Recent Advances on Stimuli-Responsive Hydrogels Based on Tissue-Derived ECMs and Their Components: Towards Improving Functionality for Tissue Engineering and Controlled Drug Delivery
Abstract
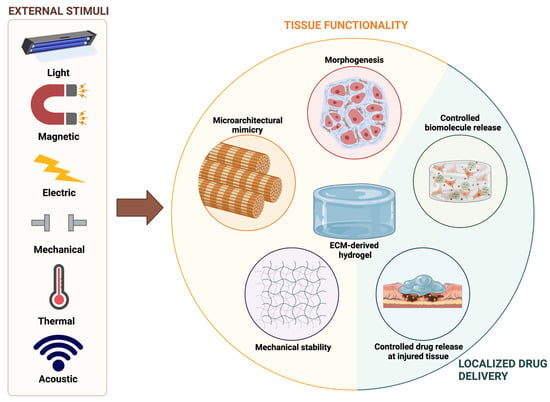
:1. Introduction
2. Enhancing the Mechanical Properties of dECM-Based Hydrogels
2.1. Improving Thermal Gelation Dynamics
2.2. Photocrosslinking
Biochemically Modified dECM-Based Hydrogels with Augmented Photosensitivity
3. Tunning Microarchitectural Characteristics of dECM-Based Hydrogels
3.1. Mechanical Stimuli
3.2. Electrical Stimuli
3.3. Magnetic Stimuli
3.4. Acoustic Stimuli
4. Improving Morphogenesis and Functionality of dECM-Based 3D Cultures with External Stimuli
4.1. Directing Stem Cell Differentiation
4.2. Maturation of Electrosensitive Tissues
4.3. Maturation of Load-Bearing Tissues
5. Stimuli-Responsive dECM-Based Hydrogels for the Delivery of Therapeutics
5.1. Light-Triggered Drug Release
5.2. Magnetic-Triggered Release
5.3. Ultrasound-Triggered Release
5.4. Electric-Triggered Release
5.5. Temperature-Triggered Release
6. Perspectives on Clinical Translation
7. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Li, Z.; Xie, M.-B.; Li, Y.; Ma, Y.; Li, J.-S.; Dai, F.-Y. Recent progress in tissue engineering and regenerative medicine. J. Biomater. Tissue Eng. 2016, 6, 755–766. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, H.-W. Emerging properties of hydrogels in tissue engineering. J. Tissue Eng. 2018, 9, 2041731418768285. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.S.; Khademhosseini, A. Advances in engineering hydrogels. Science 2017, 356, 6337. [Google Scholar] [CrossRef] [PubMed]
- Moroni, L.; Boland, T.; Burdick, J.A.; De Maria, C.; Derby, B.; Forgacs, G.; Grol, J.; Li, Q.; Malda, J.; Mironov, V.A.; et al. Biofabrication: A guide to technology and terminology. Trends Biotechnol. 2018, 36, 384–402. [Google Scholar] [CrossRef] [Green Version]
- Ozbolat, I.T.; Hospodiuk, M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials 2016, 76, 321–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyle, S.; Jessop, Z.M.; Al-Sabah, A.; Whitaker, I.S. Printability of candidate biomaterials for extrusion based 3d printing: State-of-the-Art. Adv. Healthc. Mater. 2017, 6, 1–16. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yang, F.; Zhao, H.; Gao, Q.; Xia, B.; Fu, J. Research on the printability of hydrogels in 3D bioprinting. Sci. Rep. 2016, 6, 29977. [Google Scholar] [CrossRef]
- Schwab, A.; Levato, R.; D’Este, M.; Piluso, S.; Eglin, D.; Malda, J. Printability and shape fidelity of bioinks in 3d bioprinting. Chem. Rev. 2020, 120, 11028–11055. [Google Scholar] [CrossRef]
- Theus, A.S.; Ning, L.; Hwang, B.; Gil, C.; Chen, S.; Wombwell, A.; Mehta, R.; Srepooshan, V. Bioprintability: Physiomechanical and biological requirements of materials for 3d bioprinting processes. Polymers 2020, 12, 2262. [Google Scholar] [CrossRef]
- Ouyang, L.; Yao, R.; Zhao, Y.; Sun, W. Effect of bioink properties on printability and cell viability for 3D bioplotting of embryonic stem cells. Biofabrication 2016, 8, 035020. [Google Scholar] [CrossRef]
- Hölzl, K.; Lin, S.; Tytgat, L.; van Vlierberghe, S.; Gu, L.; Ovsianikov, A. Bioink properties before, during and after 3D bioprinting. Biofabrication 2016, 8, 032002. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef] [PubMed]
