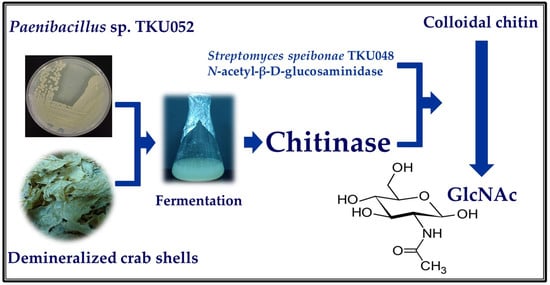
Production of Thermophilic Chitinase by Paenibacillus sp. TKU052 by Bioprocessing of Chitinous Fishery Wastes and Its Application in N-acetyl-D-glucosamine Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chitinase Assay
2.3. Chitinase Production and Purification
2.4. Effect of Temperature and pH
2.5. Effect of Various Ions and Chemicals
2.6. Substrate Specificity
2.7. The Pattern of Hydrolysis
2.8. Production of GlcNAc
2.9. HPLC Analysis
2.10. Proton Nuclear Magnetic Resonance (1H-NMR) Analysis
3. Results and Discussion
3.1. Chitinase Production
3.2. Chitinase Purification
3.3. Effect of Temperature and pH
3.4. Effect of Various Ions and Chemicals
3.5. Substrate Specificity
3.6. Hydrolysis Pattern
3.7. GlcNAc Production
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, C.; Shen, N.; Wu, J.; Jiang, M.; Shi, S.; Wang, J.; Wei, Y.; Yang, L. Cloning, expression and characterization of a chitinase from Paenibacillus chitinolyticus strain UMBR 0002. PeerJ 2020, 8, e8964. [Google Scholar] [CrossRef]
- Fu, X.; Yan, Q.; Yang, S.; Yang, X.; Guo, Y.; Jiang, Z. An acidic, thermostable exochitinase with β-N-acetylglucosaminidase activity from Paenibacillus barengoltzii converting chitin to N-acetyl glucosamine. Biotechnol. Biofuels 2014, 7, 174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadav, M.; Goswami, P.; Paritosh, K.; Kumar, M.; Pareek, N.; Vivekanand, V. Seafood waste: A source for preparation of commercially employable chitin/chitosan materials. Bioresources 2019, 6, 8–28. [Google Scholar] [CrossRef]
- Santos, V.P.; Marques, N.S.S.; Maia, P.C.S.V.; Lima, M.A.B.d.; Franco, L.d.O.; Campos-Takaki, G.M.d. Seafood waste as attractive source of chitin and chitosan production and their applications. Int. J. Mol. Sci. 2020, 21, 4290. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; He, Y.; Wei, G.; Zhou, J.; Dong, W.; Chen, K.; Quyang, P. Molecular characterization of a novel chitinase CmChi1 from Chitinolyticbacter meiyuanensis SYBC-H1 and its use in N-acetyl-d-glucosamine production. Biotechnol. Biofuels 2018, 11, 179. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.J.; Zhang, M.S.; Li, Z.W.; Lu, D.L.; Mao, H.H.; Zhu, M.J.; Li, J.Z.; Luo, X.C. One-step processing of shrimp shell waste with a chitinase fused to a carbohydrate-binding module. Green Chem. 2020, 22, 6862–6873. [Google Scholar] [CrossRef]
- Wang, C.H.; Doan, C.T.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.L. Reclamation of fishery processing waste: A mini-review. Molecules 2019, 24, 2234. [Google Scholar] [CrossRef] [Green Version]
- Doan, C.T.; Tran, T.N.; Nguyen, V.B.; Vo, T.P.K.; Nguyen, A.D.; Wang, S.L. Chitin extraction from shrimp waste by liquid fermentation using an alkaline protease-producing strain, Brevibacillus parabrevis. Int. J. Biol. Macromol. 2019, 131, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Doan, C.T.; Tran, T.N.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.-L. Utilization of seafood processing by-products for production of proteases by Paenibacillus sp. TKU052 and their application in biopeptides’ preparation. Mar. Drugs 2020, 18, 574. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Wang, S.L.; Nguyen, D.N.; Nguyen, A.D.; Nguyen, T.H.; Doan, M.D.; Ngo, V.A.; Doan, C.T.; Kuo, Y.H.; Nguyen, V.B. Bioprocessing of marine chitinous wastes for the production of bioactive prodigiosin. Molecules 2021, 26, 3138. [Google Scholar] [CrossRef]
- Das, S.; Roy, D.; Sen, R. Utilization of chitinaceous wastes for the production of chitinase. Adv. Food Nutr. Res. 2016, 78, 27–46. [Google Scholar] [PubMed]
