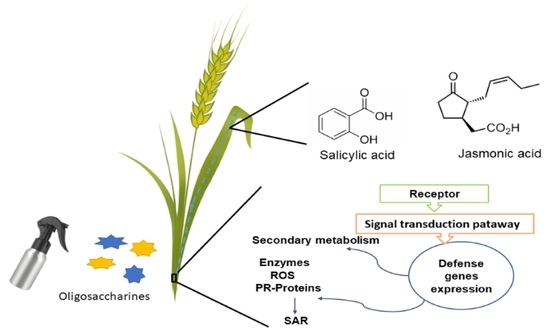
Oligosaccharins as Elicitors of Defense Responses in Wheat
Abstract
:1. Introduction
2. The Route of Plant Defense
3. Salicylic Acid: The Hormone of Plant Defense
4. Types of Elicitors
4.1. Eliciting Phytohormones
4.2. Eliciting Phytochemicals and Plant-Derived Compounds
4.3. Microorganism-Derived Elicitors
5. Elicitors and Their Effect on Wheat
6. Future Perspective and Limitations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- FAO Crop Prospects and Food Situation-Quarterly Global Report No. 4. Available online: http://www.fao.org/3/cb2334en/CB2334EN.pdf (accessed on 10 January 2021).
- Curtis, T.; Halford, N.G. Food Security: The Challenge of Increasing Wheat Yield and the Importance of Not Compromising Food Safety. Ann. Appl. Biol. 2014, 164, 354–372. [Google Scholar] [CrossRef] [Green Version]
- Buendía-Ayala, B.L. La Incidencia de Roya Amarilla y La Calidad Industrial Del Grano y La Masa En Trigo Harinero. Rev. Mex. Cienc. Agrícolas 2019, 10, 143–154. [Google Scholar]
- Zhang, P.; Li, X.; Gebrewahid, T.W.; Liu, H.; Xia, X.; He, Z.; Li, Z.; Liu, D. QTL Mapping of Adult-Plant Resistance to Leaf and Stripe Rust in Wheat Cross SW 8588/Thatcher Using the Wheat 55K SNP Array. Plant Dis. 2019, 103, 3041–3049. [Google Scholar] [CrossRef]
- Kolmer, J.A.; Herman, A.; Ordoñez, M.E.; German, S.; Morgounov, A.; Pretorius, Z.; Visser, B.; Anikster, Y.; Acevedo, M. Endemic and Panglobal Genetic Groups, and Divergence of Host-Associated Forms in Worldwide Collections of the Wheat Leaf Rust Fungus Puccinia triticina as Determined by Genotyping by Sequencing. Heredity 2020, 124, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Kirby, E.; Paulitz, T.; Murray, T.; Schroeder, K.; Chen, X. Disease management for wheat and barley. In Advances in Dryland Farming in the Inland Pacific Northwest; Yorgey, G., Kruger, C.E., Eds.; Washington State University Extension: Pullman, WA, USA, 2017; pp. 399–467. [Google Scholar]
- Chu, S.-H.; Liao, P.-H.; Chen, P.-J.; Goetz, A.K.; Dix, D.J. Toxicogenomic Effects Common to Triazole Antifungals and Conserved between Rats and Humans. Toxicol. Appl. Pharmacol. 2009, 238, 80–89. [Google Scholar] [CrossRef]
- Maurya, R.; Dubey, K.; Singh, D.; Jain, A.K.; Pandey, A.K. Effect of Difenoconazole Fungicide on Physiological Responses and Ultrastructural Modifications in Model Organism Tetrahymena Pyriformis. Ecotoxicol. Environ. Saf. 2019, 182, 109375. [Google Scholar] [CrossRef] [PubMed]
- Huerta Espino, J.; Rodríguez Contreras, M.; Rodríguez García, M.; Villaseñor Mir, H.E.; Leyva Mir, S.G.; Espitia Rangel, E. Genetic Variation of Resistence against Puccinia triticina E. in Durum Wheats from Oaxaca, México. Rev. Fitotec. Mex. 2011, 34, 35–41. [Google Scholar]
- Thakur, M.; Sohal, B.S. Role of Elicitors in Inducing Resistance in Plants against Pathogen Infection: A Review. ISRN Biochem. 2013, 2013, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Ramirez-Estrada, K.; Vidal-Limon, H.; Hidalgo, D.; Moyano, E.; Golenioswki, M.; Cusidó, R.; Palazon, J. Elicitation, an Effective Strategy for the Biotechnological Production of Bioactive High-Added Value Compounds in Plant Cell Factories. Molecules 2016, 21, 182. [Google Scholar] [CrossRef]
- Caffall, K.H.; Mohnen, D. The Structure, Function, and Biosynthesis of Plant Cell Wall Pectic Polysaccharides. Carbohydr. Res. 2009, 344, 1879–1900. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Zhao, M.; Guo, L.; Guo, X.; Zhao, D.; Batool, A.; Dong, B.; Xu, H.; Cui, S.; et al. Wheat FRIZZY PANICLE Activates VERNALIZATION1-A and HOMEOBOX4-A to Regulate Spike Development in Wheat. Plant Biotechnol. J. 2021, 19, 1141–1154. [Google Scholar] [CrossRef] [PubMed]
- Yadav, T.; Kumar, A.; Yadav, R.K.; Yadav, G.; Kumar, R.; Kushwaha, M. Salicylic Acid and Thiourea Mitigate the Salinity and Drought Stress on Physiological Traits Governing Yield in Pearl Millet- Wheat. Saudi J. Biol. Sci. 2020, 27, 2010–2017. [Google Scholar] [CrossRef]
- Gawlik-Dziki, U.; Dziki, D.; Nowak, R.; Świeca, M.; Olech, M.; Pietrzak, W. Influence of Sprouting and Elicitation on Phenolic Acids Profile and Antioxidant Activity of Wheat Seedlings. J. Cereal Sci. 2016, 70. [Google Scholar] [CrossRef]
- Arturo Enríquez-Guevara, E.; Aispuro-Hernández, E.; Martínez-Téllez, I. Oligosacarinas Derivadas de Pared Celular: Actividad Biológica y Participación En La Respuesta de Defensa de Plantas. Rev. Mex. Fitopalogía 2010, 28, 144–155. [Google Scholar]
