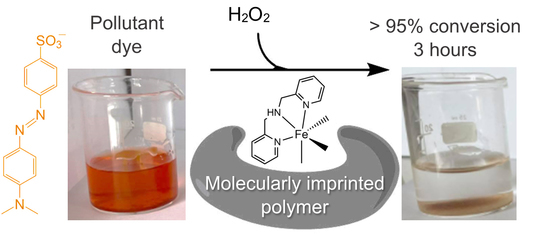
Fe(III)-Complex-Imprinted Polymers for the Green Oxidative Degradation of the Methyl Orange Dye Pollutant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. MIPs Preparation and Characterization
2.3. Kinetic and Mechanistic Characterization in Solution
3. Results and Discussion
3.1. Fe(III) Complexes Catalytic Performance
3.2. Kinetic Parameters and Mechanistic Insights
3.3. Fe(III)-Complex-Imprinted Polymers
3.4. MIPs as Fenton-like Heterogeneous Catalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Uday, U.S.P.; Bandyopadhyay, T.K.; Bhunia, B. Bioremediation and detoxification technology for treatment of dye(s) from textile efuent. In Textile Wastewater Treatment; IntechOpen: London, UK, 2016; pp. 75–92. [Google Scholar]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef]
- Allen, S.J.; McKay, G.; Porter, J.F. Adsorption isotherm models for basic dye adsorption by peat in single and binary component systems. J. Colloid Interface Sci. 2004, 280, 322–333. [Google Scholar] [CrossRef]
- Brindley, L. New Solution for Dye Wastewater Pollution. Chemistry World. 2009. Available online: https://www.chemistryworld.com/news/new-solution-for-dye-wastewater-pollution/3002870.article (accessed on 8 July 2009).
- GEA Consultores Ambientales. Gestión Ambiental en el Sector Secundario; Intendencia de Montevideo-Instituto Clemente Estable-UdelaR: Montevideo, Uruguay, 2005.
- Lalanne, A.; Carsen, A.; Lorenzo, D.; Perdomo, A.; Arriola, M. Diagnóstico de Oportunidades de Implantación del Proyecto Piloto en Uruguay; Facultad de Química: Montevideo, Uruguay, 2005. [Google Scholar]
- Chen, S.; Zhang, J.; Zhang, C.; Yue, Q.; Li, Y.; Li, C. Equilibrium and kinetic studies of methyl orange and methyl violet adsorption on activated carbon derived from Phragmites australis. Desalination 2010, 252, 149–156. [Google Scholar] [CrossRef]
- Javaid, R.; Qazi, U.Y. Catalytic Oxidation Process for the Degradation of Synthetic Dyes: An Overview. Int. J. Environ. Res. Public Health 2019, 16, 2066. [Google Scholar] [CrossRef] [Green Version]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Robinson, T.; McMullan, G.; Marchant, R.; Nigam, P. Remediation of dyes in textile effluent: A critical review on current treatment technologies with a proposed alternative. Bioresour. Technol. 2001, 77, 247–255. [Google Scholar] [CrossRef]
- Forgacs, E.; Cserháti, T.; Oros, G. Removal of synthetic dyes from wastewaters: A review. Environ. Int. 2004, 30, 953–971. [Google Scholar] [CrossRef] [PubMed]
- Nikfar, S.; Jaberidoost, M. Dyes and Colorants. In Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Ed.; Academic Press: Oxford, UK, 2014; pp. 252–261. [Google Scholar]
- Ahmadpour, A. Photocatalytic decolorization of methyl orange dye using nano-photocatalysts. Adv. Environ. Technol. 2015, 1, 121–127. [Google Scholar] [CrossRef]
- Budavari, S. The Merck Index. Encyclopedia of Chemicals, Drugs, and Biologicals; p. 1537. 1996. Available online: https://www.worldcat.org/title/merck-index-an-encyclopedia-of-chemicals-drugs-and-biologicals/oclc/34552962 (accessed on 12 September 2021).
