous CuO nanoparticle aggregates with tunable specific surface area. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geetha, M.; Nagabhushana, H.; Shivananjaiah, H. Green mediated synthesis and characterization of ZnO nanoparticles using euphorbia jatropa latex as reducing agent. J. Sci. Adv. Mater. Devices 2016, 1, 301–310. [Google Scholar] [CrossRef] [Green Version]
- Zidi, Z.; Ltifi, M.; Ben Ayadi, Z.; El Mir, L. Synthesis of nano-alumina and their effect on structure, mechanical and thermal properties of geopolymer. J. Asian Ceram. Soc. 2019, 7, 524–535. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Wu, Z.; Cheng, H.; Zhang, Z.; Frost, R.L. A Raman spectroscopic comparison of calcite and dolomite. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 117, 158–162. [Google Scholar] [CrossRef] [Green Version]
- Kosor, T.; Nakić-Alfirević, B.; Gajović, A. Geopolymerization index of fly ash geopolymers. Vib. Spectrosc. 2016, 85, 104–111. [Google Scholar] [CrossRef]
- Arnoult, M.; Perronnet, M.; Autef, A.; Rossignol, S. How to control the geopolymer setting time with the alkaline silicate solution. J. Non-Crystalline Solids 2018, 495, 59–66. [Google Scholar] [CrossRef]
- Niveditha, M.; Koniki, S. Effect of Durability properties on geopolymer concrete—A review. E3S Web Conf. 2020, 184, 01092. [Google Scholar] [CrossRef]
- Gunasekara, C.; Dirgantara, R.; Law, D.W.; Setunge, S. Effect of curing conditions on microstructure and pore-structure of brown coal fly ash geopolymers. Appl. Sci. 2019, 9, 3138. [Google Scholar] [CrossRef] [Green Version]
- Aredes, F.; Campos, T.; Machado, J.; Sakane, K.; Thim, G.; Brunelli, D. Effect of cure temperature on the formation of metakaolinite-based geopolymer. Ceram. Int. 2015, 41, 7302–7311. [Google Scholar] [CrossRef]
- Galiano, Y.L.; Pereira, C.F.; Izquierdo, M. Contributions to the study of porosity in fly ash-based geopolymers. Relationship between degree of reaction, porosity and compressive strength. Materiales Construcción 2016, 66, 098. [Google Scholar] [CrossRef]
- Lowell, S.; Shields, J.E. Interpretation of mercury porosimetry data. Powder Surf. Area Porosity 1991, 2, 99–120. [Google Scholar] [CrossRef]
- Assaedi, H.; Shaikh, F.; Low, I. Effect of nano-clay on mechanical and thermal properties of geopolymer. J. Asian Ceram. Soc. 2016, 4, 19–28. [Google Scholar] [CrossRef] [Green Version]
- Abdullah, M.M.A.B.; Sandu, A.V.; Vizureanu, P. XRD and TG-DTA study of new alkali activated materials based on fly ash with sand and glass powder. Materials 2020, 13, 343. [Google Scholar] [CrossRef] [Green Version]
- Kirboga, S.; Öner, M.; Kırboğa, S. Investigation of calcium carbonate precipitation in the presence of carboxymethyl inulin. CrystEngComm 2013, 15, 3678–3686. [Google Scholar] [CrossRef]
- Poggetto, G.D.; Catauro, M.; Crescente, G.; Leonelli, C. Efficient addition of waste glass in MK-based geopolymers: Microstructure, antibacterial and cytotoxicity investigation. Polymers 2021, 13, 1493. [Google Scholar] [CrossRef]
- Sharma, M.; Beuchat, L.R. Sensitivity of Escherichia coli O157:H7 to commercially available alkaline cleaners and subsequent resistance to heat and sanitizers. Appl. Environ. Microbiol. 2004, 70, 1795–1803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonia, S.; Jayram, N.D.; Kumar, P.S.; Mangalaraj, D.; Ponpandian, N.; Viswanathan, C. Effect of NaOH concentration on structural, surface and antibacterial activity of CuO nanorods synthesized by direct sonochemical method. Superlattices Microstruct. 2014, 66, 1–9. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [Green Version]
