Advanced Photocatalysts Based on Conducting Polymer/Metal Oxide Composites for Environmental Applications
Abstract
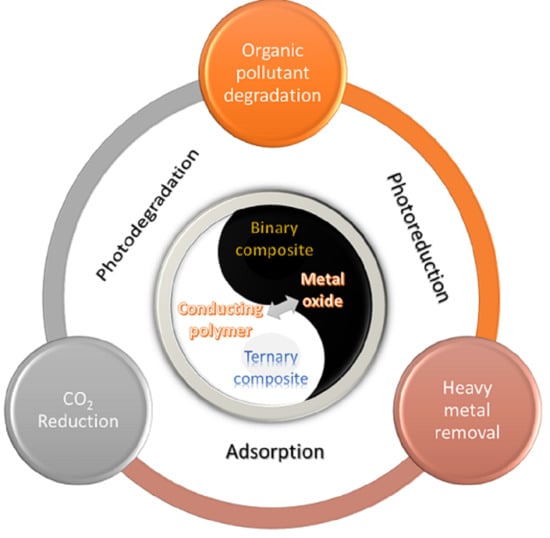
:1. Introduction
2. Synthesis and Properties of Conjugated Polymer/Metal Oxide Composites
2.1. Binary Composites of CPs and Metal Oxides
2.1.1. PANI/Metal Oxide Composites
2.1.2. Composites of PEDOT and Metal Oxides
2.1.3. Composites of PPy and Metal Oxides
2.2. Ternary Composites of CP/Metal Oxides
3. Visible-Light-Responsive Photocatalysis Mechanisms of Conducting Polymer/Metal Oxide Composites
4. Photocatalytic Applications of CP/Metal Oxide Composites in the Environment Field
4.1. Decomposition of Organic Pollutants
4.2. CO2 Reduction
4.3. Photocatalytic Oxidation of Heavy Metals
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fresno, F.; Portela, R.; Suárez, S.; Coronado, J.M. Photocatalytic materials: Recent achievements and near future trends. J. Mater. Chem. A 2014, 2, 2863–2884. [Google Scholar] [CrossRef]
- Park, H.; Park, Y.; Kim, W.; Choi, W. Surface modification of TiO2 photocatalyst for environmental applications. J. Photochem. Photobiol. 2013, 15, 1–20. [Google Scholar] [CrossRef]
- Xie, S.; Zhang, Q.; Liu, G.; Wang, Y. Photocatalytic and photoelectrocatalytic reduction of CO2 using heterogeneous catalysts with controlled nanostructures. Chem. Commun. 2016, 52, 35–59. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Zimbone, M.; Cacciato, G.; Spitaleri, L.; Egdell, R.G.; Grimaldi, M.G.; Gulino, A. Sb-Doped Titanium Oxide: A Rationale for Its Photocatalytic Activity for Environmental Remediation. ACS Omega 2018, 3, 11270–11277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiorenza, R.; Di Mauro, A.; Cantarella, M.; Gulino, A.; Spitaleri, L.; Privitera, V.; Impellizzeri, G. Molecularly imprinted N-doped TiO2 photocatalysts for the selective degradation of o-phenylphenol fungicide from water. Mater. Sci. Semicond. Process. 2020, 112, 105019. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, X.; Jia, Y.; Chen, X.; Han, H.; Li, C. Titanium Dioxide-Based Nanomaterials for Photocatalytic Fuel Generations. Chem. Rev. 2014, 114, 9987–10043. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Takata, T.; Domen, K. Particulate photocatalysts for overall water splitting. Nat. Rev. Mater. 2017, 2, 17050. [Google Scholar] [CrossRef]
- Ran, J.; Jaroniec, M.; Qiao, S.-Z. Cocatalysts in Semiconductor-based Photocatalytic CO2 Reduction: Achievements, Challenges, and Opportunities. Adv. Mater. 2018, 30, 1704649. [Google Scholar] [CrossRef]
- Zhang, Y.; He, S.; Guo, W.; Hu, Y.; Huang, J.; Mulcahy, J.R.; Wei, W.D. Surface-Plasmon-Driven Hot Electron Photochemistry. Chem. Rev. 2018, 118, 2927–2954. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K. Z-Scheme Water Splitting Using Two Different Semiconductor Photocatalysts. ACS Catal. 2013, 3, 1486–1503. [Google Scholar] [CrossRef]
- Dahl, M.; Liu, Y.; Yin, Y. Composite Titanium Dioxide Nanomaterials. Chem. Rev. 2014, 114, 9853–9889. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.V.; Tran, N.H.T.; Hwang, H.S.; Chang, M. Development strategies of conducting polymer-based electrochemical biosensors for virus biomarkers: Potential for rapid COVID-19 detection. Biosens. Bioelectron. 2021, 182, 113192. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Shi, G. Conducting Polymer-Based Catalysts. J. Am. Chem. Soc. 2016, 138, 2868–2876. [Google Scholar] [CrossRef]
- Ognibene, G.; Gangemi, C.M.A.; Spitaleri, L.; Gulino, A.; Purrello, R.; Cicala, G.; Fragalà, M.E. Role of the surface composition of the polyethersulfone–TiiP–H2T4 fibers on lead removal: From electrostatic to coordinative binding. J. Mater. Sci. 2019, 54, 8023–8033. [Google Scholar] [CrossRef]
- Zang, L.; Qiu, J.; Yang, C.; Sakai, E. Preparation and application of conducting polymer/Ag/clay composite nanoparticles formed by in situ UV-induced dispersion polymerization. Sci. Rep. 2016, 6, 20470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gangopadhyay, R.; De, A. Conducting Polymer Nanocomposites: A Brief Overview. Chem. Mater. 2000, 12, 608–622. [Google Scholar] [CrossRef]
- Jana, B.; Bhattacharyya, S.; Patra, A. Conjugated polymer P3HT–Au hybrid nanostructures for enhancing photocatalytic activity. Phys. Chem. Chem. Phys. 2015, 17, 15392–15399. [Google Scholar] [CrossRef]
- Xu, S.; Gu, L.; Wu, K.; Yang, H.; Song, Y.; Jiang, L.; Dan, Y. The influence of the oxidation degree of poly(3-hexylthiophene) on the photocatalytic activity of poly(3-hexylthiophene)/TiO2 composites. Sol. Energy Mater. Sol. Cells 2012, 96, 286–291. [Google Scholar] [CrossRef]
- Hidalgo, D.; Bocchini, S.; Fontana, M.; Saracco, G.; Hernández, S. Green and low-cost synthesis of PANI–TiO2 nanocomposite mesoporous films for photoelectrochemical water splitting. RSC Adv. 2015, 5, 49429–49438. [Google Scholar] [CrossRef] [Green Version]