- Heukelekian, H.; Rand, M.C. Biochemical Oxygen Demand of Pure Organic Compounds: A Report of the Research Committee, FSIWA. Sew. Ind. Wastes 1955, 27, 1040–1053. [Google Scholar]
- Chung, K.-T.; Fulk, G.E.; Andrews, A.W. The mutagenicity of methyl orange and metabolites produced by intestinal anaerobes. Mutat. Res./Genet. Toxicol. 1978, 58, 375–379. [Google Scholar] [CrossRef]
- Singh, O.; Maji, A.; Singh, U.P.; Ghosh, K. Water-Soluble Copper Complex Derived from Ligand TETATA Having NNN Donors: Studies on Rapid Degradation of Organic Dyes, Catecholase and Phenoxazinone Synthase Activities. ChemistrySelect 2018, 3, 2968–2975. [Google Scholar] [CrossRef]
- Ajmal, A.; Majeed, I.; Malik, R.N.; Idriss, H.; Nadeem, M.A. Principles and mechanisms of photocatalytic dye degradation on TiO2 based photocatalysts: A comparative overview. RSC Adv. 2014, 4, 37003–37026. [Google Scholar] [CrossRef]
- Gupta, V.K.; Kumar, R.; Nayak, A.; Saleh, T.A.; Barakat, M.A. Adsorptive removal of dyes from aqueous solution onto carbon nanotubes: A review. Adv. Colloid Interface Sci. 2013, 193–194, 24–34. [Google Scholar] [CrossRef]
- Ahmad, A.; Mohd-Setapar, S.H.; Chuong, C.S.; Khatoon, A.; Wani, W.A.; Kumar, R.; Rafatullah, M. Recent advances in new generation dye removal technologies: Novel search for approaches to reprocess wastewater. RSC Adv. 2015, 5, 30801–30818. [Google Scholar] [CrossRef]
- Moursy, A.S.; Abdel-Shafy, H.I. Removal of hydrocarbons from Nile water. Environ. Int. 1983, 9, 165–171. [Google Scholar] [CrossRef]
- Alebic-Juretic, A.; Cvitas, T.; Klasinc, L. Heterogeneous polycyclic aromatic hydrocarbon degradation with ozone on silica gel carrier. Environ. Sci. Technol. 1990, 24, 62–66. [Google Scholar] [CrossRef]
- Haritash, A.K.; Kaushik, C.P. Biodegradation aspects of Polycyclic Aromatic Hydrocarbons (PAHs): A review. J. Hazard. Mater. 2009, 169, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Manariotis, I.D.; Karapanagioti, H.K.; Chrysikopoulos, C.V. Degradation of PAHs by high frequency ultrasound. Water Res. 2011, 45, 2587–2594. [Google Scholar] [CrossRef]
- Chahbane, N.; Popescu, D.L.; Mitchell, A.; Chanda, A.; Lenoir, D.; Ryabov, A.D.; Schramm, K.W.; Collins, T.J. FeIII–TAML-catalyzed green oxidative degradation of the azo dye Orange II by H2O2 and organic peroxides: Products, toxicity, kinetics, and mechanisms. Green Chem. 2007, 9, 49–57. [Google Scholar] [CrossRef]
- Carvalho, S.S.F.; Carvalho, N.M.F. Degradation of organic dyes by water soluble iron(III) mononuclear complexes from bis-(2-pyridylmethyl)amine NNN-derivative ligands. Inorg. Chem. Commun. 2019, 108, 107507. [Google Scholar] [CrossRef]
- Feng, Y.; England, J.; Que, L. Iron-Catalyzed Olefin Epoxidation and cis-Dihydroxylation by Tetraalkylcyclam Complexes: The Importance of cis-Labile Sites. ACS Catal. 2011, 1, 1035–1042. [Google Scholar] [CrossRef]
- Oldenburg, P.D.; Mas-Ballesté, R.; Que, L. Bio-Inspired Iron-Catalyzed Olefin Oxidations: Epoxidation Versus cis-Dihydroxylation. In Mechanisms in Homogeneous and Heterogeneous Epoxidation Catalysis; Oyama, S.T., Ed.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 451–469. [Google Scholar]