- Asamoah, R.; Yaya, A.; Mensah, B.; Nbalayim, P.; Apalangya, V.; Bensah, Y.; Damoah, L.; Agyei-Tuffour, B.; Dodoo-Arhin, D.; Annan, E. Synthesis and characterization of zinc and copper oxide nanoparticles and their antibacteria activity. Results Mater. 2020, 7, 100099. [Google Scholar] [CrossRef]
- Meghana, S.; Kabra, P.; Chakraborty, S.; Padmavathy, N. Understanding the pathway of antibacterial activity of copper oxide nanoparticles. RSC Adv. 2015, 5, 12293–12299. [Google Scholar] [CrossRef]
- Javadhesari, S.M.; Alipour, S.; Mohammadnejad, S.; Akbarpour, M. Antibacterial activity of ultra-small copper oxide (II) nanoparticles synthesized by mechanochemical processing against S. aureus and E. coli. Mater. Sci. Eng. C 2019, 105, 110011. [Google Scholar] [CrossRef]











| Oxides/Material | SiO2 | Al2O3 | CaO | Na2O | Fe2O3 | MgO | K2O | TiO2 | MnO | P2O5 | SO3 | SrO | * LOI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GWC | 3.96 | 0.99 | 54.43 | 1.80 | 0.51 | 1.95 | 0.07 | 0.05 | 0.01 | 0.04 | 0.46 | 0.13 | 35.05 |
| Sample | GWC IPs | Cu0.5 | Zn2 | |||
|---|---|---|---|---|---|---|
| Element | Weight % | Atomic % | Weight % | Atomic % | Weight % | Atomic % |
| Ca | 26.91 | 13.60 | 22.75 | 10.92 | 15.85 | 7.39 |
| Si | 5.88 | 4.24 | 4.44 | 3.04 | 5.48 | 3.65 |
| Na | 3.36 | 2.97 | 2.62 | 2.19 | 4.05 | 3.30 |
| Mg | 1.30 | 1.08 | 1.33 | 1.05 | 1.59 | 1.23 |
| Al | 0.83 | 0.62 | 0.76 | 0.54 | 1.35 | 0.94 |
| K | 0.20 | 0.10 | 0.30 | 0.15 | 0.34 | 0.16 |
| Cu | n.d. | n.d. | 0.66 | 0.20 | n.d. | n.d. |
| Zn | n.d. | n.d. | - | n.d. | 0.03 | 0.01 |
| O | 61.12 | 77.39 | 68.12 | 81.91 | 71.35 | 83.32 |
| Total | 99.6 | 100 | 100.98 | 100 | 100.04 | 100 |
| Sample No. | Specific Surface Area, m2·g−1 | Total Pore Volume, TPV, mm3·g−1 | Average Pore Diameter, APD, Å |
|---|---|---|---|
| Control | 7.7 | 17 | 87.5 |
| Cu0.5 | 6.5 | 20 | 124.6 |
| Cu1 | 5.8 | 19 | 128.6 |
| Cu2 | 8.1 | 26 | 129 |
| Zn0.5 | 6.6 | 20 | 123 |
| Zn1 | 8.0 | 22 | 108 |
| Zn2 | 6.5 | 22 | 126 |
| Control | Cu0.5 | Zn1 | |
|---|---|---|---|
| Total Pore Area, m2·g−1 | 4.946 | 5.676 | 4.844 |
| Total Intrusion Volume, mL·g−1 | 0.155 | 0.158 | 0.112 |
| Median Pore Diameter (Volume), µm | 0.838 | 0.485 | 0.144 |
| Median Pore Diameter (Area), µm | 0.018 | 0.028 | 0.065 |
| Average Pore Diameter (4V/A), µm | 0.125 | 0.111 | 0.093 |
| Bulk Density at 1.53 psia, g·mL−1 | 1.71 | 1.17 | 2.09 |
| Apparent (skeletal) Density, g·mL−1 | 2.33 | 1.44 | 2.74 |
| Porosity, % | 26.5 | 18.5 | 23.5 |
| Steam Volume Used, % | 46 | 30 | 45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vavouraki, A.I.; Gounaki, I.; Venieri, D. Properties of Inorganic Polymers Based on Ground Waste Concrete Containing CuO and ZnO Nanoparticles. Polymers 2021, 13, 2871. https://doi.org/10.3390/polym13172871
Vavouraki AI, Gounaki I, Venieri D. Properties of Inorganic Polymers Based on Ground Waste Concrete Containing CuO and ZnO Nanoparticles. Polymers. 2021; 13(17):2871. https://doi.org/10.3390/polym13172871
Chicago/Turabian StyleVavouraki, Aikaterini I., Iosifina Gounaki, and Danae Venieri. 2021. "Properties of Inorganic Polymers Based on Ground Waste Concrete Containing CuO and ZnO Nanoparticles" Polymers 13, no. 17: 2871. https://doi.org/10.3390/polym13172871
APA StyleVavouraki, A. I., Gounaki, I., & Venieri, D. (2021). Properties of Inorganic Polymers Based on Ground Waste Concrete Containing CuO and ZnO Nanoparticles. Polymers, 13(17), 2871. https://doi.org/10.3390/polym13172871