- Sambaza, S.S.; Maity, A.; Pillay, K. Polyaniline-Coated TiO2 Nanorods for Photocatalytic Degradation of Bisphenol A in Water. ACS Omega 2020, 5, 29642–29656. [Google Scholar] [CrossRef]
- Chen, X.; Li, H.; Wu, H.; Wu, Y.; Shang, Y.; Pan, J.; Xiong, X. Fabrication of TiO2@PANI nanobelts with the enhanced absorption and photocatalytic performance under visible light. Mater. Lett. 2016, 172, 52–55. [Google Scholar] [CrossRef]
- Li, C.; Zhou, T.; Zhu, T.; Li, X. Enhanced visible light photocatalytic activity of polyaniline–crystalline TiO2–halloysite composite nanotubes by tuning the acid dopant in the preparation. RSC Adv. 2015, 5, 98482–98491. [Google Scholar] [CrossRef]
- Zhang, H.; Zong, R.; Zhu, Y. Photocorrosion Inhibition and Photoactivity Enhancement for Zinc Oxide via Hybridization with Monolayer Polyaniline. J. Phys. Chem. C 2009, 113, 4605–4611. [Google Scholar] [CrossRef]
- Gilja, V.; Vrban, I.; Mandić, V.; Žic, M.; Hrnjak-Murgić, Z. Preparation of a PANI/ZnO Composite for Efficient Photocatalytic Degradation of Acid Blue. Polymers 2018, 10, 940. [Google Scholar] [CrossRef] [Green Version]
- Alves, F.H.d.O.; Araújo, O.A.; de Oliveira, A.C.; Garg, V.K. Preparation and characterization of PAni(CA)/Magnetic iron oxide hybrids and evaluation in adsorption/photodegradation of blue methylene dye. Surf. Interfaces 2021, 23, 100954. [Google Scholar] [CrossRef]
- Rajaji, U.; Eva Gnana Dhana Rani, S.; Chen, S.-M.; Rajakumar, K.; Govindasamy, M.; Alzahrani, F.M.; Alsaiari, N.S.; Ouladsmane, M.; Sharmila Lydia, I. Synergistic photocatalytic activity of SnO2/PANI nanocomposite for the removal of direct blue 15 under UV light irradiation. Ceram. Int. 2021. [Google Scholar] [CrossRef]
- Lv, M.; Yang, L.; Wang, X.; Cheng, X.; Song, Y.; Yin, Y.; Liu, H.; Han, Y.; Cao, K.; Ma, W.; et al. Visible-light photocatalytic capability and the mechanism investigation of a novel PANI/Sn3O4 p–n heterostructure. RSC Adv. 2019, 9, 40694–40707. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.N.; Jung, H.-R.; Lee, W.-J. Hollow cobalt ferrite–polyaniline nanofibers as magnetically separable visible-light photocatalyst for photodegradation of methyl orange. J. Photochem. Photobiol. A 2016, 321, 257–265. [Google Scholar] [CrossRef]
- Katančić, Z.; Chen, W.-T.; Waterhouse, G.I.N.; Kušić, H.; Lončarić Božić, A.; Hrnjak-Murgić, Z.; Travas-Sejdic, J. Solar-active photocatalysts based on TiO2 and conductive polymer PEDOT for the removal of bisphenol A. J. Photochem. Photobiol. A 2020, 396, 112546. [Google Scholar] [CrossRef]
- Dimitrijevic, N.M.; Tepavcevic, S.; Liu, Y.; Rajh, T.; Silver, S.C.; Tiede, D.M. Nanostructured TiO2/Polypyrrole for Visible Light Photocatalysis. J. Phys. Chem. C 2013, 117, 15540–15544. [Google Scholar] [CrossRef]
- Gao, F.; Hou, X.; Wang, A.; Chu, G.; Wu, W.; Chen, J.; Zou, H. Preparation of polypyrrole/TiO2 nanocomposites with enhanced photocatalytic performance. Particuology 2016, 26, 73–78. [Google Scholar] [CrossRef]
- Yan, B.; Wang, Y.; Jiang, X.; Liu, K.; Guo, L. Flexible Photocatalytic Composite Film of ZnO-Microrods/Polypyrrole. ACS Appl. Mater. Interfaces 2017, 9, 29113–29119. [Google Scholar] [CrossRef]
- Xiong, P.; Wang, L.; Sun, X.; Xu, B.; Wang, X. Ternary Titania–Cobalt Ferrite–Polyaniline Nanocomposite: A Magnetically Recyclable Hybrid for Adsorption and Photodegradation of Dyes under Visible Light. Ind. Eng. Chem. Res. 2013, 52, 10105–10113. [Google Scholar] [CrossRef]
- Li, J.; Xiao, Q.; Li, L.; Shen, J.; Hu, D. Novel ternary composites: Preparation, performance and application of ZnFe2O4/TiO2/polyaniline. Appl. Surf. Sci. 2015, 331, 108–114. [Google Scholar] [CrossRef]
- Feng, J.; Hou, Y.; Wang, X.; Quan, W.; Zhang, J.; Wang, Y.; Li, L. In-depth study on adsorption and photocatalytic performance of novel reduced graphene oxide-ZnFe2O4-polyaniline composites. J. Alloys Compd. 2016, 681, 157–166. [Google Scholar] [CrossRef]
- Wu, H.; Lin, S.; Chen, C.; Liang, W.; Liu, X.; Yang, H. A new ZnO/rGO/polyaniline ternary nanocomposite as photocatalyst with improved photocatalytic activity. Mater. Res. Bull. 2016, 83, 434–441. [Google Scholar] [CrossRef]
- Mohammed, A.M.; Mohtar, S.S.; Aziz, F.; Aziz, M.; Ul-Hamid, A. Cu2O/ZnO-PANI ternary nanocomposite as an efficient photocatalyst for the photodegradation of Congo Red dye. J. Environ. Chem. Eng. 2021, 9, 105065. [Google Scholar] [CrossRef]
- Kumar, R.; Ansari, M.O.; Parveen, N.; Oves, M.; Barakat, M.A.; Alshahri, A.; Khan, M.Y.; Cho, M.H. Facile route to a conducting ternary polyaniline@TiO2/GN nanocomposite for environmentally benign applications: Photocatalytic degradation of pollutants and biological activity. RSC Adv. 2016, 6, 111308–111317. [Google Scholar] [CrossRef]
- Park, Y.; Numan, A.; Ponomarev, N.; Iqbal, J.; Khalid, M. Enhanced photocatalytic performance of PANI-rGO-MnO2 ternary composite for degradation of organic contaminants under visible light. J. Environ. Chem. Eng. 2021, 9, 106006. [Google Scholar] [CrossRef]