- Zipfel, C. Early Molecular Events in PAMP-Triggered Immunity. Curr. Opin. Plant Biol. 2009, 12, 414–420. [Google Scholar] [CrossRef]
- Rosa, P.; Stigliano, E. Bringing PTI into the field. In Applied Plant Biotechnology for Improving Resistance to Biotic Stress; Elsevier: Amsterdam, The Netherlands, 2020; pp. 45–61. [Google Scholar]
- Zipfel, C. Plant Pattern-Recognition Receptors. Trends Immunol. 2014, 35, 345–351. [Google Scholar] [CrossRef]
- Molina, A.; Sánchez-Vallet, A.; Sánchez-Rodríguez, C. Inmunidad Innata En Plantas y Resistencia a Patógenos: Nuevos Conceptos y Potenciales Aplicaciones En Protección Vegetal. Phytoma 2007, 192, 43–46. [Google Scholar]
- Yang, C.; Yu, Y.; Huang, J.; Meng, F.; Pang, J.; Zhao, Q.; Islam, M.A.; Xu, N.; Tian, Y.; Liu, J. Binding of the Magnaporthe Oryzae Chitinase MoChia1 by a Rice Tetratricopeptide Repeat Protein Allows Free Chitin to Trigger Immune Responses. Plant Cell 2019, 31, 172–188. [Google Scholar] [CrossRef] [Green Version]
- Feechan, A.; Turnbull, D.; Stevens, L.J.; Engelhardt, S.; Birch, P.R.J.; Hein, I.; Gilroy, E.M. The Hypersensitive Response in PAMP- and Effector-Triggered Immune Responses. In Plant Programmed Cell Death; Springer International Publishing: Cham, Switzerland, 2015; pp. 235–268. [Google Scholar]
- van de Wouw, A.P.; Idnurm, A. Biotechnological Potential of Engineering Pathogen Effector Proteins for Use in Plant Disease Management. Biotechnol. Adv. 2019, 37, 107387. [Google Scholar] [CrossRef]
- Poltronieri, P.; Brutus, A.; Reca, I.B.; Francocci, F.; Cheng, X.; Stigliano, E. Engineering plant leucine rich repeat-receptors for enhanced pattern-triggered immunity (PTI) and effector-triggered immunity (ETI). In Applied Plant Biotechnology for Improving Resistance to Biotic Stress; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–31. ISBN 9780128160305. [Google Scholar]
- Nic-Matos, G.; Narváez, M.; Peraza-Echeverría, S.; Sáenz, L.; Oropeza, C. Molecular Cloning of Two Novel NPR1 Homologue Genes in Coconut Palm and Analysis of Their Expression in Response to the Plant Defense Hormone Salicylic Acid. Genes Genom. 2017, 39, 1007–1019. [Google Scholar] [CrossRef]
- Palmer, I.A.; Chen, H.; Chen, J.; Chang, M.; Li, M.; Liu, F.; Fu, Z.Q. Novel Salicylic Acid Analogs Induce a Potent Defense Response in Arabidopsis. Int. J. Mol. Sci. 2019, 20, 3356. [Google Scholar] [CrossRef] [Green Version]
- Sanzón Gómez, D.; Zavaleta Mejía, E. Respuesta de Hipersensibilidad, Una Muerte Celular Programada Para Defenderse Del Ataque Por Fitopatógenos. Rev. Mex. Fitopatol. 2011, 29, 154–164. [Google Scholar]
- Garcia-Brugger, A.; Lamotte, O.; Vandelle, E.; Bourque, S.; Lecourieux, D.; Poinssot, B.; Wendehenne, D.; Pugin, A. Early Signaling Events Induced by Elicitors of Plant Defenses. Mol. Plant-Microbe Interact. 2006, 19, 711–724. [Google Scholar] [CrossRef] [Green Version]
- Mou, Z.; Fan, W.; Dong, X. Inducers of Plant Systemic Acquired Resistance Regulate NPR1 Function through Redox Changes. Cell 2003, 113, 935–944. [Google Scholar] [CrossRef] [Green Version]
- Benezer–Benezer, M.; Castro-Mercado, E.; García-Pineda, E. La Producción de Especies Reactivas de Oxígeno Durante La Expresión de La Resistencia a Enfermedades En Plantas. Rev. Mex. Fitopatol. 2008, 26, 56–61. [Google Scholar]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Cui, W.; Lee, J.Y. Arabidopsis Callose Synthases CalS1/8 Regulate Plasmodesmal Permeability during Stress. Nat. Plants 2016, 2. [Google Scholar] [CrossRef]
- Singh, A.; Lim, G.-H.; Kachroo, P. Transport of Chemical Signals in Systemic Acquired Resistance. J. Integr. Plant Biol. 2017, 59, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.W.; Klessig, D.F. DAMPs, MAMPs, and NAMPs in Plant Innate Immunity. BMC Plant Biol. 2016, 16, 232. [Google Scholar] [CrossRef] [Green Version]
- Beckers, G.J.M.; Spoel, S.H. Fine-Tuning Plant Defence Signalling: Salicylate versus Jasmonate. Plant Biol. 2006, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.L.; Yang, Y.; He, Z. Roles of Plant Hormones and Their Interplay in Rice Immunity. Mol. Plant 2013, 6, 675–685. [Google Scholar] [CrossRef] [Green Version]
- Tocho, E.; Lohwasser, U.; Börner, A.; Castro, A.M. Mapping and Candidate Gene Identification of Loci Induced by Phytohormones in Barley (Hordeum vulgare L.). Euphytica 2014, 195, 397–407. [Google Scholar] [CrossRef]