- Dimatteo, R.; Darling, N.J.; Segura, T. In situ forming injectable hydrogels for drug delivery and wound repair. Adv. Drug Deliv. Rev. 2018, 127, 167–184. [Google Scholar] [CrossRef]
- Xing, R.; Liu, K.; Jiao, T.; Zhang, N.; Ma, K.; Zhang, R.; Zou, Q.; Ma, G.; Yan, X. An injectable self-assembling collagen-gold hybrid hydrogel for combinatorial antitumor photothermal/photodynamic therapy. Adv. Mater. 2016, 28, 3669–3676. [Google Scholar] [CrossRef] [PubMed]
- Merino, S.; Martín, C.; Kostarelos, K.; Prato, M.; Vázquez, E. Nanocomposite Hydrogels: 3D Polymer–Nanoparticle Synergies for On-Demand Drug Delivery. ACS Nano 2015, 9, 4686–4697. [Google Scholar] [CrossRef] [Green Version]
- Hamidi, M.; Azadi, A.; Rafiei, P. Hydrogel nanoparticles in drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1638–1649. [Google Scholar] [CrossRef]
- Saldin, L.T.; Cramer, M.C.; Velankar, S.S.; White, L.J.; Badylak, S.F. Extracellular matrix hydrogels from decellularized tissues: Structure and function. Acta Biomater. 2017, 49, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Yu, C.; Ma, X.; Zhu, W.; Wang, P.; Miller, K.L.; Stupin, J.; Koroleva-Maharajh, A.; Hairabedian, A.; Chen, S. Scanningless and continuous 3D bioprinting of human tissues with decellularized extracellular matrix. Biomaterials 2018, 194, 1–13. [Google Scholar] [CrossRef]
- Spang, M.T.; Christman, K.L. Extracellular matriz hydrogel therapies: In vivo applications and development. Acta Biomater. 2018, 1, 1–14. [Google Scholar] [CrossRef]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef] [Green Version]
- Wess, T.J. Collagen fibrilar structures and hierarchies. In Collagen; Fratzl, P., Ed.; Springer: Boston, MA, USA, 2008; pp. 49–80. [Google Scholar]
- Ricard-Blum, S. The Collagen Family. Cold Spring Harb. Perspect. Biol. 2011, 3, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hynes, R.O. The extracellular matrix: Not just pretty fibrils. Science 2009, 326, 1216–1219. [Google Scholar] [CrossRef] [Green Version]
- Nusgens, B.-V. Acide hyaluronique et matrice extracellulaire: Une molécule primitive? Ann. Dermatol. Venereol. 2010, 137, S3–S8. [Google Scholar] [CrossRef] [Green Version]
- Schaefer, L.; Schaefer, R.M. Proteoglycans: From structural compounds to signaling molecules. Cell Tissue Res. 2010, 339, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Giobbe, G.G.; Crowley, C.; Luni, C.; Campinoti, S.; Khedr, M.; Kretzschmar, K.; de Santis, M.M.; Zambaiti, E.; Michielin, F.; Meran, L.; et al. Extracellular matrix hydrogel derived from decellularized tissues enables endodermal organoid culture. Nat. Commun. 2019, 10, 5658. [Google Scholar] [CrossRef] [Green Version]
- Yamaoka, T. Preparation Methods for Tissue/Organ-derived dECMs–Effects on Cell Removal and ECM Changes. In Decellularized Extracellular Matrix: Characterization, Fabrication and Applications; Hoshiba, T., Yamaoka, T., Eds.; Royal Society of Chemistry: London, UK, 2019; pp. 15–28. [Google Scholar]
- Fernández-Pérez, J.; Ahearne, M. The impact of decellularization methods on extracellular matrix derived hydrogels. Sci. Rep. 2019, 9, 14933. [Google Scholar] [CrossRef] [Green Version]
- Dzobo, K.; Motaung, K.S.C.M.; Adesida, A. Recent Trends in Decellularized Extracellular Matrix Bioinks for 3D Printing: An Updated Review. Int. J. Mol. Sci. 2019, 20, 4628. [Google Scholar] [CrossRef] [Green Version]
- Annabi, N.; Nichol, J.W.; Zhong, X.; Ji, C.; Koshy, S.; Khademhosseini, A.; Dehghani, F. Controlling the porosity and microarchitecture of hydrogels for tissue engineering. Tissue Eng. Part B Rev. 2010, 16, 371–383. [Google Scholar] [CrossRef]