- Alenizi, M.A.; Kumar, R.; Aslam, M.; Alseroury, F.A.; Barakat, M.A. Construction of a ternary g-C3N4/TiO2@polyaniline nanocomposite for the enhanced photocatalytic activity under solar light. Sci. Rep. 2019, 9, 12091. [Google Scholar] [CrossRef]
- Riaz, U.; Ashraf, S.M.; Kashyap, J. Enhancement of photocatalytic properties of transitional metal oxides using conducting polymers: A mini review. Mater. Res. Bull. 2015, 71, 75–90. [Google Scholar] [CrossRef]
- Wang, Q.; Hui, J.; Li, J.; Cai, Y.; Yin, S.; Wang, F.; Su, B. Photodegradation of methyl orange with PANI-modified BiOCl photocatalyst under visible light irradiation. Appl. Surf. Sci. 2013, 283, 577–583. [Google Scholar] [CrossRef]
- Lee, K.; Cho, S.; Heum Park, S.; Heeger, A.J.; Lee, C.-W.; Lee, S.-H. Metallic transport in polyaniline. Nature 2006, 441, 65–68. [Google Scholar] [CrossRef]
- Nguyen, T.N.; Tran, V.V.; Bui, V.K.H.; Kim, M.; Park, D.; Hur, J.; Kim, I.T.; Lee, H.U.; Ko, S.; Lee, Y.-C. A Novel Photocatalyst Composite of Magnesium Aminoclay and TiO2 Immobilized into Activated Carbon Fiber (ACF) Matrix for Pollutant Removal. J. Nanosci. Nanotech. 2020, 20, 6844–6849. [Google Scholar] [CrossRef]
- Bui, V.K.H.; Tran, V.V.; Moon, J.-Y.; Park, D.; Lee, Y.-C. Titanium Dioxide Microscale and Macroscale Structures: A Mini-Review. Nanomaterials 2020, 10, 1190. [Google Scholar] [CrossRef]
- Yadav, A.; Kumar, H.; Sharma, R.; Kumari, R. Influence of polyaniline on the photocatalytic properties of metal nanocomposites: A review. Colloids Interface Sci. Commun. 2021, 40, 100339. [Google Scholar] [CrossRef]
- Gilja, V.; Novaković, K.; Travas-Sejdic, J.; Hrnjak-Murgić, Z.; Kraljić Roković, M.; Žic, M. Stability and Synergistic Effect of Polyaniline/TiO2 Photocatalysts in Degradation of Azo Dye in Wastewater. Nanomaterials 2017, 7, 412. [Google Scholar] [CrossRef] [Green Version]
- Mirzaeifard, Z.; Shariatinia, Z.; Jourshabani, M.; Rezaei Darvishi, S.M. ZnO Photocatalyst Revisited: Effective Photocatalytic Degradation of Emerging Contaminants Using S-Doped ZnO Nanoparticles under Visible Light Radiation. Ind. Eng. Chem. Res. 2020, 59, 15894–15911. [Google Scholar] [CrossRef]
- Guo, M.Y.; Ng, A.M.C.; Liu, F.; Djurišić, A.B.; Chan, W.K.; Su, H.; Wong, K.S. Effect of Native Defects on Photocatalytic Properties of ZnO. J. Phys. Chem. C 2011, 115, 11095–11101. [Google Scholar] [CrossRef]
- Pei, Z.; Ding, L.; Hu, J.; Weng, S.; Zheng, Z.; Huang, M.; Liu, P. Defect and its dominance in ZnO films: A new insight into the role of defect over photocatalytic activity. Appl. Catal. B 2013, 142–143, 736–743. [Google Scholar] [CrossRef]
- Pei, Z.; Ding, L.; Lu, M.; Fan, Z.; Weng, S.; Hu, J.; Liu, P. Synergistic Effect in Polyaniline-Hybrid Defective ZnO with Enhanced Photocatalytic Activity and Stability. J. Phys. Chem. C 2014, 118, 9570–9577. [Google Scholar] [CrossRef]
- Yang, C.; Du, J.; Peng, Q.; Qiao, R.; Chen, W.; Xu, C.; Shuai, Z.; Gao, M. Polyaniline/Fe3O4 Nanoparticle Composite: Synthesis and Reaction Mechanism. J. Phys. Chem. B 2009, 113, 5052–5058. [Google Scholar] [CrossRef] [PubMed]
- Xuan, S.; Wang, Y.-X.J.; Leung, K.C.-F.; Shu, K. Synthesis of Fe3O4@Polyaniline Core/Shell Microspheres with Well-Defined Blackberry-Like Morphology. J. Phys. Chem. C 2008, 112, 18804–18809. [Google Scholar] [CrossRef]
- Luo, Q.; Wang, L.; Wang, D.; Yin, R.; Li, X.; An, J.; Yang, X. Preparation, characterization and visible-light photocatalytic performances of composite films prepared from polyvinyl chloride and SnO2 nanoparticles. J. Environ. Chem. Eng. 2015, 3, 622–629. [Google Scholar] [CrossRef]
- Babu, B.; Cho, M.; Byon, C.; Shim, J. Sunlight-driven photocatalytic activity of SnO2 QDs-g-C3N4 nanolayers. Mater. Lett. 2018, 212, 327–331. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, F.; Huang, Z.; Chen, H.; Zhuang, Z.; Pan, Z.; Long, J.; Gu, F. Photochemical fabrication of SnO2 dense layers on reduced graphene oxide sheets for application in photocatalytic degradation of p-Nitrophenol. Appl. Catal. B 2017, 215, 8–17. [Google Scholar] [CrossRef]
- Ma, H.; Li, C.; Yin, J.; Pu, X.; Zhang, D.; Su, C.; Wang, X.; Shao, X. Polyoxometalate enhances the photocatalytic performance of polyaniline/SnO2 composites. Mater. Lett. 2016, 168, 103–106. [Google Scholar] [CrossRef]
- Li, J.; Peng, T.; Zhang, Y.; Zhou, C.; Zhu, A. Polyaniline modified SnO2 nanoparticles for efficient photocatalytic reduction of aqueous Cr(VI) under visible light. Sep. Purif. Technol. 2018, 201, 120–129. [Google Scholar] [CrossRef]
- Kefeni, K.K.; Mamba, B.B. Photocatalytic application of spinel ferrite nanoparticles and nanocomposites in wastewater treatment: Review. Sustain. Mater. Technol. 2020, 23, e00140. [Google Scholar] [CrossRef]
- Suresh, R.; Rajendran, S.; Kumar, P.S.; Vo, D.-V.N.; Cornejo-Ponce, L. Recent advancements of spinel ferrite based binary nanocomposite photocatalysts in wastewater treatment. Chemosphere 2021, 274, 129734. [Google Scholar] [CrossRef]