- Bürger, M.; Chory, J. Stressed Out About Hormones: How Plants Orchestrate Immunity. Cell Host Microbe 2019, 26, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Ramos, O.F.; Smith, C.M.; Fritz, A.K.; Madl, R.L. Salicylic Acid-Mediated Synthetic Elicitors of Systemic Acquired Resistance Administered to Wheat Plants at Jointing Stage Induced Phenolics in Mature Grains. Crop. Sci. 2017, 57, 3122–3128. [Google Scholar] [CrossRef]
- Kohli, S.K.; Handa, N.; Sharma, A.; Kumar, V.; Kaur, P.; Bhardwaj, R. Synergistic Effect of 24-Epibrassinolide and Salicylic Acid on Photosynthetic Efficiency and Gene Expression in Brassica juncea L. Under Pb Stress. Turk. J. Biol. 2017, 41, 943–953. [Google Scholar] [CrossRef]
- Ali, B. Salicylic Acid Induced Antioxidant System Enhances the Tolerence to Aluminium in Mung Bean (Vigna radiata L. Wilczek) Plants. Indian J. Plant Physiol. 2017, 22, 178–189. [Google Scholar] [CrossRef]
- van Butselaar, T.; van den Ackerveken, G. Salicylic Acid Steers the Growth–Immunity Tradeoff. Trends Plant Sci. 2020, 25, 566–576. [Google Scholar] [CrossRef]
- Ding, P.; Ding, Y. Stories of Salicylic Acid: A Plant Defense Hormone. Trends Plant Sci. 2020, 25, 549–565. [Google Scholar] [CrossRef] [PubMed]
- Sarowar, S.; Alam, S.T.; Makandar, R.; Lee, H.; Trick, H.N.; Dong, Y.; Shah, J. Targeting the Pattern-triggered Immunity Pathway to Enhance Resistance to Fusarium graminearum. Mol. Plant Pathol. 2019, 20, 626–640. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Li, X. Salicylic Acid: Biosynthesis, Perception, and Contributions to Plant Immunity. Curr. Opin. Plant Biol. 2019, 50, 29–36. [Google Scholar] [CrossRef]
- Rangel Sánchez, G.; Castro Mercado, E.; Beltran Peña, E.; Reyes de la Cruz, H.; García Pineda, E. El Acido Salicílico y Su Participación En La Resistencia a Patógenos En Plantas. Biológicas 2010, 12, 90–95. [Google Scholar]
- Garcion, C.; Lohmann, A.; Lamodière, E.; Catinot, J.; Buchala, A.; Doermann, P.; Métraux, J.P. Characterization and Biological Function of the Isochorismate Synthase2 Gene of Arabidopsis. Plant Physiol. 2008, 147, 1279–1287. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Wang, X.; Zhang, X.; Wang, Y.; Huo, Z.; Huang, M.; Cai, J.; Zhou, Q.; Jiang, D. Involvement of Salicylic Acid in Cold Priming-Induced Freezing Tolerance in Wheat Plants. Plant Growth Regul. 2020, 93, 117–130. [Google Scholar] [CrossRef]
- Yokoo, S.; Inoue, S.; Suzuki, N.; Amakawa, N.; Matsui, H.; Nakagami, H.; Takahashi, A.; Arai, R.; Katou, S. Comparative Analysis of Plant Isochorismate Synthases Reveals Structural Mechanisms Underlying Their Distinct Biochemical Properties. Biosci. Rep. 2018, 38. [Google Scholar] [CrossRef] [Green Version]
- He, C.; Duan, K.; Zhang, L.; Zhang, L.; Song, L.; Yang, J.; Zou, X.; Wang, Y.; Gao, Q. Fast Quenching the Burst of Host Salicylic Acid Is Common in Early Strawberry/Colletotrichum fructicola Interaction. Phytopathology 2019, 109, 531–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shine, M.B.; Yang, J.-W.; El-Habbak, M.; Nagyabhyru, P.; Fu, D.-Q.; Navarre, D.; Ghabrial, S.; Kachroo, P.; Kachroo, A. Cooperative Functioning between Phenylalanine Ammonia Lyase and Isochorismate Synthase Activities Contributes to Salicylic Acid Biosynthesis in Soybean. New Phytol. 2016, 212, 627–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno-Escamilla, J.O.; Alvarez-Parrilla, E.; de la Rosa, L.A.; Núñez-Gastélum, J.A.; González-Aguilar, G.A.; Rodrigo-García, J. Effect of Elicitors in the Nutritional and Sensorial Quality of Fruits and Vegetables. In Preharvest Modulation of Postharvest Fruit and Vegetable Quality; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 71–91. ISBN 9780128098080. [Google Scholar]
- Narasimhamurthy, K.; Soumya, K.; Udayashankar, A.C.; Srinivas, C.; Niranjana, S.R. Elicitation of Innate Immunity in Tomato by Salicylic Acid and Amomum nilgiricum against Ralstonia solanacearum. Biocatal. Agric. Biotechnol. 2019, 22, 101414. [Google Scholar] [CrossRef]
- van Aubel, G.; Cambier, P.; Dieu, M.; van Cutsem, P. Plant Immunity Induced by COS-OGA Elicitor Is a Cumulative Process That Involves Salicylic Acid. Plant Sci. 2016, 247, 60–70. [Google Scholar] [CrossRef]