- Aubin, H.; Nichol, J.W.; Hutson, C.B.; Bae, H.; Sieminski, A.L.; Cropek, D.M.; Akhyari, P.; Khademhosseini, A. Directed 3D cell alignment and elongation in microengineered hydrogels. Biomaterials 2010, 31, 6941–6951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.-C.; Zhang, Y.S.; Akpek, A.; Shin, S.R.; Khademhosseini, A. 4D bioprinting: The next-generation technology for biofabrication enabled by stimuli-responsive materials. Biofabrication 2016, 9, 012001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasiński, A.; Zielińska-Pisklak, M.; Oledzka, E.; Sobczak, M. Smart hydrogels–synthetic stimuli-responsive antitumor drug release systems. Int. J. Nanomed. 2020, 15, 4541–4572. [Google Scholar] [CrossRef]
- Skardal, A.; Devarasetty, M.; Kang, H.-W.; Mead, I.; Bishop, C.; Shupe, T.; Lee, S.L.; Jackson, J.; Yoo, J.; Soker, S. A hydrogel bioink toolkit for mimicking native tissue biochemical and mechanical properties in bioprinted tissue constructs. Acta Biomater. 2015, 25, 24–34. [Google Scholar] [CrossRef]
- Bejleri, D.; Streeter, B.W.; Nachlas, A.L.; Broen, M.E.; Gaetani, R.; Christman, K.L.; Davis, M.E. A bioprinted cardiac patch composed of cardiac-specific extracellular matrix and progenitor cells for heart repair. Adv. Healthc. Mater. 2018, 7, 1800672. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Fernandes-Cunha, G.M.; Myung, D. In situ-forming hyaluronic acid hydrogel through visible light-induced thiol-ene reaction. React. Funct. Polym. 2018, 131, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Liu, S.; Le, Y.; Qin, Z.; He, M.; Xu, F.; Zhu, Y.; Zhao, J.; Mao, C.; Zheng, L. An injectable collagen-genipin-carbon dot hydrogel combined with photodynamic therapy to enhance chondrogenesis. Biomaterials 2019, 218, 119190. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Pr, A.K.; Yoo, J.J.; Zahran, F.; Atala, A.; Lee, S.J. A photo-crosslinkable kidney ecm-derived bioink accelerates renal tissue formation. Adv. Healthc. Mater. 2019, 8, 1800992. [Google Scholar] [CrossRef] [PubMed]
- Parthiban, S.P.; Athirasala, A.; Tahayeri, A.; Abdelmoniem, R.; George, A.; Bertassoni, L.E. BoneMA—synthesis and characterization of a methacrylated bone-derived hydrogel for bioprinting of in vitro vascularized tissue constructs. Biofabrication 2021, 13, 035031. [Google Scholar] [CrossRef]
- Rueda-Gensini, L.; Serna, J.A.; Cifuentes, J.; Cruz, J.C.; Muñoz-Camargo, C. Graphene oxide-embedded extracellular matrix- derived hydrogel as a multiresponsive platform for 3d bioprinting applications. Int. J. Bioprint. 2021, 7. [Google Scholar] [CrossRef]
- Kim, H.; Kang, B.; Cui, X.; Lee, S.-H.; Lee, K.; Cho, D.-W.; Hwang, W.; Woodfield, T.B.F.; Lim, K.S.; Jang, J. Light-activated decellularized extracellular matrix-based bioinks for volumetric tissue analogs at the centimeter scale. Adv. Funct. Mater. 2021, 31, 2011252. [Google Scholar] [CrossRef]
- Keating, M.; Lim, M.; Hu, Q.; Botvinick, E. Selective stiffening of fibrin hydrogels with micron resolution via photocrosslinking. Acta Biomater. 2019, 87, 88–96. [Google Scholar] [CrossRef]
- Xu, X.; Zeng, Z.; Huang, Z.; Sun, Y.; Huang, Y.; Chen, J.; Ye, J.; Yang, H.; Yang, C.; Zhao, C. Near-infrared light-triggered degradable hyaluronic acid hydrogel for on-demand drug release and combined chemo-photodynamic therapy. Carbohydr. Polym. 2020, 229, 115394. [Google Scholar] [CrossRef]