- Rashad, M.M.; Mohamed, R.M.; Ibrahim, M.A.; Ismail, L.F.M.; Abdel-Aal, E.A. Magnetic and catalytic properties of cubic copper ferrite nanopowders synthesized from secondary resources. Adv. Powder Technol. 2012, 23, 315–323. [Google Scholar] [CrossRef]
- Li, X.; Lu, H.; Zhang, Y.; He, F. Efficient removal of organic pollutants from aqueous media using newly synthesized polypyrrole/CNTs-CoFe2O4 magnetic nanocomposites. Chem. Eng. J. 2017, 316, 893–902. [Google Scholar] [CrossRef]
- Laokul, P.; Arthan, S.; Maensiri, S.; Swatsitang, E. Magnetic and Optical Properties of CoFe2O4 Nanoparticles Synthesized by Reverse Micelle Microemulsion Method. J. Supercond. Nov. Magn. 2015, 28, 2483–2489. [Google Scholar] [CrossRef]
- Ghosh, S.; Kouame, N.A.; Remita, S.; Ramos, L.; Goubard, F.; Aubert, P.-H.; Dazzi, A.; Deniset-Besseau, A.; Remita, H. Visible-light active conducting polymer nanostructures with superior photocatalytic activity. Sci. Rep. 2015, 5, 18002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Neal Tugaoen, H.; Garcia-Segura, S.; Hristovski, K.; Westerhoff, P. Compact light-emitting diode optical fiber immobilized TiO2 reactor for photocatalytic water treatment. Sci. Total Environ. 2018, 613–614, 1331–1338. [Google Scholar] [CrossRef]
- Liu, J.; McCarthy, D.L.; Tong, L.; Cowan, M.J.; Kinsley, J.M.; Sonnenberg, L.; Skorenko, K.H.; Boyer, S.M.; DeCoste, J.B.; Bernier, W.E.; et al. Poly(3,4-ethylenedioxythiophene) (PEDOT) infused TiO2 nanofibers: The role of hole transport layer in photocatalytic degradation of phenazopyridine as a pharmaceutical contaminant. RSC Adv. 2016, 6, 113884–113892. [Google Scholar] [CrossRef]
- Abdiryim, T.; Ali, A.; Jamal, R.; Osman, Y.; Zhang, Y. A facile solid-state heating method for preparation of poly(3,4-ethelenedioxythiophene)/ZnO nanocomposite and photocatalytic activity. Nanoscale Res. Lett. 2014, 9, 89. [Google Scholar] [CrossRef] [Green Version]
- Yan, H.; Zhang, L.; Shen, J.; Chen, Z.; Shi, G.; Zhang, B. Synthesis, property and field-emission behaviour of amorphous polypyrrole nanowires. Nanotechnology 2006, 17, 3446–3450. [Google Scholar] [CrossRef]
- Ferreira, C.A.; Domenech, S.C.; Lacaze, P.C. Synthesis and characterization of polypyrrole/TiO2 composites on mild steel. J. Appl. Electrochem. 2001, 31, 49–56. [Google Scholar] [CrossRef]
- Tai, H.; Jiang, Y.; Xie, G.; Yu, J.; Zhao, M. Self-assembly of TiO2/polypyrrole nanocomposite ultrathin films and application for an NH3 gas sensor. Int. J. Environ. Anal. Chem. 2007, 87, 539–551. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, C.; Wang, X.; Xu, H.; Wang, H.; Zhao, X.; Feng, J.; Yan, W.; Ren, Z. Preparation of PPy/TiO2 core-shell nanorods film and its photocathodic protection for 304 stainless steel under visible light. Mater. Res. Bull. 2020, 124, 110751. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Y.; Poyraz, S.; Zhang, X. Green-nano approach to nanostructured polypyrrole. Chem. Commun. 2011, 47, 4421–4423. [Google Scholar] [CrossRef]
- Ahmad, N.; Sultana, S.; Faisal, S.M.; Ahmed, A.; Sabir, S.; Khan, M.Z. Zinc oxide-decorated polypyrrole/chitosan bionanocomposites with enhanced photocatalytic, antibacterial and anticancer performance. RSC Adv. 2019, 9, 41135–41150. [Google Scholar] [CrossRef] [Green Version]
- Chougule, M.A.; Sen, S.; Patil, V.B. Facile and efficient route for preparation of polypyrrole-ZnO nanocomposites: Microstructural, optical, and charge transport properties. J. Appl. Polym. Sci. 2012, 125, E541–E547. [Google Scholar] [CrossRef]
- Yang, Y.; Wen, J.; Wei, J.; Xiong, R.; Shi, J.; Pan, C. Polypyrrole-Decorated Ag-TiO2 Nanofibers Exhibiting Enhanced Photocatalytic Activity under Visible-Light Illumination. ACS Appl. Mater. Interfaces 2013, 5, 6201–6207. [Google Scholar] [CrossRef]
- Wang, B.; Li, C.; Pang, J.; Qing, X.; Zhai, J.; Li, Q. Novel polypyrrole-sensitized hollow TiO2/fly ash cenospheres: Synthesis, characterization, and photocatalytic ability under visible light. Appl. Surf. Sci. 2012, 258, 9989–9996. [Google Scholar] [CrossRef]
- Pruna, A.; Shao, Q.; Kamruzzaman, M.; Li, Y.Y.; Zapien, J.A.; Pullini, D.; Busquets Mataix, D.; Ruotolo, A. Effect of ZnO core electrodeposition conditions on electrochemical and photocatalytic properties of polypyrrole-graphene oxide shelled nanoarrays. Appl. Surf. Sci. 2017, 392, 801–809. [Google Scholar] [CrossRef]
- Ong, W.L.; Low, Q.X.; Huang, W.; van Kan, J.A.; Ho, G.W. Patterned growth of vertically-aligned ZnO nanorods on a flexible platform for feasible transparent and conformable electronics applications. J. Mater. Chem. 2012, 22, 8518–8524. [Google Scholar] [CrossRef]
- Silvestri, S.; Ferreira, C.D.; Oliveira, V.; Varejão, J.M.T.B.; Labrincha, J.A.; Tobaldi, D.M. Synthesis of PPy-ZnO composite used as photocatalyst for the degradation of diclofenac under simulated solar irradiation. J. Photochem. Photobiol. A 2019, 375, 261–269. [Google Scholar] [CrossRef]