- Tran, T.N.; Doan, C.T.; Nguyen, V.B.; Nguyen, A.D. The isolation of chitinase from Streptomyces thermocarboxydus and its application in the preparation of chitin oligomers. Res. Chem. Intermed. 2019, 45, 727–742. [Google Scholar] [CrossRef]
- Wang, S.L.; Yu, H.T.; Tsai, M.H.; Doan, C.T.; Nguyen, V.B.; Do, V.C.; Nguyen, A.D. Conversion of squid pens to chitosanases and dye adsorbents via Bacillus cereus. Res. Chem. Intermed. 2018, 44, 4903–4911. [Google Scholar] [CrossRef]
- Doan, C.T.; Tran, T.N.; Nguyen, M.T.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.L. Anti-α-glucosidase activity by a protease from Bacillus licheniformis. Molecules 2019, 24, 691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, T.W.; Tseng, S.C.; Wang, S.L. Production and characterization of antioxidant properties of exopolysaccharides from Paenibacillus mucilaginosus TKU032. Mar. Drugs 2016, 14, 40. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.H.; Nguyen, A.D.; Chen, Y.W.; Wang, S.L. Tyrosinase inhibitors and insecticidal materials produced by Burkholderia cepacia using squid pen as the sole carbon and nitrogen source. Res. Chem. Intermed. 2014, 40, 2249–2258. [Google Scholar] [CrossRef]
- Nguyen, V.B.; Wang, S.L. Reclamation of marine chitinous materials for the production of α-glucosidase inhibitors via microbial conversion. Mar. Drugs 2017, 15, 350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, T.W.; Wu, C.C.; Cheng, W.T.; Chen, Y.C.; Wang, C.L.; Wang, I.L.; Wang, S.L. Exopolysaccharides and antimicrobial biosurfactants produced by Paenibacillus macerans TKU029. Appl. Biochem. Biotechnol. 2014, 172, 933–950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shereena, E.K.; Nisha, M.K.; Gaayathiri Devi, E. Chitinase production by Aspergillus terreus from marine wastes and its efficacy in antifungal activity. Int. J. Adv. Res. 2020, 8, 1399–1404. [Google Scholar]
- Chen, J.K.; Shen, C.R.; Liu, C.L. N-acetylglucosamine: Production and applications. Mar. Drugs 2010, 8, 2493–2516. [Google Scholar] [CrossRef] [Green Version]
- Tran, T.N.; Doan, C.T.; Nguyen, M.T.; Nguyen, V.B.; Vo, T.P.K.; Nguyen, A.D.; Wang, S.L. An exochitinase with N-acetyl-β- glucosaminidase-like activity from shrimp head conversion by Streptomyces speibonae and its application in hydrolyzing β-chitin powder to produce N-acetyl-D-glucosamine. Polymers 2019, 11, 1600. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; Wang, D.; Liu, T.; Yang, Q. Production of N-Acetyl-d-glucosamine from mycelial waste by a combination of bacterial chitinases and an insect N-acetyl-D-glucosaminidase. J. Agric. Food Chem. 2016, 64, 6738–6744. [Google Scholar] [CrossRef]
- Salas-Ovilla, R.; Gálvez-López, D.; Vázquez-Ovando, A.; Salvador-Figueroa, M.; Rosas-Quijano, R. Isolation and identification of marine strains of Stenotrophomona maltophilia with high chitinolytic activity. PeerJ. 2019, 7, e6102. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya D, Nagpure A, Gupta RK: Bacterial chitinases: Properties and potential. Crit. Rev. Biotechnol. 2007, 27, 21–28. [CrossRef]
- Dahiya, N.; Tewari, R.; Hoondal, G.S. Biotechnological aspects of chitinolytic enzymes: A review. Appl. Microbiol. Biotechnol. 2006, 71, 773–782. [Google Scholar] [CrossRef]
- Ben Rebah, F.; Miled, N. Fish processing wastes for microbial enzyme production: A review. 3 Biotech 2013, 3, 255–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghorbel-Bellaaj, O.; Manni, L.; Jellouli, K.; Hmidet, N.; Nasri, M. Optimization of protease and chitinase production by Bacillus cereus SV1 on shrimp shell waste using statistical experimental design. Biochemical and molecular characterization of the chitinase. Ann. Microbiol. 2021, 62, 1255–1268. [Google Scholar] [CrossRef]