- Cho, S.-H.; Kim, A.; Shin, W.; Heo, M.B.; Noh, H.J.; Hong, K.S.; Cho, J.-H.; Lim, Y.T. Photothermal-modulated drug delivery and magnetic relaxation based on collagen/poly(γ-glutamic acid) hydrogel. Int. J. Nanomed. 2017, 12, 2607–2620. [Google Scholar] [CrossRef] [Green Version]
- Sun, P.; Huang, T.; Wang, X.; Wang, G.; Liu, Z.; Chen, G.; Fan, Q. Dynamic-Covalent Hydrogel with NIR-Triggered Drug Delivery for Localized Chemo-Photothermal Combination Therapy. Biomacromolecules 2020, 21, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.R.; Zihlmann, C.; Akbari, M.; Assawes, P.; Cheung, L.; Zhang, K.; Manoharan, V.; Zhang, Y.S.; Yüksekkaya, M.; Wan, K.-T. Reduced Graphene Oxide-GelMA Hybrid Hydrogels as Scaffolds for Cardiac Tissue Engineering. Small 2016, 12, 3677–3689. [Google Scholar] [CrossRef] [Green Version]
- Ahadian, S.; Yamada, S.; Ramón-Azcón, J.; Estili, M.; Liang, X.; Nakajima, K.; Shiku, H.; Khademhosseini, A.; Matsue, T. Hybrid hydrogel-aligned carbon nanotube scaffolds to enhance cardiac differentiation of embryoid bodies. Acta Biomater. 2016, 31, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-P.; Qu, K.-Y.; Zhou, B.; Zhang, F.; Wang, Y.-Y.; Abodunrin, O.D.; Zhu, Z.; Huang, N.-P. Electrical stimulation of neonatal rat cardiomyocytes using conductive polydopamine-reduced graphene oxide-hybrid hydrogels for constructing cardiac microtissues. Colloids Surf. B Biointerfaces 2021, 205, 111844. [Google Scholar] [CrossRef]
- Tsui, J.H.; Leonard, A.; Camp, N.D.; Long, J.T.; Nawas, Z.Y.; Chavanachat, R.; Smith, A.S.T.; Choi, J.S.; Dong, Z.; Ahn, E.H.; et al. Tunable electroconductive decellularized extracellular matrix hydrogels for engineering human cardiac microphysiological systems. Biomaterials 2021, 272, 120764. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Xu, Y.; Yang, M.; Chang, Y.; Nie, A.; Liu, Z.; Wang, J.; Luo, Z. Black-phosphorus-incorporated hydrogel as a conductive and biodegradable platform for enhancement of the neural differentiation of mesenchymal stem cells. Adv. Funct. Mater. 2020, 30, 2000177. [Google Scholar] [CrossRef]
- Kim, W.; Jang, C.H.; Kim, G.H. A myoblast-laden collagen bioink with fully aligned au nanowires for muscle-tissue regeneration. Nano Lett. 2019, 19, 8612–8620. [Google Scholar] [CrossRef] [PubMed]
- Koppes, A.N.; Keating, K.W.; McGregor, A.L.; Koppes, R.A.; Kearns, K.R.; Ziemba, A.M.; McKay, C.A.; Zuidema, J.M.; Rivet, C.J.; Gilbert, R.J.; et al. Robust neurite extension following exogenous electrical stimulation within single walled carbon nanotube-composite hydrogels. Acta Biomater. 2016, 39, 34–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ha, J.H.; Lim, J.H.; Kim, J.W.; Cho, H.-Y.; Jo, S.G.; Lee, S.H.; Eom, J.Y.; Lee, J.M.; Chung, B.G. Conductive gelma–collagen–agnw blended hydrogel for smart actuator. Polymers 2021, 13, 1217. [Google Scholar] [CrossRef] [PubMed]
- Betsch, M.; Cristian, C.; Lin, Y.-Y.; Blaeser, A.; Schöneberg, J.; Vogt, M.; Buhl, E.M.; Fischer, H.; Duarte Campos, D.F. Incorporating 4d into bioprinting: Real-time magnetically directed collagen fiber alignment for generating complex multilayered tissues. Adv. Healthc. Mater. 2018, 7, 1800894. [Google Scholar] [CrossRef] [PubMed]
- Rose, J.C.; Cámara-Torres, M.; Rahimi, K.; Köhler, J.; Möller, M.; de Laporte, L. Nerve Cells Decide to Orient inside an injectable hydrogel with minimal structural guidance. Nano Lett. 2017, 17, 3782–3791. [Google Scholar] [CrossRef]