- Kalaivani, K.; Kalaiselvi, M.M.; Senthil-Nathan, S. Effect of Methyl Salicylate (MeSA), an Elicitor on Growth, Physiology and Pathology of Resistant and Susceptible Rice Varieties. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cawood, M.E.; Pretorius, J.C.; van der Westhuizen, J.H.; van Heerden, F.R. A Saponin Isolated from Agapanthus africanus Differentially Induces Apoplastic Peroxidase Activity in Wheat and Displays Fungicidal Properties. Acta Physiol. Plant. 2015, 37, 1–8. [Google Scholar] [CrossRef]
- Vera-Guzman, A.M.; Lafuente, M.T.; Aispuro-Hernandez, E.; Vargas-Arispuro, I.; Martinez-Tellez, M.A. Pectic and Galacturonic Acid Oligosaccharides on the Postharvest Performance of Citrus Fruits. HortScience 2017, 52, 264–270. [Google Scholar] [CrossRef]
- Zabotin, A.I.; Barisheva, T.S.; Trofimova, O.I.; Toroschina, T.E.; Larskaya, I.A.; Zabotina, O.A. Oligosaccharin and ABA Synergistically Affect the Acquisition of Freezing Tolerance in Winter Wheat. Plant Physiol. Biochem. 2009, 47, 854–858. [Google Scholar] [CrossRef]
- Clinckemaillie, A.; Decroës, A.; van Aubel, G.; Carrola dos Santos, S.; Renard, M.E.; van Cutsem, P.; Legrève, A. The Novel Elicitor COS-OGA Enhances Potato Resistance to Late Blight. Plant Pathol. 2017, 66, 818–825. [Google Scholar] [CrossRef]
- Singh, R.R.; Chinnasri, B.; de Smet, L.; Haeck, A.; Demeestere, K.; van Cutsem, P.; van Aubel, G.; Gheysen, G.; Kyndt, T. Systemic Defense Activation by COS-OGA in Rice against Root-Knot Nematodes Depends on Stimulation of the Phenylpropanoid Pathway. Plant Physiol. Biochem. 2019, 142, 202–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jatoi, G.H.; Lihua, G.; Xiufen, Y.; Gadhi, M.A.; Keerio, A.U.; Abdulle, Y.A.; Qiu, D. A Novel Protein Elicitor PeBL2, from Brevibacillus laterosporus A60, Induces Systemic Resistance against Botrytis cinerea in Tobacco Plant. Plant Pathol. J. 2019, 35, 208–218. [Google Scholar] [CrossRef]
- Li, S.; Nie, H.; Qiu, D.; Shi, M.; Yuan, Q. A Novel Protein Elicitor PeFOC1 from Fusarium oxysporum Triggers Defense Response and Systemic Resistance in Tobacco. Biochem. Biophys. Res. Commun. 2019, 514, 1074–1080. [Google Scholar] [CrossRef] [PubMed]
- Xoca-Orozco, L.-Á.; Aguilera-Aguirre, S.; Vega-Arreguín, J.; Acevedo-Hernández, G.; Tovar-Pérez, E.; Stoll, A.; Herrera-Estrella, L.; Chacón-López, A. Activation of the Phenylpropanoid Biosynthesis Pathway Reveals a Novel Action Mechanism of the Elicitor Effect of Chitosan on Avocado Fruit Epicarp. Food Res. Int. 2019, 121, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Rendina, N.; Nuzzaci, M.; Scopa, A.; Cuypers, A.; Sofo, A. Chitosan-Elicited Defense Responses in Cucumber Mosaic Virus (CMV)-Infected Tomato Plants. J. Plant Physiol. 2019, 234–235, 9–17. [Google Scholar] [CrossRef]
- Zhou, W.; Qian, C.; Li, R.; Zhou, S.; Zhang, R.; Xiao, J.; Wang, X.; Zhang, S.; Xing, L.; Cao, A. TaNAC6s Are Involved in the Basal and Broad-Spectrum Resistance to Powdery Mildew in Wheat. Plant Sci. 2018, 277. [Google Scholar] [CrossRef]
- de Azevedo Souza, C.; Li, S.; Lin, A.Z.; Boutrot, F.; Grossmann, G.; Zipfel, C.; Somerville, S.C. Cellulose-Derived Oligomers Act as Damage-Associated Molecular Patterns and Trigger Defense-like Responses. Plant Physiol. 2017, 173. [Google Scholar] [CrossRef] [Green Version]
- Selim, S.; Sanssené, J.; Rossard, S.; Courtois, J. Systemic Induction of the Defensin and Phytoalexin Pisatin Pathways in Pea (Pisum sativum) against Aphanomyces euteiches by Acetylated and Nonacetylated Oligogalacturonides. Molecules 2017, 22, 1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Zhou, P.; Mo, X.; Hu, L.; Jin, N.; Chen, X.; Yu, Z.; Meng, J.; Erb, M.; Shang, Z.; et al. Induction of Defense in Cereals by 4-Fluorophenoxyacetic Acid Suppresses Insect Pest Populations and Increases Crop Yields in the Field. Proc. Natl. Acad. Sci. USA. 2020, 117. [Google Scholar] [CrossRef]
- Cui, K.; Shu, C.; Zhao, H.; Fan, X.; Cao, J.; Jiang, W. Preharvest Chitosan Oligochitosan and Salicylic Acid Treatments Enhance Phenol Metabolism and Maintain the Postharvest Quality of Apricots (Prunus armeniaca L.). Sci. Hortic. 2020, 267. [Google Scholar] [CrossRef]
- Shi, Z.; Wang, F.; Lu, Y.; Deng, J. Combination of Chitosan and Salicylic Acid to Control Postharvest Green Mold Caused by Penicillium digitatum in Grapefruit Fruit. Sci. Hortic. 2018, 233, 54–60. [Google Scholar] [CrossRef]
- Xin, P.; Guo, Q.; Li, B.; Cheng, S.; Yan, J.; Chu, J. A Tailored High-Efficiency Sample Pretreatment Method for Simultaneous Quantification of 10 Classes of Known Endogenous Phytohormones. Plant Commun. 2020, 1, 100047. [Google Scholar] [CrossRef] [PubMed]
