Effect of pH on the In Vitro Biocompatibility of Surfactant-Assisted Synthesis and Hydrothermal Precipitation of Rod-Shaped Nano-Hydroxyapatite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
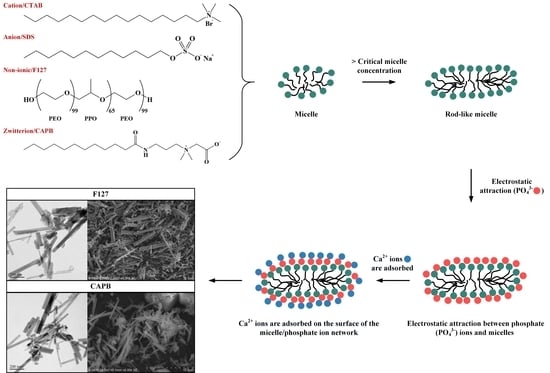
2.2. Surfactant-Assisted HA Powder Synthesis
2.3. Morphological Observation and Aspect Ratio
2.4. X-ray Diffraction (XRD) Analysis
2.5. Fourier Transform Infrared Spectroscopy–Attenuated Total Reflection (FTIR-ATR)
2.6. Biocompatible Evaluation
2.7. Statistical Analysis
3. Results and Discussion
3.1. Characterization of the Hydrothermal Synthesis of HA by XRD
3.2. Characterization of HA by FTIR
3.3. Morphology and Aspect Ratio
3.4. TEM Analysis
3.5. Biocompatibility
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sadat-Shojai, M.; Khorasani, M.-T.; Dinpanah-Khoshdargi, E.; Jamshidi, A. Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta Biomater. 2013, 9, 7591–7621. [Google Scholar] [CrossRef]
- Miranda, M.; Torrecillas, R.; Fernández, A. Reactivity of Ca and P precursors to form hydroxyapatite and its influence on the properties of the obtained powders. Ceram. Int. 2020, 46, 27860–27865. [Google Scholar] [CrossRef]
- Kumar, A.; Kargozar, S.; Baino, F.; Han, S.S. Additive manufacturing methods for producing hydroxyapatite and hydroxyapatite-based composite scaffolds: A review. Front. Mater. 2019, 6, 313. [Google Scholar] [CrossRef]
- Luo, Y.; Chen, S.; Shi, Y.; Ma, J. 3D printing of strontium-doped hydroxyapatite based composite scaffolds for repairing critical-sized rabbit calvarial defects. Biomed. Mater. 2018, 13, 065004. [Google Scholar] [CrossRef]
- Chen, W.-C.; Cheng, I.-T.; Chang, K.-C.; Haung, S.-M.; Chen, J.-C.; Shih, C.-J. Heparin as a biomimetic template on nanoapatite rods with tunable aspect ratio: Synthesis and biocompatibility. J. Aust. Ceram. Soc. 2021, 57, 825–834. [Google Scholar] [CrossRef]
- Vallet-Regi, M.; González-Calbet, J.M. Calcium phosphates as substitution of bone tissues. Prog. Solid. State Chem. 2004, 32, 1–31. [Google Scholar] [CrossRef]
- Wu, Y.R.; Chang, C.W.; Chang, K.C.; Lin, D.J.; Ko, C.L.; Wu, H.Y.; Chen, W.C. Effect of micro-/nano-hybrid hydroxyapatite rod reinforcement in composite resins on strength through thermal cycling. Polym. Compos. 2019, 40, 3703–3710. [Google Scholar] [CrossRef]
- Dorozhkin, S.V.; Epple, M. Biological and medical significance of calcium phosphates. Angew. Chem. Int. Ed. 2002, 41, 3130–3146. [Google Scholar] [CrossRef]
- Amer, W.; Abdelouahdi, K.; Ramananarivo, H.R.; Zahouily, M.; Fihri, A.; Djessas, K.; Zahouily, K.; Varma, R.S.; Solhy, A. Microwave-assisted synthesis of mesoporous nano-hydroxyapatite using surfactant templates. CrystEngComm 2014, 16, 543–549. [Google Scholar] [CrossRef]
- Zhan, J.; Tseng, Y.H.; Chan, J.C.; Mou, C.Y. Biomimetic formation of hydroxyapatite nanorods by a single-crystal-to-single-crystal transformation. Adv. Funct. Mater. 2005, 15, 2005–2010. [Google Scholar] [CrossRef]
- Utku, F.; Seckin, E.; Goller, G.; Tamerler, C.; Urgen, M. Electrochemically designed interfaces: Hydroxyapatite coated macro-mesoporous titania surfaces. Appl. Surf. Sci. 2015, 350, 62–68. [Google Scholar] [CrossRef]
- Sánchez-Campos, D.; Mendoza-Anaya, D.; Reyes-Valderrama, M.; Esteban-Gómez, S.; Rodríguez-Lugo, V. Cationic surfactant at high pH in microwave HAp synthesis. Mater. Lett. 2020, 265, 127416. [Google Scholar] [CrossRef]
- Prakash, V.C.A.; Venda, I.; Thamizharasi, V.; Sathya, E. A comparative study on microemulsion synthesis of hydroxyapatite powders by ionic and Non-Ionic surfactants. Mater. Today Proc. 2020. [Google Scholar] [CrossRef]