- Dai, G.; Sun, L.; Xu, J.; Zhao, G.; Tan, Z.; Wang, C.; Sun, X.; Xu, K.; Zhong, W. Catechol–metal coordination-mediated nanocomposite hydrogels for on-demand drug delivery and efficacious combination therapy. Acta Biomater. 2021, 129, 84–95. [Google Scholar] [CrossRef]
- Bettini, S.; Bonfrate, V.; Syrgiannis, Z.; Sannino, A.; Salvatore, L.; Madaghiele, M.; Valli, L.; Giancane, G. Biocompatible collagen paramagnetic scaffold for controlled drug release. Biomacromolecules 2015, 16, 2599–2608. [Google Scholar] [CrossRef]
- Dong, X.; Lu, X.; Kingston, K.; Brewer, E.; Juliar, B.A.; Kripfgans, O.D.; Fowlkes, J.B.; Franceschi, R.T.; Putnam, A.J.; Liu, Z.; et al. Controlled delivery of basic fibroblast growth factor (bFGF) using acoustic droplet vaporization stimulates endothelial network formation. Acta Biomater. 2019, 97, 409–419. [Google Scholar] [CrossRef]
- Aliabouzar, M.; Jivani, A.; Lu, X.; Kripfgans, O.D.; Fowlkes, J.B.; Fabiilli, M.L. Standing wave-assisted acoustic droplet vaporization for single and dual payload release in acoustically responsive scaffolds. Ultrason. Sonochem. 2020, 66, 105109. [Google Scholar] [CrossRef]
- Chameettachal, S.; Sasikumar, S.; Sethi, S.; Sriya, Y.; Pati, F. Tissue/organ-derived bioink formulation for 3D bioprinting. J. 3D Print. Med. 2019, 3, 39–54. [Google Scholar] [CrossRef]
- Unagolla, J.M.; Jayasuriya, A.C. Hydrogel-based 3D bioprinting: A comprehensive review on cell-laden hydrogels, bioink formulations, and future perspectives. Appl. Mater. Today 2020, 18, 100479. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.Y.; Park, S.-H. ECM Based bioink for tissue mimetic 3d bioprinting. In Biomimetic Medical Materials; Springer: Berlin/Heidelberg, Germany, 2018; pp. 335–353. [Google Scholar]
- Kabirian, F.; Mozafari, M. Decellularized ECM-derived bioinks: Prospects for the future. Methods 2020, 171, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Yang, C. Enhanced physicochemical properties of collagen by using EDC/NHS-crosslinking. Bull. Mater. Sci. 2012, 35, 913–918. [Google Scholar] [CrossRef]
- Chimene, D.; Lennox, K.K.; Kaunas, R.R.; Gaharwar, A.K. Advanced Bioinks for 3D Printing: A Materials Science Perspective. Ann. Biomed. Eng. 2016, 44, 2090–2102. [Google Scholar] [CrossRef]
- Jang, J.; Kim, T.G.; Kim, B.S.; Kim, S.W.; Kwon, S.M.; Cho, D.W. Tailoring mechanical properties of decellularized extracellular matrix bioink by vitamin B2-induced photo-crosslinking. Acta Biomater. 2016, 33, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Hospodiuk, M.; Dey, M.; Sosnoski, D.; Ozbolat, I.T. The bioink: A comprehensive review on bioprintable materials. Biotechnol. Adv. 2017, 35, 217–239. [Google Scholar] [CrossRef] [Green Version]
- Ahn, G.; Min, K.-H.; Kim, C.; Lee, J.-S.; Kang, D.; Won, J.-Y.; Cho, D.-W.; Kim, J.-Y.; Jin, S.; Yun, W.-S.; et al. Precise stacking of decellularized extracellular matrix based 3D cell-laden constructs by a 3D cell printing system equipped with heating modules. Sci. Rep. 2017, 7, 8624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuss, M.A.; Harms, R.; Wu, S.; Wang, Y.; Untrauer, J.B.; Carlson, M.A.; Duan, B. Short-term hypoxic preconditioning promotes prevascularization in 3D bioprinted bone constructs with stromal vascular fraction derived cells. RSC Adv. 2017, 7, 29312–29320. [Google Scholar] [CrossRef] [Green Version]