- Maag, D.; Erb, M.; Köllner, T.G.; Gershenzon, J. Defensive Weapons and Defense Signals in Plants: Some Metabolites Serve Both Roles. BioEssays 2015, 37, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Jayaraj, J.; Muthukrishnan, S.; Liang, G.H.; Velazhahan, R. Jasmonic Acid and Salicylic Acid Induce Accumulation of β-1,3-Glucanase and Thaumatin-like Proteins in Wheat and Enhance Resistance against Stagonospora nodorum. Biol. Plant. 2004, 48, 425–430. [Google Scholar] [CrossRef]
- Kumar, R.R.; Sharma, S.K.; Goswami, S.; Verma, P.; Singh, K.; Dixit, N.; Pathak, H.; Viswanathan, C.; Rai, R.D. Salicylic Acid Alleviates the Heat Stress-Induced Oxidative Damage of Starch Biosynthesis Pathway by Modulating the Expression of Heat-Stable Genes and Proteins in Wheat (Triticum aestivum). Acta Physiol. Plant. 2015, 37, 1–12. [Google Scholar] [CrossRef]
- Tayeh, C.; Randoux, B.; Laruelle, F.; Bourdon, N.; Reignault, P. Phosphatidic Acid Synthesis, Octadecanoic Pathway and Fatty Acids Content as Lipid Markers of Exogeneous Salicylic Acid-Induced Elicitation in Wheat. Funct. Plant Biol. 2016, 43, 512. [Google Scholar] [CrossRef]
- Twamley, T.; Gaffney, M.; Feechan, A. A Microbial Fermentation Mixture Primes for Resistance against Powdery Mildew in Wheat. Front. Plant Sci. 2019, 10, 1241. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, S.; Yang, X.; Francis, F.; Qiu, D. Protein Elicitor PeaT1 Enhanced Resistance against Aphid (Sitobion avenae) in wheat. Pest. Manag. Sci. 2020, 76, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Antalová, Z.; Bleša, D.; Martinek, P.; Matušinsky, P. Transcriptional Analysis of Wheat Seedlings Inoculated with Fusarium culmorum under Continual Exposure to Disease Defence Inductors. PLoS ONE 2020, 15, e0224413. [Google Scholar] [CrossRef]
- Pazarlar, S.; Cetinkaya, N.; Bor, M.; Ozdemir, F. Ozone Triggers Different Defence Mechanisms against Powdery Mildew (Blumeria graminis DC. Speer f. Sp. tritici) in Susceptible and Resistant Wheat Genotypes. Funct. Plant Biol. 2017, 44, 1016–1028. [Google Scholar] [CrossRef]
- Phuong, L.T.; Zhao, L.; Fitrianti, A.N.; Matsui, H.; Noutoshi, Y.; Yamamoto, M.; Ichinose, Y.; Shiraishi, T.; Toyoda, K. The Plant Activator Saccharin Induces Resistance to Wheat Powdery Mildew by Activating Multiple Defense-Related Genes. J. Gen. Plant. Pathol. 2020, 86, 107–113. [Google Scholar] [CrossRef]
- Sorahinobar, M.; Niknam, V.; Ebrahimzadeh, H.; Soltanloo, H.; B2ehmanesh, M.; Enferadi, S.T. Central Role of Salicylic Acid in Resistance of Wheat against Fusarium graminearum. J. Plant. Growth Regul. 2016, 35, 477–491. [Google Scholar] [CrossRef]
- le Mire, G.; Siah, A.; Marolleau, B.; Gaucher, M.; Maumené, C.; Brostaux, Y.; Massart, S.; Brisset, M.N.; Haissam Jijakli, M. Evaluation of L-Carrageenan, CpG-ODN, Glycine Betaine, Spirulina Platensis, and Ergosterol as Elicitors for Control of Zymoseptoria tritici in Wheat. Phytopathology 2019, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ameye, M.; Audenaert, K.; de Zutter, N.; Steppe, K.; van Meulebroek, L.; Vanhaecke, L.; de Vleesschauwer, D.; Haesaert, G.; Smagghe, G. Priming of Wheat with the Green Leaf Volatile Z-3-Hexenyl Acetate Enhances Defense against Fusarium graminearum but Boosts Deoxynivalenol Production. Plant Physiol. 2015, 167, 1671–1684. [Google Scholar] [CrossRef] [Green Version]
- Mercier, L.; Lafitte, C.; Borderies, G.; Briand, X.; Esquerré-Tugayé, M.T.; Fournier, J. The Algal Polysaccharide Carrageenans Can Act as an Elicitor of Plant Defence. New Phytol. 2001, 149, 43–51. [Google Scholar] [CrossRef]
- Choi, J.; Huh, S.U.; Kojima, M.; Sakakibara, H.; Paek, K.H.; Hwang, I. The Cytokinin-Activated Transcription Factor ARR2 Promotes Plant Immunity via TGA3/NPR1-Dependent Salicylic Acid Signaling in Arabidopsis. Dev. Cell 2010, 19, 284–295. [Google Scholar] [CrossRef] [Green Version]
- Ridley, B.L.; O’Neill, M.A.; Mohnen, D. Pectins: Structure, Biosynthesis, and Oligogalacturonide-Related Signaling. Phytochemistry 2001, 57, 929–967. [Google Scholar] [CrossRef]