- Kermanian, M.; Naghibi, M.; Sadighian, S. One-pot hydrothermal synthesis of a magnetic hydroxyapatite nanocomposite for MR imaging and pH-Sensitive drug delivery applications. Heliyon 2020, 6, e04928. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Ng, S.; Heng, B.C.; Guo, J.; Ma, L.; Tan, T.T.Y.; Ng, K.W.; Loo, S.C.J. Cytotoxicity of hydroxyapatite nanoparticles is shape and cell dependent. Arch. Toxicol. 2013, 87, 1037–1052. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Li, Y.; Luo, W.; Jiang, J.; Zhao, J.; Liu, C. Controllable synthesis of biomimetic hydroxyapatite nanorods with high osteogenic bioactivity. ACS Biomater. Sci. Eng. 2019, 6, 320–328. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, J.; Yang, S.; Yu, Q.; Wang, Z.; Zhang, Q. Preparation of amino-acid-regulated hydroxyapatite particles by hydrothermal method. Mater. Lett. 2011, 65, 572–574. [Google Scholar] [CrossRef]
- Sadat-Shojai, M.; Khorasani, M.-T.; Jamshidi, A. Hydrothermal processing of hydroxyapatite nanoparticles—A Taguchi experimental design approach. J. Cryst. Growth 2012, 361, 73–84. [Google Scholar] [CrossRef]
- Shanthi, P.M.S.; Mangalaraja, R.; Uthirakumar, A.; Velmathi, S.; Balasubramanian, T.; Ashok, M. Synthesis and characterization of porous shell-like nano hydroxyapatite using Cetrimide as template. J. Colloid Interface Sci. 2010, 350, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Salarian, M.; Solati-Hashjin, M.; Shafiei, S.S.; Salarian, R.; Nemati, Z.A. Template-directed hydrothermal synthesis of dandelion-like hydroxyapatite in the presence of cetyltrimethylammonium bromide and polyethylene glycol. Ceram. Int. 2009, 35, 2563–2569. [Google Scholar] [CrossRef]
- Mohammad, N.F.; Othman, R.; Yeoh, F.Y. Controlling the pore characteristics of mesoporous apatite materials: Hydroxyapatite and carbonate apatite. Ceram. Int. 2015, 41, 10624–10633. [Google Scholar] [CrossRef]
- Goh, K.W.; Wong, Y.H.; Ramesh, S.; Chandran, H.; Krishnasamy, S.; Sidhu, A.; Teng, W. Effect of pH on the properties of eggshell-derived hydroxyapatite bioceramic synthesized by wet chemical method assisted by microwave irradiation. Ceram. Int. 2021, 47, 8879–8887. [Google Scholar] [CrossRef]
- Yao, J.; Tjandra, W.; Chen, Y.Z.; Tam, K.C.; Ma, J.; Soh, B. Hydroxyapatite nanostructure material derived using cationic surfactant as a template. J. Mater. Chem. 2003, 13, 3053–3057. [Google Scholar] [CrossRef]
- Verma, G.; Barick, K.; Manoj, N.; Sahu, A.; Hassan, P. Rod-like micelle templated synthesis of porous hydroxyapatite. Ceram. Int. 2013, 39, 8995–9002. [Google Scholar] [CrossRef]
- Ortiz, G.M.H.; Parra, R.; Fanovich, M.A. Comparative hydrothermal synthesis of hydroxyapatite by using cetyltrimethylammonium bromide and hexamethylenetetramine as additives. Ceram. Int. 2018, 44, 3658–3663. [Google Scholar] [CrossRef]
- Wei, K.; Lai, C.; Wang, Y. Formation of monetite nanoparticles and nanofibers in reverse micelles. J. Mater. Sci. 2007, 42, 5340–5346. [Google Scholar] [CrossRef]
- Scherrer, P. Nachrichten von der Gesellschaft der Wissenschaften zu Göttingen. Math.-Phys. Kl. 1918, 2, 98–100. [Google Scholar]
- Wang, H.; Yuan, L.; An, J. Crystallographic characteristics of hydroxylapatite in hard tissues of cololabis saira. Crystals 2017, 7, 103. [Google Scholar] [CrossRef] [Green Version]
- Manafi, S.; Yazdani, B.; Rahimiopour, M.; Sadrnezhaad, S.; Amin, M.H.; Razavi, M. Synthesis of nano-hydroxyapatite under a sonochemical/hydrothermal condition. Biomed. Mater. 2008, 3, 025002. [Google Scholar] [CrossRef]
- Méndez-Lozano, N.; Velázquez-Castillo, R.; Rivera-Muñoz, E.M.; Bucio-Galindo, L.; Mondragón-Galicia, G.; Manzano-Ramírez, A.; Ocampo, M.Á.; Apátiga-Castro, L.M. Crystal growth and structural analysis of hydroxyapatite nanofibers synthesized by the hydrothermal microwave-assisted method. Ceram. Int. 2017, 43, 451–457. [Google Scholar] [CrossRef]