- Balakumar, V.; Sekar, K.; Chuaicham, C.; Manivannan, R.; Sasaki, K. Synergistic ternary porous CN–PPy–MMt nanocomposite for efficient photocatalytic metronidazole mineralization: Performance, mechanism, and pathways. Environ. Sci. Nano 2021, 8, 2261–2276. [Google Scholar] [CrossRef]
- Ali, H. Facile synthesis of mesoporous TiO2-CdS-polyaniline ternary system with improved optical properties. Mater. Res. Express 2019, 6, 115529. [Google Scholar] [CrossRef]
- Meng, S.; Zhang, J.; Chen, S.; Zhang, S.; Huang, W. Perspective on construction of heterojunction photocatalysts and the complete utilization of photogenerated charge carriers. Appl. Surf. Sci. 2019, 476, 982–992. [Google Scholar] [CrossRef]
- Shylesh, S.; Schünemann, V.; Thiel, W.R. Magnetically Separable Nanocatalysts: Bridges between Homogeneous and Heterogeneous Catalysis. Angew. Chem. Int. Ed. 2010, 49, 3428–3459. [Google Scholar] [CrossRef]
- Zhang, Q.; Fan, W.; Gao, L. Anatase TiO2 nanoparticles immobilized on ZnO tetrapods as a highly efficient and easily recyclable photocatalyst. Appl. Catal. B 2007, 76, 168–173. [Google Scholar] [CrossRef]
- Zhou, M.; Yu, J.; Liu, S.; Zhai, P.; Jiang, L. Effects of calcination temperatures on photocatalytic activity of SnO2/TiO2 composite films prepared by an EPD method. J. Hazard. Mater. 2008, 154, 1141–1148. [Google Scholar] [CrossRef]
- Berger, C.; Song, Z.; Li, X.; Wu, X.; Brown, N.; Naud, C.; Mayou, D.; Li, T.; Hass, J.; Marchenkov, A.N.; et al. Electronic Confinement and Coherence in Patterned Epitaxial Graphene. Science 2006, 312, 1191–1196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, S.; Ding, X.; Guo, T.; Yue, X.; Han, X.; Sun, J. Self-assembled hollow sphere shaped Bi2WO6/RGO composites for efficient sunlight-driven photocatalytic degradation of organic pollutants. Chem. Eng. J. 2017, 316, 778–789. [Google Scholar] [CrossRef]
- Akhavan, O. Graphene Nanomesh by ZnO Nanorod Photocatalysts. ACS Nano 2010, 4, 4174–4180. [Google Scholar] [CrossRef]
- Jiang, G.; Lin, Z.; Chen, C.; Zhu, L.; Chang, Q.; Wang, N.; Wei, W.; Tang, H. TiO2 nanoparticles assembled on graphene oxide nanosheets with high photocatalytic activity for removal of pollutants. Carbon 2011, 49, 2693–2701. [Google Scholar] [CrossRef]
- Wang, J.; Tsuzuki, T.; Tang, B.; Hou, X.; Sun, L.; Wang, X. Reduced Graphene Oxide/ZnO Composite: Reusable Adsorbent for Pollutant Management. ACS Appl. Mater. Interfaces 2012, 4, 3084–3090. [Google Scholar] [CrossRef]
- Williams, G.; Seger, B.; Kamat, P.V. TiO2-Graphene Nanocomposites. UV-Assisted Photocatalytic Reduction of Graphene Oxide. ACS Nano 2008, 2, 1487–1491. [Google Scholar] [CrossRef]
- Xie, X.; Kretschmer, K.; Wang, G. Advances in graphene-based semiconductor photocatalysts for solar energy conversion: Fundamentals and materials engineering. Nanoscale 2015, 7, 13278–13292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, C.; Zhang, N.; Xu, Y.-J. Structural diversity of graphene materials and their multifarious roles in heterogeneous photocatalysis. Nano Today 2016, 11, 351–372. [Google Scholar] [CrossRef]
- Ameen, S.; Seo, H.-K.; Shaheer Akhtar, M.; Shin, H.S. Novel graphene/polyaniline nanocomposites and its photocatalytic activity toward the degradation of rose Bengal dye. Chem. Eng. J. 2012, 210, 220–228. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, Z.; Xu, L.; Wang, H.; Fu, N. Preparation of reduced graphene oxide/meso-TiO2/AuNPs ternary composites and their visible-light-induced photocatalytic degradation n of methylene blue. Appl. Surf. Sci. 2016, 369, 576–583. [Google Scholar] [CrossRef]
- Miao, J.; Xie, A.; Li, S.; Huang, F.; Cao, J.; Shen, Y. A novel reducing graphene/polyaniline/cuprous oxide composite hydrogel with unexpected photocatalytic activity for the degradation of Congo red. Appl. Surf. Sci. 2016, 360, 594–600. [Google Scholar] [CrossRef]
- Pandiselvi, K.; Fang, H.; Huang, X.; Wang, J.; Xu, X.; Li, T. Constructing a novel carbon nitride/polyaniline/ZnO ternary heterostructure with enhanced photocatalytic performance using exfoliated carbon nitride nanosheets as supports. J. Hazard. Mater. 2016, 314, 67–77. [Google Scholar] [CrossRef]
- Liras, M.; Barawi, M.; de la Peña O’Shea, V.A. Hybrid materials based on conjugated polymers and inorganic semiconductors as photocatalysts: From environmental to energy applications. Chem. Soc. Rev. 2019, 48, 5454–5487. [Google Scholar] [CrossRef]
- Deng, X.; Chen, Y.; Wen, J.; Xu, Y.; Zhu, J.; Bian, Z. Polyaniline-TiO2 composite photocatalysts for light-driven hexavalent chromium ions reduction. Sci. Bull. 2020, 65, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Han, Y.; Xu, Y.; Meng, H.; Xu, J.; Wu, J.; Xu, Y.; Zhang, X. Fabrication of polyaniline sensitized grey-TiO2 nanocomposites and enhanced photocatalytic activity. Sep. Purif. Technol. 2017, 184, 248–256. [Google Scholar] [CrossRef]