- Ferrari, S.; Savatin, D.V.; Sicilia, F.; Gramegna, G.; Cervone, F.; de Lorenzo, G. Oligogalacturonides: Plant Damage-Associated Molecular Patterns and Regulators of Growth and Development. Front. Plant Sci. 2013, 4, 49. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Wu, Q.; Cao, S.; Zhao, T.; Chen, L.; Zhuang, P.; Zhou, X.; Gao, Z. A Novel Protein Elicitor (SsCut) from Sclerotinia sclerotiorum Induces Multiple Defense Responses in Plants. Plant Mol. Biol. 2014, 86, 495–511. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Lichtfouse, E.; Torri, G.; Crini, G. Fundamentals and Applications of Chitosan. In Sustainable Agriculture Reviews 35; Crini, G., Lichtfouse, E., Eds.; Springer: Cham, Switzerland, 2019; pp. 49–123. [Google Scholar]
- Knogge, W.; Scheel, D. LysM Receptors Recognize Friend and Foe. Proc. Natl. Acad. Sci. USA 2006, 103, 10829–10830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, D.; Jiao, S.; Cheng, G.; Li, X.; Pei, Z.; Pei, Y.; Yin, H.; Du, Y. Identification of Chitosan Oligosaccharides Binding Proteins from the Plasma Membrane of Wheat Leaf Cell. Int. J. Biol. Macromol. 2018, 111, 1083–1090. [Google Scholar] [CrossRef]
- Poudel, R.S.; Richards, J.; Shrestha, S.; Solanki, S.; Brueggeman, R. Transcriptome-Wide Association Study Identifies Putative Elicitors/Suppressor of Puccinia graminis f. Sp. tritici That Modulate Barley Rpg4-Mediated Stem Rust Resistance. BMC Genom. 2019, 20, 985. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; Rabea, E.I. Chitosan and Its Derivatives as Active Ingredients against Plant Pests and Diseases. In Chitosan in the Preservation of Agricultural Commodities; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 179–219. ISBN 9780128027356. [Google Scholar]
- Aljawish, A.; Chevalot, I.; Piffaut, B.; Rondeau-Mouro, C.; Girardin, M.; Jasniewski, J.; Scher, J.; Muniglia, L. Functionalization of Chitosan by Laccase-Catalyzed Oxidation of Ferulic Acid and Ethyl Ferulate under Heterogeneous Reaction Conditions. Carbohydr. Polym. 2012, 87, 537–544. [Google Scholar] [CrossRef]
- Salazar-Leyva, J.A.; Lizardi-Mendoza, J.; Ramırez-Suarez, J.C.; Garcıa-Sánchez, G.; Ezquerra-Brauer, J.M.; Valenzuela-Soto, E.M.; Pacheco-Aguilar, R. Utilización de Materiales a Base de Quitina y Quitosano Para La Inmovilización Proteasa: Efectos y Aplicaciones de Estabilización. Rev. Mex. Ing. Química 2014, 13, 129–150. [Google Scholar]
- Sharma, S.; Barman, K.; Siddiqui, M.W. Chitosan: Properties and roles in postharvest quality preservation of horticultural crops. In Eco-Friendly Technology for Postharvest Produce Quality; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 269–296. ISBN 9780128043844. [Google Scholar]
- Falcón-Rodríguez, A.B.; Costales, D.; Gónzalez-Peña, D.; Morales, D.; Mederos, Y.; Jerez, E.; Cabrera, J.C. Chitosans of Different Molecular Weight Enhance Potato (Solanum tuberosum L.) Yield in a Field Trial-Dialnet. Span. J. Agric. Res. 2017, 15, 25. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.; Yang, L.; Yan, H.; Kennedy, J.F.; Meng, X. Chitosan and Oligochitosan Enhance the Resistance of Peach Fruit to Brown Rot. Carbohydr. Polym. 2013, 94, 272–277. [Google Scholar] [CrossRef]
- Ahmed, A.B.A.; Taha, R.M.; Mohajer, S.; Elaagib, M.E.; Kim, S.K. Preparation, Properties and Biological Applications of Water Soluble Chitin Oligosaccharides from Marine Organisms. Russ. J. Mar. Biol. 2012, 38, 351–358. [Google Scholar] [CrossRef]
- Masuelli, M.; Renard, D. Section A. Specials Cases Polysaccharides. In Advances in Physicochemical Properties of Biopolymers (Part 2); Bentham Science Publishers: Sharjah, United Arab Emirates, 2017; pp. 7–8. [Google Scholar]
- Kim, S.K.; Rajapakse, N. Enzymatic Production and Biological Activities of Chitosan Oligosaccharides (COS): A Review. Carbohydr. Polym. 2005, 62, 357–368. [Google Scholar] [CrossRef]
- Zou, P.; Tian, X.; Dong, B.; Zhang, C. Size Effects of Chitooligomers with Certain Degrees of Polymerization on the Chilling Tolerance of Wheat Seedlings. Carbohydr. Polym. 2017, 160, 194–202. [Google Scholar] [CrossRef]
- Hamel, L.-P.; Beaudoin, N. Chitooligosaccharide Sensing and Downstream Signaling: Contrasted Outcomes in Pathogenic and Beneficial Plant–Microbe Interactions. Planta 2010, 232, 787–806. [Google Scholar] [CrossRef] [PubMed]
- Mélida, H.; Sopeña-Torres, S.; Bacete, L.; Garrido-Arandia, M.; Jordá, L.; López, G.; Muñoz-Barrios, A.; Pacios, L.F.; Molina, A. Non-Branched β-1,3-Glucan Oligosaccharides Trigger Immune Responses in Arabidopsis. Plant J. 2018, 93, 34–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Pedroso, A.T.; Ramírez-Arrebato, M.A.; Cárdenas-Travieso, R.M.; Falcón-Rodríguez, A.; Bautista-Baños, S. Efecto de La Quitosana En La Inducción de La Actividad de Enzimas Relacionadas Con La Defensa y Protección de Plántulas de Arroz (Oryza sativa L.) Contra Pyricularia grisea Sacc. Rev. Mex. Fitopatol. 2006, 24, 1–7. [Google Scholar]