- Wang, S.L.; Li, J.Y.; Liang, T.W.; Hsieh, J.L.; Tseng, W.N. Conversion of shrimp shell by using Serratia sp. TKU017 fermentation for the production of enzymes and antioxidants. J. Microbiol. Biotechnol. 2010, 20, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Cheba, B.A.; Zaghloul, T.I.; El-Mahdy, A.R. Demineralized crab and shrimp shell powder: Cost effective medium for Bacillus Sp. R2 growth and chitinase production. Procedia Manuf. 2018, 22, 413–419. [Google Scholar] [CrossRef]
- Doan, C.T.; Tran, T.N.; Nguyen, V.B.; Tran, T.D.; Nguyen, A.D.; Wang, S.-L. Bioprocessing of squid pens waste into chitosanase by Paenibacillus sp. TKU047 and its application in low-molecular weight chitosan oligosaccharides production. Polymers 2020, 12, 1163. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.L.; Hsu, W.H.; Liang, T.W. Conversion of squid pen by Pseudomonas aeruginosa K187 fermentation for the production of N-acetyl chitooligosaccharides and biofertilizers. Carbohydr. Res. 2010, 345, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Tariq, A.L.; Sarath Chandran, R.; Reyaz, A.L. Extracellular chitinolytic enzyme producing Paenibacillus elgii TS33 isolated from shrimp shell waste. Int. J. Pharma. Res. Health Sci. 2017, 5, 2064–2069. [Google Scholar]
- Kim, Y.H.; Park, S.K.; Hur, J.Y.; Kim, Y.C. Purification and characterization of a major extracellular chitinase from a biocontrol bacterium, Paenibacillus elgii HOA73. Plant Pathol. J. 2017, 33, 318–328. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.H.; Doan, C.T.; Tran, T.N.; Nguyen, V.B.; Nguyen, A.D.; Wang, C.L.; Wang, S.L. Proteases production and chitin preparation from the liquid fermentation of chitinous fishery by-products by Paenibacillus elgii. Mar. Drugs 2021, 19, 477. [Google Scholar] [CrossRef]
- Doan, C.T.; Tran, T.N.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.L. Conversion of squid pens to chitosanases and proteases via Paenibacillus sp. TKU042. Mar. Drugs 2018, 16, 83. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, V.B.; Nguyen, D.N.; Nguyen, A.D.; Ngo, V.A.; Ton, T.Q.; Doan, C.T.; Pham, T.P.; Tran, T.P.H.; Wang, S.L. Utilization of crab waste for cost-effective bioproduction of prodigiosin. Mar. Drugs 2020, 18, 523. [Google Scholar] [CrossRef]
- Seo, D.J.; Lee, Y.S.; Kim, K.Y.; Jung, W.J. Antifungal activity of chitinase obtained from Paenibacillus ehimensis MA2012 against conidial of Collectotrichum gloeosporioides in vitro. Microb. Pathog. 2016, 96, 10–14. [Google Scholar] [CrossRef]
- Yahiaoui, M.; Laribi-Habchi, H.; Bouacem, K.; Asmani, K.L.; Mechri, S.; Harir, M.; Bendif, H.; Fertas, R.A.E.; Jaouadi, B. Purification and biochemical characterization of a new organic solvent-tolerant chitinase from Paenibacillus timonensis strain LK-DZ15 isolated from the Djurdjura Mountains in Kabylia, Algeria. Carbohydr. Res. 2019, 483, 107747. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Xu, P.; Zong, M.; Lou, W. Purification and characterization of alkaline chitinase from Paenibacillus pasadenensis CS0611. Chin. J. Catal. 2017, 38, 665–672. [Google Scholar] [CrossRef]
- Ueda, J.; Kurosawa, N. Characterization of an extracellular thermophilic chitinase from Paenibacillus thermoaerophilus strain TC22-2b isolated from compost. World J. Microbiol. Biotechnol. 2015, 31, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Mao, X.; Guo, N.; Zhao, L.; Cao, R.; Liu, Q. Discovery and characterization of a novel chitosanase from Paenibacillus dendritiformis by phylogeny-based enzymatic product specificity prediction. J. Agric. Food Chem. 2018, 66, 4645–4651. [Google Scholar] [CrossRef]