- Carvalho, N.M.F.; Horn, A., Jr.; Antunes, O.A.C. Cyclohexane oxidation catalyzed by mononuclear iron(III) complexes. Appl. Catal. A Gen. 2006, 305, 140–145. [Google Scholar] [CrossRef]
- Silva, G.C.; Carvalho, N.M.F.; Horn, A., Jr.; Lachter, E.R.; Antunes, O.A.C. Oxidation of aromatic compounds by hydrogen peroxide catalyzed by mononuclear iron(III) complexes. J. Mol. Catal. A Chem. 2017, 426, 564–571. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, C.; Lyu, S.; Liu, H.; Jiang, C.; Lei, Y. Enhancement of Fenton degradation by catechol in a wide initial pH range. Sep. Purif. Technol. 2016, 169, 202–209. [Google Scholar] [CrossRef]
- Bai, C.; Xiao, W.; Feng, D.; Xian, M.; Guo, D.; Ge, Z.; Zhou, Y. Efficient decolorization of Malachite Green in the Fenton reaction catalyzed by [Fe(III)-salen]Cl complex. Chem. Eng. J. 2013, 215–216, 227–234. [Google Scholar] [CrossRef]
- Xu, H.-Y.; Wang, Y.; Shi, T.-N.; Zhao, H.; Tan, Q.; Zhao, B.-C.; He, X.-L.; Qi, S.-Y. Heterogeneous Fenton-like discoloration of methyl orange using Fe3O4/MWCNTs as catalyst: Kinetics and Fenton-like mechanism. Front. Mater. Sci. 2018, 12, 34–44. [Google Scholar] [CrossRef]
- Lan, H.; Wang, A.; Liu, R.; Liu, H.; Qu, J. Heterogeneous photo-Fenton degradation of acid red B over Fe2O3 supported on activated carbon fiber. J. Hazard. Mater. 2015, 285, 167–172. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, C.S.; Li, F.B.; Liu, C.P.; Liang, J.B. Photodegradation of polycyclic aromatic hydrocarbon pyrene by iron oxide in solid phase. J. Hazard. Mater. 2009, 162, 716–723. [Google Scholar] [CrossRef]
- Shilpa, E.R.; Gayathri, V. Polymer immobilized Fe(III) complex of 2-phenylbenzimidazole: An efficient catalyst for photodegradation of dyes under UV/Visible light irradiation. J. Saudi Chem. Soc. 2018, 22, 678–691. [Google Scholar] [CrossRef]
- Huang, D.; Wang, C.; Song, Y. Immobilized complexes of the salen Schiff’s base with metal as oxidation catalysts. Russ. J. Gen. Chem. 2013, 83, 2361–2369. [Google Scholar] [CrossRef]
- Haupt, K.; Linares, A.V.; Bompart, M.; Bui, B.T.S. Molecularly imprinted polymers. Molecular Imprinting. 2012, pp. 1–28. Available online: https://www.springer.com/gp/book/9783642284205 (accessed on 12 September 2021).
- Sun, W.; Tan, R.; Zheng, W.; Yin, D. Molecularly imprinted polymer containing Fe(III) catalysts for specific substrate recognition. Cuihua Xuebao/Chin. J. Catal. 2013, 34, 1589–1598. [Google Scholar] [CrossRef]
- Chen, Z.; Huang, S.; Zhao, M. Molecularly Imprinted Polymers for Biomimetic Catalysts. In Molecularly Imprinted Catalysts; Li, S., Cao, S., Piletsky, S.A., Turner, A.P.F., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 229–239. [Google Scholar]
- Cornish-Bowden, A. Fundamentals of Enzyme Kinetics; Wiley: Weinheim, Germany, 2013. [Google Scholar]
- The IUPAC Stability Constants Database, SC-Database, 5.83; Academic Software: Yorks, UK, 2013.