- Chang, K.-C.; Chen, J.-C.; Cheng, I.-T.; Haung, S.-M.; Liu, S.-M.; Ko, C.-L.; Sun, Y.-S.; Shih, C.-J.; Chen, W.-C. Strength and biocompatibility of heparin-based calcium phosphate cement grafted with ferulic acid. Polymers 2021, 13, 2219. [Google Scholar] [CrossRef]
- Haung, S.-M.; Chen, J.-C.; Chang, K.-C.; Ko, C.-L.; Lin, D.-J.; Chen, W.-C. Synthesis of nanorod apatites with templates at critical micelle concentrations and in vitro evaluation of cytotoxicity and antimicrobial activity. J. Asian Ceram. Soc. 2021, 1–12. [Google Scholar] [CrossRef]
- Foley, B.; Greiner, M.; McGlynn, G.; Schmahl, W.W. Anatomical variation of human bone bioapatite crystallography. Crystals 2020, 10, 859. [Google Scholar] [CrossRef]
- Savchyn, P.; Karbovnyk, I.; Vistovskyy, V.; Voloshinovskii, A.; Pankratov, V.; Cestelli Guidi, M.; Mirri, C.; Myahkota, O.; Riabtseva, A.; Mitina, N. Vibrational properties of LaPO4 nanoparticles in mid-and far-infrared domain. J. Appl. Phys. 2012, 112, 124309. [Google Scholar] [CrossRef]
- Bystrova, A.; Dekhtyar, Y.D.; Popov, A.; Coutinho, J.; Bystrov, V. Modified hydroxyapatite structure and properties: Modeling and synchrotron data analysis of modified hydroxyapatite structure. Ferroelectrics 2015, 475, 135–147. [Google Scholar] [CrossRef]
- Meejoo, S.; Maneeprakorn, W.; Winotai, P. Phase and thermal stability of nanocrystalline hydroxyapatite prepared via microwave heating. Thermochim. Acta 2006, 447, 115–120. [Google Scholar] [CrossRef]
- Xu, G.; Aksay, I.A.; Groves, J.T. Continuous crystalline carbonate apatite thin films. A biomimetic approach. J. Am. Chem. Soc. 2001, 123, 2196–2203. [Google Scholar] [CrossRef]







| Surfactants | Synthetic Product Phases and Length-to-Thickness Ratio | |||||||
|---|---|---|---|---|---|---|---|---|
| pH 4 | pH 9 | |||||||
| Product Orginal Phase | Phase after Calcination | The c-axis (002) Plane/(100) Thickness of HA Aspect Ratio | Product Orginal Phase | Phase after Calcination | The c-axis (002) Plane/(100]) Thickness of HA Aspect Ratio | |||
| Original | Calcined | Original | Calcined | |||||
| Surfactant-free P | DCPA | HA + β-TCP | 0.50 | 0.68 | HA | HA + β-TCP | 0.63 | 0.90 |
| Cationic, CTAB | HA | HA + β-TCP | 1.03 | 0.80 | HA | HA + β-TCP | 0.74 | 0.87 |
| Anionic, SDS | DCPA + (HA) a | HA + β-TCP | 0.63 | 0.53 | HA + (DCPA) a | HA + β-TCP | 0.82 | 0.78 |
| Non-ionic, F127 | HA | HA + β-TCP | 0.68 | 0.89 | HA | HA + β-TCP | 0.63 | 1.19 |
| Zwitterionic, CAPB | HA | HA + β-TCP | 0.72 | 0.72 | HA | HA + β-TCP | 0.81 | 1.68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, D.-J.; Lin, H.-L.; Haung, S.-M.; Liu, S.-M.; Chen, W.-C. Effect of pH on the In Vitro Biocompatibility of Surfactant-Assisted Synthesis and Hydrothermal Precipitation of Rod-Shaped Nano-Hydroxyapatite. Polymers 2021, 13, 2994. https://doi.org/10.3390/polym13172994
Lin D-J, Lin H-L, Haung S-M, Liu S-M, Chen W-C. Effect of pH on the In Vitro Biocompatibility of Surfactant-Assisted Synthesis and Hydrothermal Precipitation of Rod-Shaped Nano-Hydroxyapatite. Polymers. 2021; 13(17):2994. https://doi.org/10.3390/polym13172994
Chicago/Turabian StyleLin, Dan-Jae, Hao-Lian Lin, Ssu-Meng Haung, Shih-Ming Liu, and Wen-Cheng Chen. 2021. "Effect of pH on the In Vitro Biocompatibility of Surfactant-Assisted Synthesis and Hydrothermal Precipitation of Rod-Shaped Nano-Hydroxyapatite" Polymers 13, no. 17: 2994. https://doi.org/10.3390/polym13172994
APA StyleLin, D. -J., Lin, H. -L., Haung, S. -M., Liu, S. -M., & Chen, W. -C. (2021). Effect of pH on the In Vitro Biocompatibility of Surfactant-Assisted Synthesis and Hydrothermal Precipitation of Rod-Shaped Nano-Hydroxyapatite. Polymers, 13(17), 2994. https://doi.org/10.3390/polym13172994