- Doan, C.T.; Tran, T.N.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.L. Reclamation of marine chitinous materials for chitosanase production via microbial conversion by Paenibacillus macerans. Mar. Drugs 2018, 16, 429. [Google Scholar] [CrossRef] [Green Version]
- Loni, P.P.; Patil, J.U.; Phugare, S.S.; Bajekal, S.S. Purification and characterization of alkaline chitinase from Paenibacillus pasadenensis NCIM 5434. J. Basic Microbiol. 2014, 54, 1080–1089. [Google Scholar] [CrossRef] [PubMed]
- Mathew, G.M.; Madhavan, A.; Arun, K.B.; Sindhu, R.; Binod, P.; Singhania, R.R.; Sukumaran, R.K.; Pandey, A. Thermophilic chitinases: Structural, functional and engineering attributes for industrial applications. Appl. Biochem. Biotechnol. 2021, 193, 142–164. [Google Scholar] [CrossRef] [PubMed]
- Doan, C.T.; Tran, T.N.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.L. Production of a thermostable chitosanase from shrimp heads via Paenibacillus mucilaginosus TKU032 conversion and its application in the preparation of bioactive chitosan oligosaccharides. Mar. Drugs 2019, 17, 217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zitouni, M.; Fortin, M.; Scheerle, R.K.; Letzel, T.; Matteau, D.; Rodrigue, S.; Brzezinski, R. Biochemical and molecular characterization of a thermostable chitosanase produced by the strain Paenibacillus sp. 1794 newly isolated from compost. Appl. Microbiol. Biotechnol. 2013, 97, 5801–5813. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, J.; Yan, Q.; Jiang, Z.; Yang, S. Biochemical characterization of a novel acidic chitinase with antifungal activity from Paenibacillus xylanexedens Z2-4. Int. J. Biol. Macromol. 2021, 182, 1528–1536. [Google Scholar] [CrossRef]
- Jung, W.J.; Kuk, J.K.; Kim, K.Y.; Kim, T.H.; Park, R.D. Purification and characterization of chitinase from Paenibacillus illinoisensis KJA-424. J. Microbiol. Biotechnol. 2005, 15, 274–280. [Google Scholar]
- Fu, X.; Yan, Q.; Wang, J.; Yang, S.; Jiang, Z. Purification and biochemical characterization of novel acidic chitinase from Paenibacillus barengoltzii. Int. J. Biol. Macromol. 2016, 91, 973–979. [Google Scholar] [CrossRef]
- Singh, A.K.; Mehta, G.; Chhatpar, H.S. Optimization of medium constituents for improved chitinase production by Paenibacillus sp. D1 using statistical approach. Lett. Appl. Microbiol. 2009, 49, 708–714. [Google Scholar] [CrossRef]
- Meena, S.; Gothwal, R.K.; Saxena, J.; Nehra, S.; Mohan, M.K.; Ghosh, P. Effect of metal ions and chemical compounds on chitinase produced by a newly isolated thermotolerant Paenibacillus sp. BISR-047 and its shelf-life. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 872–881. [Google Scholar]
- Alves, T.B.; de Oliveira Ornela, P.H.; de Oliveira, A.H.C.; Jorge, J.A.; Guimarães, L.H.S. Production and characterization of a thermostable antifungal chitinase secreted by the filamentous fungus Aspergillus niveus under submerged fermentation. 3 Biotech 2018, 8, 369. [Google Scholar] [CrossRef]
- Yang, S.; Fu, X.; Yan, Q.; Guo, Y.; Liu, Z.; Jiang, Z. Cloning, expression, purification and application of a novel chitinase from a thermophilic marine bacterium Paenibacillus barengoltzii. Food Chem. 2016, 192, 1041–1048. [Google Scholar] [CrossRef]