- Theodoridis, A.; Maigut, J.; Puchta, R.; Kudrik, E.V.; Van Eldik, R. Novel iron(III) porphyrazine complex. Complex speciation and reactions with NO and H2O2. Inorg. Chem. 2008, 47, 2994–3013. [Google Scholar] [CrossRef]
- Arshadi, M.; Abdolmaleki, M.K.; Mousavinia, F.; Khalafi-Nezhad, A.; Firouzabadi, H.; Gil, A. Degradation of methyl orange by heterogeneous Fenton-like oxidation on a nano-organometallic compound in the presence of multi-walled carbon nanotubes. Chem. Eng. Res. Des. 2016, 112, 113–121. [Google Scholar] [CrossRef]
- Guivarch, E.; Trevin, S.; Lahitte, C.; Oturan, M.A. Degradation of azo dyes in water by Electro-Fenton process. Environ. Chem. Lett. 2003, 1, 38–44. [Google Scholar] [CrossRef]
- Campi, E.; Ferguson, J.; Tobe, M.L. Mechanism and steric course of octahedral aquation. XIII. Kinetics and steric course of the acid and base hydrolysis of cis- and trans-dichloro(1,4,8,11-tetraazacyclotetradecane)chromium(III) cations. Inorg. Chem. 1970, 9, 1781–1784. [Google Scholar] [CrossRef]
- Wagenknecht, P.S.; Hu, C.; Ferguson, D.; Nathan, L.C.; Hancock, R.D.; Whitehead, J.R.; Wright-Garcia, K.; Vagnini, M.T. Effects of Steric Constraint on Chromium(III) Complexes of Tetraazamacrocycles, 2. Comparison of the Chemistry and Photobehavior of the trans-Dichloro- and trans-Dicyano-Complexes of Cyclam, 1,4-C2-Cyclam, and 1,11-C3-Cyclam. Inorg. Chem. 2005, 44, 9518–9526. [Google Scholar] [CrossRef]
- Roushani, M.; Beygi, T.M.; Saedi, Z. Synthesis and application of ion-imprinted polymer for extraction and pre-concentration of iron ions in environmental water and food samples. Spectrochim. Acta A 2016, 153, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Mitreva, M.; Dakova, I.; Karadjova, I. Iron(II) ion imprinted polymer for Fe(II)/Fe(III) speciation in wine. Microchem. J. 2017, 132, 238–244. [Google Scholar] [CrossRef]
- Olivo, G.; Cussó, O.; Borrell, M.; Costas, M. Oxidation of alkane and alkene moieties with biologically inspired nonheme iron catalysts and hydrogen peroxide: From free radicals to stereoselective transformations. JBIC J. Biol. Inorg. Chem. 2017, 22, 425–452. [Google Scholar] [CrossRef]
- Chen, K.; Costas, M.; Kim, J.; Tipton, A.K.; Que, L., Jr. Olefin cis-dihydroxylation versus epoxidation by non-heme iron catalysts: Two faces of an FeIII-OOH coin. J. Am. Chem. Soc. 2002, 124, 3026–3035. [Google Scholar] [CrossRef] [PubMed]
- McDonald, A.R.; Que, L. Elusive iron(V) species identified. Nat. Chem. 2011, 3, 761–762. [Google Scholar] [CrossRef] [PubMed]
- Yusof, N.A.; Rahman, S.K.A.B.; Hussein, M.Z.; Ibrahim, N.A. Preparation and characterization of molecularly imprinted polymer as SPE sorbent for melamine isolation. Polymers 2013, 5, 1215–1228. [Google Scholar] [CrossRef] [Green Version]
- Noorhidayah, I.; Mohd Noor, A.; Azalina Mohamed, N.; Islam, A.K.M.S. Computational Modeling and Synthesis of Molecular Imprinted Polymer for Recognition of Nitrate Ion. Malays. J. Anal. Sci. 2015, 19, 866–873. [Google Scholar]