- Deng, L.; Zhou, Y.; Zeng, K. Pre-Harvest Spray of Oligochitosan Induced the Resistance of Harvested Navel Oranges to Anthracnose during Ambient Temperature Storage. Crop. Prot. 2015, 70, 70–76. [Google Scholar] [CrossRef]
- Samain, E.; van Tuinen, D.; Jeandet, P.; Aussenac, T.; Selim, S. Biological Control of Septoria Leaf Blotch and Growth Promotion in Wheat by Paenibacillus Sp. Strain B2 and Curtobacterium plantarum Strain EDS. Biol. Control. 2017, 114, 87–96. [Google Scholar] [CrossRef]
- Orzali, L.; Forni, C.; Riccioni, L. Effect of Chitosan Seed Treatment as Elicitor of Resistance to Fusarium graminearum in wheat. Seed Sci. Technol. 2014, 42, 132–149. [Google Scholar] [CrossRef]
- Díaz-Martínez, J.M.; Aispuro-Hernández, E.; Vargas-Arispuro, I.; Falcón-Rodríguez, A.B.; Martínez-Téllez, M. Chitosan Derivatives Induce Local and Distal Expression of Defence-Related Genes in Wheat (Triticum aestivum L.) Seedlings. Agrociencia 2018, 52, 497–509. [Google Scholar]
- Liu, H.; Carvalhais, L.C.; Schenk, P.M.; Dennis, P.G. Activation of the Salicylic Acid Signalling Pathway in Wheat Had No Significant Short-Term Impact on the Diversity of Root-Associated Microbiomes. Pedobiologia 2018, 70, 6–11. [Google Scholar] [CrossRef] [Green Version]
- Randoux, B.; Renard-Merlier, D.; Mulard, G.; Rossard, S.; Duyme, F.; Sanssené, J.; Courtois, J.; Durand, R.; Reignault, P. Distinct Defenses Induced in Wheat Against Powdery Mildew by Acetylated and Nonacetylated Oligogalacturonides. Phytopathology 2010, 100, 1352–1363. [Google Scholar] [CrossRef] [PubMed]
| Crop | Stressor | Elicitor | Elicitor Type | Concentration | Mode of Application | Triggered Response | Reference |
|---|---|---|---|---|---|---|---|
| Tomato | Ralstonia solanacearum | SA | Phytohormone | 1 µM | Soaked seeds | Increased the activities of peroxidase and polyphenol oxidase enzymes | [53] |
| Tomato | Leveillula taurica | COS + OGA | Fragments of cell wall + fungal cell wall | 50 ppm | Foliar spray | Upregulation of PR proteins and salicylic acid (SA)-related genes | [54] |
| Rice | Xanthomonas oryzae pv. Oryzae | Methyl salicylate | Phytohormone | 75 and 100 mg L−1 | Soaked seeds | Promoted early growth and provided better protection against diseases | [55] |
| Wheat | Fusarium oxysporum. | Saponin isolated from Agapanthus africanus | Phytochemical | 125 µg mL−1 | Foliar spray | Stimulation of peroxidase enzyme activity | [56] |
| Citrus | Low temperature | Pectic oligosaccharides | Fragments of cell wall | 10 g L−1 | Postharvest spray application on fruits | Early defense signals | [57] |
| Wheat | Low temperature | GXAG + ABA | Fragments of cell wall | 5 µg mL−1 (GXAG) + 50 µM (ABA) | Application in roots | Initiation of freezing tolerance acquisition in winter plants | [58] |
| Potato | Phytophthora infestans | FytoSave (COS-OGAS) | Fungal cell wall—Fragments of cell wall | 12.5 g L−1 | Foliar spray | Upregulation of defense genes PI-1, PR-1, and acidic PR-2 in potato | [59] |
| Rice | Meloidogyne graminicola | Induced defense dependent on OsPAL4 gene expression in rice | [60] | ||||
| Tobacco | Botrytis cinerea | PeBL2 | Microorganism-Derived | 50 µM | Infiltrated leaves | Generation of ROS (H2O2 and O2−) and systemic resistance activation | [61] |
| Tobacco | tobacco mosaic virus and Pseudomonas syringae pv. tabaci. | PeFOC1 | Microorganism-Derived | 5 µM | Infiltrated leaves | Upregulation of NtPAL, NtEDS1, NtLOX, and NtPDF, activated SA and JA/Et signaling pathways, induced callose, and phenolic compounds deposition | [62] |
| Avocado | Colletotrichum gloeosporioides | Chitosan | Fungal cell wall | 16 mg mL−1 | In vitro | Induce specific accumulation of phenylpropanoids and an antifungal diene | [63] |
| Tomato | Cucumber mosaic virus (CMV) | Chitosan | Fungal cell wall | 10 mL. plant−1 | Foliar spray | Reduced viral load and upregulated PAL5 expression. | [64] |
| Citrus | Geotrichum candidum | SA | Phytohormone | 2.5 mmol L−1 | Wounded fruit | Enhanced phenylpropanoid pathway-related enzyme activities and stimulated the synthesis of phenolic acids and lignin | [65] |
| P. membranaefaciens | Microorganism-derivate | 1 × 108 cells mL−1 | |||||
| Chitooligosaccharide | Fungal cell wall | 15 g L−1 | |||||