- Ma, J.; Dai, J.; Duan, Y.; Zhang, J.; Qiang, L.; Xue, J. Fabrication of PANI-TiO2/rGO hybrid composites for enhanced photocatalysis of pollutant removal and hydrogen production. Renew. Energy 2020, 156, 1008–1018. [Google Scholar] [CrossRef]
- Wu, J.; Feng, Y.; Logan, B.E.; Dai, C.; Han, X.; Li, D.; Liu, J. Preparation of Al–O-Linked Porous-g-C3N4/TiO2-Nanotube Z-Scheme Composites for Efficient Photocatalytic CO2 Conversion and 2,4-Dichlorophenol Decomposition and Mechanism. ACS Sustain. Chem. Eng. 2019, 7, 15289–15296. [Google Scholar] [CrossRef]
- Ding, Y.; Zhu, L.; Wang, N.; Tang, H. Sulfate radicals induced degradation of tetrabromobisphenol A with nanoscaled magnetic CuFe2O4 as a heterogeneous catalyst of peroxymonosulfate. Appl. Catal. B 2013, 129, 153–162. [Google Scholar] [CrossRef]
- Guan, Y.-H.; Ma, J.; Ren, Y.-M.; Liu, Y.-L.; Xiao, J.-Y.; Lin, L.-q.; Zhang, C. Efficient degradation of atrazine by magnetic porous copper ferrite catalyzed peroxymonosulfate oxidation via the formation of hydroxyl and sulfate radicals. Water Res. 2013, 47, 5431–5438. [Google Scholar] [CrossRef] [PubMed]
- Nath, B.K.; Chaliha, C.; Kalita, E.; Kalita, M.C. Synthesis and characterization of ZnO:CeO2:nanocellulose:PANI bionanocomposite. A bimodal agent for arsenic adsorption and antibacterial action. Carbohydr. Polym. 2016, 148, 397–405. [Google Scholar] [CrossRef]
- Faisal, M.; Harraz, F.A.; Ismail, A.A.; Alsaiari, M.A.; Al-Sayari, S.A.; Al-Assiri, M.S. Novel synthesis of Polyaniline/SrSnO3 nanocomposites with enhanced photocatalytic activity. Ceram. Int. 2019, 45, 20484–20492. [Google Scholar] [CrossRef]
- Kharazi, P.; Rahimi, R.; Rabbani, M. Copper ferrite-polyaniline nanocomposite: Structural, thermal, magnetic and dye adsorption properties. Solid State Sci. 2019, 93, 95–100. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, Y.; Xiao, J.; Zhan, Y. Preparation of magnetic core-shell Fe3O4@polyaniline composite material and its application in adsorption and removal of tetrabromobisphenol A and decabromodiphenyl ether. Ecotoxicol. Environ. Saf. 2019, 183, 109471. [Google Scholar] [CrossRef]
- Hayashi, H.; Lightcap, I.V.; Tsujimoto, M.; Takano, M.; Umeyama, T.; Kamat, P.V.; Imahori, H. Electron Transfer Cascade by Organic/Inorganic Ternary Composites of Porphyrin, Zinc Oxide Nanoparticles, and Reduced Graphene Oxide on a Tin Oxide Electrode that Exhibits Efficient Photocurrent Generation. J. Am. Chem. Soc. 2011, 133, 7684–7687. [Google Scholar] [CrossRef]
- Iwase, A.; Ng, Y.H.; Ishiguro, Y.; Kudo, A.; Amal, R. Reduced Graphene Oxide as a Solid-State Electron Mediator in Z-Scheme Photocatalytic Water Splitting under Visible Light. J. Am. Chem. Soc. 2011, 133, 11054–11057. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhu, Y. Significant Visible Photoactivity and Antiphotocorrosion Performance of CdS Photocatalysts after Monolayer Polyaniline Hybridization. J. Phys. Chem. C 2010, 114, 5822–5826. [Google Scholar] [CrossRef]
- Lin, Y.; Li, D.; Hu, J.; Xiao, G.; Wang, J.; Li, W.; Fu, X. Highly Efficient Photocatalytic Degradation of Organic Pollutants by PANI-Modified TiO2 Composite. J. Phys. Chem. C 2012, 116, 5764–5772. [Google Scholar] [CrossRef]
- Mikkelsen, M.; Jørgensen, M.; Krebs, F.C. The teraton challenge. A review of fixation and transformation of carbon dioxide. Energy Environ. Sci. 2010, 3, 43–81. [Google Scholar] [CrossRef]
- Mao, J.; Li, K.; Peng, T. Recent advances in the photocatalytic CO2 reduction over semiconductors. Catal. Sci. Technol. 2013, 3, 2481–2498. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Navalon, S.; Corma, A.; Garcia, H. Photocatalytic CO2 reduction by TiO2 and related titanium containing solids. Energy Environ. Sci. 2012, 5, 9217–9233. [Google Scholar] [CrossRef]
- Wang, S.; Han, X.; Zhang, Y.; Tian, N.; Ma, T.; Huang, H. Inside-and-Out Semiconductor Engineering for CO2 Photoreduction: From Recent Advances to New Trends. Small Struct. 2021, 2, 2000061. [Google Scholar] [CrossRef]
- Liu, G.; Xie, S.; Zhang, Q.; Tian, Z.; Wang, Y. Carbon dioxide-enhanced photosynthesis of methane and hydrogen from carbon dioxide and water over Pt-promoted polyaniline–TiO2 nanocomposites. Chem. Commun. 2015, 51, 13654–13657. [Google Scholar] [CrossRef]
- Indrakanti, V.P.; Kubicki, J.D.; Schobert, H.H. Photoinduced activation of CO2 on Ti-based heterogeneous catalysts: Current state, chemical physics-based insights and outlook. Energy Environ. Sci. 2009, 2, 745–758. [Google Scholar] [CrossRef]
- Roy, S.C.; Varghese, O.K.; Paulose, M.; Grimes, C.A. Toward Solar Fuels: Photocatalytic Conversion of Carbon Dioxide to Hydrocarbons. ACS Nano 2010, 4, 1259–1278. [Google Scholar] [CrossRef]
- Califano, M.; Zhou, Y. Inverse-designed semiconductor nanocatalysts for targeted CO2 reduction in water. Nanoscale 2021, 13, 10024–10034. [Google Scholar] [CrossRef]