- Pati, F.; Jang, J.; Ha, D.-H.; Won Kim, S.; Rhie, J.-W.; Shim, J.-H.; Kim, D.-H.; Cho, D.-W. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat. Commun. 2014, 5, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Han, W.; Kim, H.; Ha, D.-H.; Jang, J.; Kim, B.S.; Cho, D.-W. Development of Liver Decellularized Extracellular Matrix Bioink for Three-Dimensional Cell Printing-Based Liver Tissue Engineering. Biomacromolecules 2017, 18, 1229–1237. [Google Scholar] [CrossRef]
- Pereira, R.F.; Bártolo, P.J. 3D bioprinting of photocrosslinkable hydrogel constructs. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.; Rizzo, R.; Surman, F.; Zenobi-Wong, M. Guiding Lights: Tissue Bioprinting Using Photoactivated Materials. Chem. Rev. 2020, 120, 10950–11027. [Google Scholar] [CrossRef]
- Lim, K.S.; Galarraga, J.H.; Cui, X.; Lindberg, G.C.J.; Burdick, J.A.; Woodfield, T.B.F. Fundamentals and Applications of Photo-Cross-Linking in Bioprinting. Chem. Rev. 2020, 120, 10662–10694. [Google Scholar] [CrossRef]
- Serna, J.A.; Florez, S.L.; Talero, V.A.; Briceño, J.C.; Muñoz-Camargo, C.; Cruz, J.C. Formulation and characterization of a SIS-Based photocrosslinkable bioink. Polymers 2019, 11, 569. [Google Scholar] [CrossRef] [Green Version]
- Kato, Y.; Uchida, K.; Kawakishi, S. Aggregation of collagen exposed to UVA in the presence of riboflavin: A plausible role of tyrosine modification. Photochem. Photobiol. 1994, 59, 343–349. [Google Scholar] [CrossRef]
- Au, V.; Madison, S.A. Effects of singlet oxygen on the extracellular matrix protein collagen: Oxidation of the collagen crosslink histidinohydroxylysinonorleucine and histidine. Arch. Biochem. Biophys. 2000, 384, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Mao, X.; Schwend, T.; Littlechild, S.; Conrad, G.W. Resistance of Corneal RFUVA–Cross-Linked Collagens and Small Leucine-Rich Proteoglycans to Degradation by Matrix Metalloproteinases. Investig. Opthalmol. Vis. Sci. 2013, 54, 1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heo, J.; Koh, R.H.; Shim, W.; Kim, H.D.; Yim, H.-G.; Hwang, N.S. Riboflavin-induced photo-crosslinking of collagen hydrogel and its application in meniscus tissue engineering. Drug Deliv. Transl. Res. 2016, 6, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Tirella, A.; Liberto, T.; Ahluwalia, A. Riboflavin and collagen: New crosslinking methods to tailor the stiffness of hydrogels. Mater. Lett. 2012, 74, 58–61. [Google Scholar] [CrossRef]
- Diamantides, N.; Wang, L.; Pruiksma, T.; Siemiatkoski, J.; Dugopolski, C.; Shortkroff, S.; Kennedy, S.; Bonassar, L.J. Correlating rheological properties and printability of collagen bioinks: The effects of riboflavin photocrosslinking and pH. Biofabrication 2017, 9, 34102. [Google Scholar] [CrossRef]
- Rich, H.; Odlyha, M.; Cheema, U.; Mudera, V.; Bozec, L. Effects of photochemical riboflavin-mediated crosslinks on the physical properties of collagen constructs and fibrils. J. Mater. Sci. Mater. Med. 2014, 25, 11–21. [Google Scholar] [CrossRef]
- Mao, Q.; Wang, Y.; Li, Y.; Juengpanich, S.; Li, W.; Chen, M.; Yin, J.; Fu, J.; Cai, X. Fabrication of liver microtissue with liver decellularized extracellular matrix (dECM) bioink by digital light processing (DLP) bioprinting. Mater. Sci. Eng. C 2020, 109, 110625. [Google Scholar] [CrossRef]
- Acosta-Vélez, G.; Linsley, C.; Zhu, T.; Wu, W.; Wu, B. Photocurable Bioinks for the 3D Pharming of Combination Therapies. Polymers 2018, 10, 1372. [Google Scholar] [CrossRef] [Green Version]