- Nguyen-Thi, N.; Doucet, N. Combining chitinase C and N-acetylhexosaminidase from Streptomyces coelicolor A3(2) provides an efficient way to synthesize N-acetylglucosamine from crystalline chitin. J. Biotechnol. 2016, 220, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Gao, C.; Wang, J.; Chen, K.; Quyang, P. An efficient enzymatic production of N-acetyl-D-glucosamine from crude chitin powders. Green Chem. 2016, 18, 2147–2154. [Google Scholar] [CrossRef]
- Krolicka, M.; Hinz, S.W.A.; Koetsier, M.J.; Eggink, G.; van den Broek, L.A.M.; Boeriu, C.G. β-N-Acetylglucosaminidase MthNAG from Myceliophthora thermophila C1, a thermostable enzyme for production of N-acetylglucosamine from chitin. Appl. Microbiol. Biotechnol. 2018, 102, 7441–7454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, S.; Dey, P.; Roy, D.; Maiti, M.K.; Sen, R. N-Acetyl-D-glucosamine production by a chitinase of marine fungal origin: A case study of potential industrial significance for valorization of waste chitins. Appl. Biochem. Biotechnol. 2019, 187, 407–423. [Google Scholar] [CrossRef]
- Jamialahmadi, K.; Behravan, J.; Fathi Najafi, M.; Tabatabaei Yazdi, M.; Shahverdi, A.R.; Faramarzi, M.A. Enzymatic production of N-Acetyl-D-glucosamine from chitin using crude enzyme preparation of Aeromonas sp. PTCC1691. Biotechnology 2011, 10, 292–297. [Google Scholar] [CrossRef] [Green Version]
- Cardozo, F.A.; Facchinatto, W.M.; Colnago, L.A.; Campana-Filho, S.P.; Pessoa, A. Bioproduction of N-acetyl-glucosamine from colloidal α-chitin using an enzyme cocktail produced by Aeromonas caviae CHZ306. World J. Microbiol. Biotechnol. 2019, 35, 114. [Google Scholar] [CrossRef]
- Kuk, J.H.; Jung, W.J.; Jo, G.H.; Kim, Y.C.; Kim, K.Y.; Park, R.D. Production of N-acetyl-beta-D-glucosamine from chitin by Aeromonas sp. GJ-18 crude enzyme. Appl. Microbiol. Biotechnol. 2005, 68, 384–389. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, Z.; Ma, J.; Ma, S.; Yan, Q.; Yang, S. Biochemical characterization and structural analysis of a β-N-acetylglucosaminidase from Paenibacillus barengoltzii for efficient production of N-acetyl-d-glucosamine. J. Agric. Food Chem. 2020, 68, 5648–5657. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.J.; Souleimanov, A.; Park, R.D.; Smith, D.L. Enzymatic production of N-acetyl chitooligosaccharides by crude enzyme derived from Paenibacillus illioisensis KJA-424. Carbohydr. Polym. 2007, 67, 256–259. [Google Scholar] [CrossRef]
- Lv, C.; Gu, T.; Xu, K.; Gu, J.; Li, L.; Liu, X.; Zhang, A.; Gao, S.; Li, W.; Zhao, G. Biochemical characterization of a β-N-acetylhexosaminidase from Streptomyces alfalfae and its application in the production of N-acetyl-D-glucosamine. J. Biosci. Bioeng. 2019, 128, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gao, K.; Secundo, F.; Mao, X. Biochemical characterization of two β-N-acetylglucosaminidases from Streptomyces violascens for efficient production of N-acetyl-d-glucosamine. Food Chem. 2021, 364, 130393. [Google Scholar] [CrossRef] [PubMed]









| Step | Total Protein (mg) | Total Activity (U) | Specific Activity (U/mg) | Recovery (%) | Purification (Fold) |
|---|---|---|---|---|---|
| Cultural supernatant | 2731.95 | 358.00 | 0.13 | 100.00 | 1.00 |
| (NH4)2SO4 precipitation | 395.94 | 164.40 | 0.42 | 45.92 | 3.17 |
| Macro-Prep High S | 39.67 | 43.56 | 1.10 | 12.17 | 8.38 |
| KW-802.5 | 5.63 | 14.36 | 2.55 | 4.01 | 19.47 |
| Chemical | Relative Activity (%) |
|---|---|
| None | 100.00 ± 7.23 |
| Fe2+ | 102.31 ± 3.85 |
| Fe3+ | 26.72 ± 4.12 |
| Ca2+ | 102.13 ± 6.37 |
| Ba2+ | 107.57 ± 3.12 |
| Mn2+ | 142.57 ± 5.02 |
| Mg2+ | 102.65 ± 2.18 |
| Cu2+ | 109.93 ± 6.82 |
| Zn2+ | 59.51 ± 4.30 |