- Yoshimi, Y.; Ishii, N. Improved gate effect enantioselectivity of phenylalanine-imprinted polymers in water by blending crosslinkers. Anal. Chim. Acta 2015, 862, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Patras, G.; Qiao, G.G.; Solomon, D.H. Characterization of the pore structure of aqueous three-dimensional polyacrylamide gels with a novel cross-linker. Electrophoresis 2000, 21, 3843–3850. [Google Scholar] [CrossRef]






| Catalyst | Initial pH | Optimum Kinetic Model a | Conversion (%) (3 h) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MO Degradation | Intermediate L-Fe(III)-OOH | ||||||||
| R2 | First-Order | Carvalho et al. | R2 | Carvalho et al. | |||||
| k (min−1) | k1 (min−1) | k2 (min−1) | k3 (min−1) | k4 (min−1) | |||||
| Fe(III) | 3.9 | 0.99 | 0.027 ± 0.001 | --- | --- | 0.99 | 0.047 ± 0.003 | 0.023 ± 0.001 | 94.7 |
| Fe–BMPA | 4.6 | 0.99 | --- | 0.13 ± 0.01 | 0.0040 ± 0.0007 | 0.98 | 0.16 ± 0.01 | 0.0012 ± 0.0001 | 84.4 |
| Fe–TPA | 3.8 | 0.96 | --- | 0.29 ± 0.04 | 1.8 × 10−14 c | 0.99 | 0.14 ± 0.01 | 0.00025 ± 0.00009 | 28.0 |
| Fe–NTP | 3.4 | 0.98 | 0.0058 ± 0.0003 | --- | --- | 0.98 | 0.019 ± 0.005 | 0.016 ± 0.004 | 73.0 |
| Fe–NTA | 3.5 | 0.99 | 0.00097 ± 0.00005 | --- | --- | --- b | --- | --- | 17.4 |
| Polymeric Matrix | Template | Mass (mg) | Fe Content (mg/g MIP) a | Fe Released (mg/g MIP) a | Fe Released (%) | MO Degradation (%) (3 h) |
|---|---|---|---|---|---|---|
| MIP-MBAA | Fe-BMPA | 175 | 2.41 ± 0.01 | 0.31 ± 0.05 | 12.9 | 95.7 |
| Fe-NTP | 255 | 1.33 ± 0.06 | 0.23 ± 0.02 | 17.3 | 98.7 | |
| MIP-EDMA | Fe-BMPA | 474 | 1.26 ± 0.06 | 0.342 ± 0.001 | 0.1 | 28.4 |
| Fe-NTP | 226 | 11.13 ± 0.03 | 0.18 ± 0.03 | 5.3 | 41.8 | |
| MIP-TAT | Fe-BMPA | 253 | 9.42 ± 0.15 | 0.012 ± 0.001 | 27.1 | 23.5 |
| Fe-NTP | 279 | 1.54 ± 0.03 | 0.082 ± 0.001 | 1.6 | 7.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haller, P.; Machado, I.; Torres, J.; Vila, A.; Veiga, N. Fe(III)-Complex-Imprinted Polymers for the Green Oxidative Degradation of the Methyl Orange Dye Pollutant. Polymers 2021, 13, 3127. https://doi.org/10.3390/polym13183127
Haller P, Machado I, Torres J, Vila A, Veiga N. Fe(III)-Complex-Imprinted Polymers for the Green Oxidative Degradation of the Methyl Orange Dye Pollutant. Polymers. 2021; 13(18):3127. https://doi.org/10.3390/polym13183127
Chicago/Turabian StyleHaller, Paulina, Ignacio Machado, Julia Torres, Agustina Vila, and Nicolás Veiga. 2021. "Fe(III)-Complex-Imprinted Polymers for the Green Oxidative Degradation of the Methyl Orange Dye Pollutant" Polymers 13, no. 18: 3127. https://doi.org/10.3390/polym13183127
APA StyleHaller, P., Machado, I., Torres, J., Vila, A., & Veiga, N. (2021). Fe(III)-Complex-Imprinted Polymers for the Green Oxidative Degradation of the Methyl Orange Dye Pollutant. Polymers, 13(18), 3127. https://doi.org/10.3390/polym13183127