- Yue, K.; de Santiago, G.T.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef] [Green Version]
- Datta, P.; Vyas, V.; Dhara, S.; Chowdhury, A.R.; Barui, A. Anisotropy properties of tissues: A basis for fabrication of biomimetic anisotropic scaffolds for tissue engineering. J. Bionic Eng. 2019, 16, 842–868. [Google Scholar] [CrossRef]
- Mauck, R.L.; Baker, B.M.; Nerurkar, N.L.; Burdick, J.A.; Li, W.-J.; Tuan, R.S.; Elliott, D.M. Engineering on the Straight and Narrow: The Mechanics of Nanofibrous Assemblies for Fiber-Reinforced Tissue Regeneration. Tissue Eng. Part B Rev. 2009, 15, 171–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, R.R.; Edgerton, V.R. Skeletal Muscle Architecture. In Encyclopedia of Neuroscience; Springer: Berlin/Heidelberg, Germany, 2009; pp. 3702–3707. [Google Scholar]
- Kim, H.; Jang, J.; Park, J.; Lee, K.-P.; Lee, S.; Lee, D.-M.; Kim, K.H.; Kim, H.K.; Cho, D.-W. Shear-induced alignment of collagen fibrils using 3D cell printing for corneal stroma tissue engineering. Biofabrication 2019, 11, 035017. [Google Scholar] [CrossRef] [PubMed]
- Torricelli, A.A.M.; Wilson, S.E. Cellular and extracellular matrix modulation of corneal stromal opacity. Exp. Eye Res. 2014, 129, 151–160. [Google Scholar] [CrossRef] [Green Version]
- Schwab, A.; Hélary, C.; Richards, R.G.; Alini, M.; Eglin, D.; D’Este, M. Tissue mimetic hyaluronan bioink containing collagen fibers with controlled orientation modulating cell migration and alignment. Mater. Today Bio 2020, 7, 100058. [Google Scholar] [CrossRef]
- Kim, W.; Kim, G. 3D bioprinting of functional cell-laden bioinks and its application for cell-alignment and maturation. Appl. Mater. Today 2020, 19, 100588. [Google Scholar] [CrossRef]
- Das, S.; Kim, S.-W.; Choi, Y.-J.; Lee, S.; Lee, S.-H.; Kong, J.-S.; Park, H.-J.; Cho, D.-W.; Jang, J. Decellularized extracellular matrix bioinks and the external stimuli to enhance cardiac tissue development in vitro. Acta Biomater. 2019, 95, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-J.; Kim, T.G.; Jeong, J.; Yi, H.-G.; Park, J.W.; Hwang, W.; Cho, D.-W. 3D Cell Printing of Functional Skeletal Muscle Constructs Using Skeletal Muscle-Derived Bioink. Adv. Healthc. Mater. 2016, 5, 2636–2645. [Google Scholar] [CrossRef]
- Puetzer, J.L.; Koo, E.; Bonassar, L.J. Induction of fiber alignment and mechanical anisotropy in tissue engineered menisci with mechanical anchoring. J. Biomech. 2015, 48, 1436–1443. [Google Scholar] [CrossRef] [Green Version]
- Murphy, S.; Ohlendieck, K. The extracellular matrix complexome from skeletal muscle. In Composition and Function of the Extracellular Matrix in the Human Body; Travacio, F., Ed.; InTech: London, UK, 2016. [Google Scholar]
- Gillies, A.R.; Lieber, R.L. Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve 2011, 44, 318–331. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Kim, M.-C.; Asada, H.H. Extracellular matrix remodelling induced by alternating electrical and mechanical stimulations increases the contraction of engineered skeletal muscle tissues. Sci. Rep. 2019, 9, 2732. [Google Scholar] [CrossRef]
- Yang, G.; Long, H.; Ren, X.; Ma, K.; Xiao, Z.; Wang, Y.; Guo, Y. Regulation of adipose-tissue-derived stromal cell orientation and motility in 2D- and 3D-cultures by direct-current electrical field. Dev. Growth Differ. 2017, 59, 70–82. [Google Scholar] [CrossRef]