- Fiorenza, R.; Bellardita, M.; Balsamo, S.A.; Spitaleri, L.; Gulino, A.; Condorelli, M.; D’Urso, L.; Scirè, S.; Palmisano, L. A solar photothermocatalytic approach for the CO2 conversion: Investigation of different synergisms on CoO-CuO/brookite TiO2-CeO2 catalysts. Chem. Eng. J. 2021, 428, 131249. [Google Scholar] [CrossRef]
- Kim, Y.; Choi, K.; Jung, J.; Park, S.; Kim, P.-G.; Park, J. Aquatic toxicity of acetaminophen, carbamazepine, cimetidine, diltiazem and six major sulfonamides, and their potential ecological risks in Korea. Environ. Int. 2007, 33, 370–375. [Google Scholar] [CrossRef]
- Van Tran, V.; Park, D.; Lee, Y.-C. Hydrogel applications for adsorption of contaminants in water and wastewater treatment. Environ. Sci. Pollut. Res. Int. 2018, 25, 24569–24599. [Google Scholar] [CrossRef]
- Zhu, J.; Zhu, Z.; Zhang, H.; Lu, H.; Zhang, W.; Qiu, Y.; Zhu, L.; Küppers, S. Calcined layered double hydroxides/reduced graphene oxide composites with improved photocatalytic degradation of paracetamol and efficient oxidation-adsorption of As(III). Appl. Catal. B 2018, 225, 550–562. [Google Scholar] [CrossRef]
- Nicomel, N.R.; Leus, K.; Folens, K.; Van Der Voort, P.; Du Laing, G. Technologies for Arsenic Removal from Water: Current Status and Future Perspectives. Int. J. Environ. Res. Public Health 2016, 13, 62. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Fang, W.; Wang, Y.; Xing, M.; Zhang, J. Gold-loaded graphene oxide/PDPB composites for the synchronous removal of Cr(VI) and phenol. Chinese J. Catal. 2018, 39, 8–15. [Google Scholar] [CrossRef]
- Liu, F.; Yu, J.; Tu, G.; Qu, L.; Xiao, J.; Liu, Y.; Wang, L.; Lei, J.; Zhang, J. Carbon nitride coupled Ti-SBA15 catalyst for visible-light-driven photocatalytic reduction of Cr (VI) and the synergistic oxidation of phenol. Appl. Catal. B 2017, 201, 1–11. [Google Scholar] [CrossRef]
- Yang, X.; Wang, X.; Liu, X.; Zhang, Y.; Song, W.; Shu, C.; Jiang, L.; Wang, C. Preparation of graphene-like iron oxide nanofilm/silica composite with enhanced adsorption and efficient photocatalytic properties. J. Mater. Chem. A 2013, 1, 8332–8337. [Google Scholar] [CrossRef]
- Mou, F.; Guan, J.; Xiao, Z.; Sun, Z.; Shi, W.; Fan, X.-a. Solvent-mediated synthesis of magnetic Fe2O3 chestnut-like amorphous-core/γ-phase-shell hierarchical nanostructures with strong As(v) removal capability. J. Mater. Chem. 2011, 21, 5414–5421. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, P.; Zhang, T.C.; Xiang, G.; Wang, X.; Pehkonen, S.; Yuan, S. A magnetic γ-Fe2O3@PANI@TiO2 core–shell nanocomposite for arsenic removal via a coupled visible-light-induced photocatalytic oxidation–adsorption process. Nanoscale Adv. 2020, 2, 2018–2024. [Google Scholar] [CrossRef] [Green Version]
- Vellaichamy, B.; Periakaruppan, P.; Nagulan, B. Reduction of Cr6+ from Wastewater Using a Novel in Situ-Synthesized PANI/MnO2/TiO2 Nanocomposite: Renewable, Selective, Stable, and Synergistic Catalysis. ACS Sustain. Chem. Eng. 2017, 5, 9313–9324. [Google Scholar] [CrossRef]
- Lin, C.J.; Wang, S.L.; Huang, P.M.; Tzou, Y.M.; Liu, J.C.; Chen, C.C.; Chen, J.H.; Lin, C. Chromate reduction by zero-valent Al metal as catalyzed by polyoxometalate. Water Res. 2009, 43, 5015–5022. [Google Scholar] [CrossRef]
- Guo, L.; Xiao, Y.; Wang, Y. Hexavalent Chromium-induced Alteration of Proteomic Landscape in Human Skin Fibroblast Cells. J. Proteome Res. 2013, 12, 3511–3518. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Jin, P.; Duan, S.; She, H.; Huang, J.; Wang, Q. In-situ incorporation of Copper(II) porphyrin functionalized zirconium MOF and TiO2 for efficient photocatalytic CO2 reduction. Sci. Bull. 2019, 64, 926–933. [Google Scholar] [CrossRef] [Green Version]
- Bui, V.K.H.; Park, D.; Tran, V.V.; Lee, G.-W.; Oh, S.Y.; Huh, Y.S.; Lee, Y.-C. One-Pot Synthesis of Magnesium Aminoclay-Titanium Dioxide Nanocomposites for Improved Photocatalytic Performance. J. Nanosci. Nanotech. 2018, 18, 6070–6074. [Google Scholar] [CrossRef]
- Qiu, B.; Xu, C.; Sun, D.; Yi, H.; Guo, J.; Zhang, X.; Qu, H.; Guerrero, M.; Wang, X.; Noel, N.; et al. Polyaniline Coated Ethyl Cellulose with Improved Hexavalent Chromium Removal. ACS Sustain. Chem. Eng. 2014, 2, 2070–2080. [Google Scholar] [CrossRef]
- Xiong, S.; Phua, S.L.; Dunn, B.S.; Ma, J.; Lu, X. Covalently Bonded Polyaniline−TiO2 Hybrids: A Facile Approach to Highly Stable Anodic Electrochromic Materials with Low Oxidation Potentials. Chem. Mater. 2010, 22, 255–260. [Google Scholar] [CrossRef]
- Ansari, M.O.; Khan, M.M.; Ansari, S.A.; Raju, K.; Lee, J.; Cho, M.H. Enhanced Thermal Stability under DC Electrical Conductivity Retention and Visible Light Activity of Ag/TiO2@Polyaniline Nanocomposite Film. ACS Appl. Mater. Interfaces 2014, 6, 8124–8133. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Bao, S.-J.; Li, C.M.; Cui, X.-Q.; Lu, Z.-S.; Guo, J. Nanostructured Polyaniline/Titanium Dioxide Composite Anode for Microbial Fuel Cells. ACS Nano 2008, 2, 113–119. [Google Scholar] [CrossRef] [PubMed]