| SDS | 28.84 ± 3.30 |
| Triton X-100 | 78.96 ± 7.48 |
| Tween 40 | 65.71 ± 11.06 |
| Tween 20 | 101.16 ±12.15 |
| EDTA | 108.69 ± 7.21 |
| 2-ME | 129.70 ± 5.45 |
| Substrate | Relative Activity (%) |
|---|---|
| CC | 100.00 ± 8.37 |
| α-chitin powder | 6.65 ± 0.48 |
| β-chitin powder | 16.20 ± 6.36 |
| 75% DDA chitosan | 8.64 ± 4.46 |
| 100% DDA chitosan | N.A. |
| Pectin | N.A. |
| Starch | N.A. |
| Xylan | N.A. |
| Carboxymethyl cellulose | N.A. |
| Gum arabic | N.A. |
| β-1,3-glucan | N.A. |
| Dextran | N.A |
| Enzyme | Substrate | Time Consumed | Yield (%) | Ref. |
|---|---|---|---|---|
| Paenibacillus sp. TKU052 chitinase and S. speibonae TKU048 N-acetyl-β-D-glucosaminidase | CC | 12–24 h | 94.35–98.60 | This study |
| S. speibonae TKU048 N-acetyl-β-D-glucosaminidase | β-chitin powder | 96 h | 73.64 | [20] |
| Serratia marcescens chitinases (SmChiA, SmChiB, and SmChiC) and Ostrinia furnacalis N-acetyl-d-glucosaminidase (OfHex1) | Asperillus niger mycelia powder | 24 h | 93 | [22] |
| Streptomyces coelicolor A3(2) chitinase C (ScChiC) and N-acetylhexosaminidase (ScHEX) | Crystalline chitin | 8 h | 90 | [54] |
| Chitinolyticbacter meiyuanensis SYBC-H1 chitinase (CmChi1) | CC | 24 h | 98 | [5] |
| C. meiyuanensis SYBC-H1 chitinase | Chitin powder | 4 days | near 100 | [55] |
| Myceliophthora thermophila C1 β-N-acetylglucosaminidase (MthNAG) and chitinase Chi1 | Swollen chitin | 24 h | 37.8 | [56] |
| Aspergillus terreus chitinase | Ground prawn shell | 5 days | 30 | [57] |
| Chitin flakes | 73 | |||
| Colloidal prawn shell | 80 | |||
| Swollen chitin | 92 | |||
| Aeromonas sp. PTCC1691 crude enzyme | CC | 24 h | 79 | [58] |
| Aeromonas caviae CHZ306 enzyme cocktail | CC | 6 h | 90 | [59] |
| Aeromonas sp. GJ-18 crude enzyme | Swollen chitin | 5–9 days | 83.0–94.9 | [60] |
| P. barengoltzii β-N-acetylglucosaminidase (PbNag39) and chitinase (PbChi70) | Powdery chitin | 24 h | 75.3 | [61] |
| CC | 97.0 | |||
| P. barengoltzii chitinase (PbChi74) and Rhizomucor miehei β-N-acetylglucosaminidase (RmNAG) | CC | 24 h | 92.6 | [2] |
| P. illinoisensis KJA-424 crude enzyme | Swollen chitin | 24 h | 62.2 | [62] |
| Streptomyces alfalae β-N-acetylhexosaminidase (SaHEX) and a commercial chitinase (SgCtn) | CC | 6 h | 93.7 | [63] |
| Streptomyces violascens β-N-acetylglucosaminidases (SvNag2557 and SvNag4755) | CC | 80.2 | [64] | |
| Ionic liquid pretreated chitin | 73.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doan, C.T.; Tran, T.N.; Wang, S.-L. Production of Thermophilic Chitinase by Paenibacillus sp. TKU052 by Bioprocessing of Chitinous Fishery Wastes and Its Application in N-acetyl-D-glucosamine Production. Polymers 2021, 13, 3048. https://doi.org/10.3390/polym13183048
Doan CT, Tran TN, Wang S-L. Production of Thermophilic Chitinase by Paenibacillus sp. TKU052 by Bioprocessing of Chitinous Fishery Wastes and Its Application in N-acetyl-D-glucosamine Production. Polymers. 2021; 13(18):3048. https://doi.org/10.3390/polym13183048
Chicago/Turabian StyleDoan, Chien Thang, Thi Ngoc Tran, and San-Lang Wang. 2021. "Production of Thermophilic Chitinase by Paenibacillus sp. TKU052 by Bioprocessing of Chitinous Fishery Wastes and Its Application in N-acetyl-D-glucosamine Production" Polymers 13, no. 18: 3048. https://doi.org/10.3390/polym13183048
APA StyleDoan, C. T., Tran, T. N., & Wang, S. -L. (2021). Production of Thermophilic Chitinase by Paenibacillus sp. TKU052 by Bioprocessing of Chitinous Fishery Wastes and Its Application in N-acetyl-D-glucosamine Production. Polymers, 13(18), 3048. https://doi.org/10.3390/polym13183048