| Arabidopsis | Pseudomonas syringae pv. tomato | Cellobiose | Fragments of cell wall | 100 µM | In vitro | Signaling similar to other PAMPs/DAMPs, | [66] |
| Pea | Aphanomyces euteiches | Oligogalacturonides | Fragments of cell wall | 80 µg | Injected plants | Stimulated defense mechanisms, including the SA pathway | [67] |
| Rice | Sogatella furcifera | 4-Fluorophenoxyacetic | Synthetic chemical | 0.5 to 5 mg. L−1 | Root application and Foliar spray | Modulated the production of peroxidases, H2O2, and flavonoids | [68] |
| Apricot | Low temperature | SA + COS | Phytohormones+ fungal cell wall | SA (1 mmol L−1) + 0.05% COS (w/v) | Foliar spray | Reduced chilling injury and improved fruit quality | [69] |
| Grapefruits | Penicillium digitatum | SA + Chitosan | Phytohormones+ fungal cell wall | SA (2 mM) + 10 g L−1 chitosan (w/v) | Fruit dipped | Enhanced the chitinase, β-1,3-glucanase, peroxidase, phenylalanine ammonia-lyase, and polyphenoloxidase activities and stimulated the synthesis of total phenolic compounds content | [70] |
| Pathogen | Elicitor | Mode of Application | Gene Up-Regulation | Suggested Mechanisms | Reference |
|---|---|---|---|---|---|
| Blumeria graminis f. sp. tritici | SA | Foliar infiltration | PI-PLC2, LOX, | Induction of the octadecanoid pathway | [75] |
| Stagonospora nodorum. | SA/JA | Foliar spray | GLU, TLPs | Induced in response to infection | [73] |
| Blumeria graminis f. sp. tritici | MFP | Foliar spray | PR1, PR4, PR5, and PR9 | Induction of plant defense systems | [76] |
| Sitobion avenae | PeaT1 | Seed immersion and foliar spray | ICS, PR1, | Increased number of trichomes and higher accumulation of wax. Induced SA and JA levels | [77] |
| Fusarium culmorum | Sodium bicarbonate | Seed immersion | B2H2, PAL | Induction of plant defense systems | [78] |
| Blumeria graminis f. sp. tritici | Ozone | Gas | PR1, LOX, PAL | Expression induced via the SA pathway | [79] |
| Blumeria graminis f. sp. tritici | Saccharin/PBZ | Foliar spray | PR1.1, PR2, PR4, CHI3 CHI4, TaNPR1, PAL, LOX, AOS, WCI2, WCI3, WRKY72a/b e | Induced expression of defense-related genes, including a WRKY-type transcription factor. Increased SA and JA biosynthesis | [80] |
| Fusarium graminearum | SA | Soil drench | PAL | Activated antioxidant defense responses and possible induced systemic acquired resistance | [81] |
| Zymoseptoria tritici | λ-Carrageenan | Foliar spray | PR1, PR4, PR5, PR8,13-lipoxygenase 2, PAL, PR15 | Displayed antimicrobial activities, increased antioxidative processes, and plant defense signaling of SA and JA | [82] |
| Cytosine-phosphate guanine oligodesoxynucleotide motifs (CpG ODN) | Foliar spray | PR4, PR5,13-lipoxygenase 2 | |||
| Spirulina platensis | Foliar spray | PR1,13-lipoxygenase 2, PAL, PR15 | |||
| Glycine betaine | Foliar spray | PR4, PR5,13-lipoxygenase 2, PAL, PR15 | |||
| Ergosterol | Foliar spray | PR1, PR4, PR5, PR8,13-lipoxygenase 2, PAL, PR15 | |||
| Fusarium graminearum | Green Leaf Volatile Z-3-Hexenyl Acetate | Cuvette System | PR1, PR4, PR5, peroxidase | Enhanced defense against the hemibiotrophic fungus F. graminearum, resulting in slower disease progress, reduced symptom development, and lower fungal growth | [83] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ochoa-Meza, L.C.; Quintana-Obregón, E.A.; Vargas-Arispuro, I.; Falcón-Rodríguez, A.B.; Aispuro-Hernández, E.; Virgen-Ortiz, J.J.; Martínez-Téllez, M.Á. Oligosaccharins as Elicitors of Defense Responses in Wheat. Polymers 2021, 13, 3105. https://doi.org/10.3390/polym13183105
Ochoa-Meza LC, Quintana-Obregón EA, Vargas-Arispuro I, Falcón-Rodríguez AB, Aispuro-Hernández E, Virgen-Ortiz JJ, Martínez-Téllez MÁ. Oligosaccharins as Elicitors of Defense Responses in Wheat. Polymers. 2021; 13(18):3105. https://doi.org/10.3390/polym13183105
Chicago/Turabian StyleOchoa-Meza, Laura Celina, Eber Addí Quintana-Obregón, Irasema Vargas-Arispuro, Alejandro Bernardo Falcón-Rodríguez, Emmanuel Aispuro-Hernández, José J. Virgen-Ortiz, and Miguel Ángel Martínez-Téllez. 2021. "Oligosaccharins as Elicitors of Defense Responses in Wheat" Polymers 13, no. 18: 3105. https://doi.org/10.3390/polym13183105
APA StyleOchoa-Meza, L. C., Quintana-Obregón, E. A., Vargas-Arispuro, I., Falcón-Rodríguez, A. B., Aispuro-Hernández, E., Virgen-Ortiz, J. J., & Martínez-Téllez, M. Á. (2021). Oligosaccharins as Elicitors of Defense Responses in Wheat. Polymers, 13(18), 3105. https://doi.org/10.3390/polym13183105