| Composite | Band-Gap Energy (eV) | Photocatalytic Properties | Applications | Reference |
|---|---|---|---|---|
| Binary Composite of CPs and Metal Oxides | ||||
| Mesoporous PANI/TiO2 | - | Enhanced water oxidation efficiency under sunlight irradiation, reaching about two-fold higher photocurrent densities than pure TiO2 nanoparticles | Water splitting | [20] |
| PANI/TiO2 nanorods | 3.1 |
| Degradation of organic pollutants (Bisphenol A) | [21] |
| PANI nanobelt/TiO2 | 2.77 | The photocatalytic degradation rate of rhodamine B was 99% | Degradation of rhodamine B | [22] |
| PANI/TiO2 nanotubes | - | The photocatalytic activity can easily be tuned using a particular type and concentration of the acid dopant in the redoping process | Degradation of rhodamine B | [23] |
| PANI/ZnO | - |
| Degradation of methylene blue (MB) | [24] |
| PANI/ZnO | 2.13–2.22 | The composite photocatalysts’ activity was broadened into the Vis region | Degradation of acid blue | [25] |
| PANI/Fe3O4 | - | The adsorption process prevails in relation to photodegradation | Degradation of MB | [26] |
| PANI/SnO2 | 2.7 | Increased photocatalytic activity for visible light is due to its electrical conductivity and efficient charge separation | Degradation of direct blue 15 | [27] |
| PANI/Sn3O4 | 2.06 | Photocatalytic activity for visible light is 2.27 times higher than that of Sn3O4 alone | Degradation of rhodamine B | [28] |
| PANI/CoFe2O4 | - | Photocatalytic activity under visible-light irradiation with CoFe2O4/PANI was 80 times greater than for CoFe2O4 | Degradation of methyl orange (MO) | [29] |
| PEDOT/TiO2 | 3.01–3.05 | PEDOT infused TiO2 nanofiber, exhibits the highest degradation enhancement (125%) | Degradation of phenazopyridine | [30] |
| PPy/TiO2 | - | The photoactivity of the nanocomposite arises from the electron transfer from excited PPy to TiO2 nanoparticles and further across the nanocomposite interface | Degradation of MB | [31] |
| PPy/TiO2 | 3.08–3.11 | The photoactivity of nanocomposites increased by 41% compared with pure TiO2 | Degradation of RhB and CO2 | [32] |
| ZnO-microrods/PPy | 1.7 | Composite films achieve a much higher photocatalytic efficiency in comparison with pure ZnO-microrod arrays (a rate of 22%/min MB degradation) | Degradation of MB | [33] |
| Ternary Composites | ||||
| TiO2-CoFe2O4-PANI | - | The ternary TiO2-CoFe2O4-PANI composite shows a highly enhanced photocatalytic activity in the range of visible light, compared with the binary TiO2-CoFe2O4, CoFe2O4-PANI, or TiO2-PANI composites | Degradation of methyl orange | [34] |
| ZnFe2O4-TiO2-PANI | - | The decontaminating efficiency of composites on MO and RhB reached up to 98% | Degradation and adsorption of MO and RhB | [35] |
| rGO-ZnFe2O4-PANI | - | The photocatalytic activity still stays above 90% after five recycles | Degradation of RhB | [36] |
| ZnO/rGO/PANI | - | The photocatalyst shows an enhanced photocatalytic performance in the photodegradation of MO (almost 100%) | Degradation of methyl orange | [37] |
| Cu2O/ZnO-PANI | 2.68 | The ternary composite with Z-scheme heterojunction properties displayed outstanding adsorption properties, super-fast photocatalytic activities as well as enhanced stability | Degradation of congo red | [38] |
| PANI/TiO2/graphene | 2.1 | High photocatalytic activity is partly due to the sensitizing effect of PANI and the low recombination rate due to the graphene electron scavenging property | Degradation of MB | [39] |
| PANI-rGO-MnO2 | 1.92 | The ternary composite exhibited significantly enhanced catalytic and photocatalytic activity under visible-light irradiation within 2 h | Degradation of MB | [40] |
| g-C3N4/TiO2/PANI | 2.58 | Greatly enhanced photocatalytic degradation and high reusability | Degradation of congo red | [41] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, V.V.; Nu, T.T.V.; Jung, H.-R.; Chang, M. Advanced Photocatalysts Based on Conducting Polymer/Metal Oxide Composites for Environmental Applications. Polymers 2021, 13, 3031. https://doi.org/10.3390/polym13183031
Tran VV, Nu TTV, Jung H-R, Chang M. Advanced Photocatalysts Based on Conducting Polymer/Metal Oxide Composites for Environmental Applications. Polymers. 2021; 13(18):3031. https://doi.org/10.3390/polym13183031
Chicago/Turabian StyleTran, Vinh Van, Truong Thi Vu Nu, Hong-Ryun Jung, and Mincheol Chang. 2021. "Advanced Photocatalysts Based on Conducting Polymer/Metal Oxide Composites for Environmental Applications" Polymers 13, no. 18: 3031. https://doi.org/10.3390/polym13183031
APA StyleTran, V. V., Nu, T. T. V., Jung, H. -R., & Chang, M. (2021). Advanced Photocatalysts Based on Conducting Polymer/Metal Oxide Composites for Environmental Applications. Polymers, 13(18), 3031. https://doi.org/10.3390/polym13183031