- Chen, C.; Bai, X.; Ding, Y.; Lee, I.-S. Electrical stimulation as a novel tool for regulating cell behavior in tissue engineering. Biomater. Res. 2019, 23, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, T.; Hattori-Aramaki, N.; Sunohara, A.; Okabe, K.; Sakamoto, Y.; Ochiai, H.; Hayashi, R.; Kishi, K. Alignment of Skeletal Muscle Cells Cultured in Collagen Gel by Mechanical and Electrical Stimulation. Int. J. Tissue Eng. 2014, 2014, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Zhou, J.; Huang, Z.; Qu, L.; Lin, N.; Liang, C.; Dai, R.; Tang, L.; Tian, F. Carbon nanotube-incorporated collagen hydrogels improve cell alignment and the performance of cardiac constructs. Int. J. Nanomed. 2017, 12, 3109–3120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lenshof, A.; Laurell, A. Acoustophoresis. In Encyclopedia of Nanotechnology; Springer: Dordrecht, The Netherlands, 2015; pp. 1–6. [Google Scholar]
- Cohen, S.; Sazan, H.; Kenigsberg, A.; Schori, H.; Piperno, S.; Shpaisman, H.; Shefi, O. Large-scale acoustic-driven neuronal patterning and directed outgrowth. Sci. Rep. 2020, 10, 4932. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.; Shin, J.; Park, H.-J.; Rhyou, C.; Kang, D.; Lee, S.-J.; Yoon, Y.; Cho, S.-W.; Lee, H. High-resolution acoustophoretic 3D cell patterning to construct functional collateral cylindroids for ischemia therapy. Nat. Commun. 2018, 9, 5402. [Google Scholar] [CrossRef] [Green Version]
- Petta, D.; Basoli, V.; Pellicciotta, D.; Tognato, R.; Barcik, J.; Arrigoni, C.; Bella, E.D.; Armiento, A.R.; Candrian, C.; Richards, R.G.; et al. Sound-induced morphogenesis of multicellular systems for rapid orchestration of vascular networks. Biofabrication 2020, 13, 015004. [Google Scholar] [CrossRef]
- Ma, Z.; Holle, A.W.; Melde, K.; Qiu, T.; Poeppel, K.; Kadiri, V.M.; Fischer, P. Acoustic Holographic Cell Patterning in a Biocompatible Hydrogel. Adv. Mater. 2020, 32, 1904181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malda, J.; Visser, J.; Melchels, F.P.; Jüngst, T.; Hennink, W.E.; Dhert, W.J.A.; Groll, J.; Hutmacher, D.W. 25th anniversary article: Engineering hydrogels for biofabrication. Adv. Mater. 2013, 25, 5011–5028. [Google Scholar] [CrossRef]
- Williams, D.; Thayer, P.; Martinez, H.; Gatenholm, E.; Khademhosseini, A. A perspective on the physical, mechanical and biological specifications of bioinks and the development of functional tissues in 3D bioprinting. Bioprinting 2018, 9, 19–36. [Google Scholar] [CrossRef]
- Lutolf, M.P.; Hubbell, J.A. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol. 2005, 23, 47–55. [Google Scholar] [CrossRef]
- Groll, J.; Boland, T.; Blunk, T.; Burdick, J.A.; Cho, D.-W.; Dalton, P.D.; Derby, B.; Forgacs, G.; Li, Q.; Mironov, V.A.; et al. Biofabrication: Reappraising the definition of an evolving field. Biofabrication 2016, 8, 013001. [Google Scholar] [CrossRef] [Green Version]
- Farhat, W.; Hasan, A.; Lucia, L.; Becquart, F.; Ayoub, A.; Kobeissy, F. Hydrogels for Advanced Stem Cell Therapies: A Biomimetic Materials Approach for Enhancing Natural Tissue Function. IEEE Rev. Biomed. Eng. 2019, 12, 333–351. [Google Scholar] [CrossRef]
- Hiemer, B.; Krogull, M.; Bender, T.; Ziebart, J.; Krueger, S.; Bader, R.; Jonitz-Heincke, A. Effect of electric stimulation on human chondrocytes and mesenchymal stem cells under normoxia and hypoxia. Mol. Med. Rep. 2018, 18, 2133–2141. [Google Scholar] [CrossRef] [Green Version]
- Matta, C. Calcium signalling in chondrogenesis implications for cartilage repair. Front. Biosci. 2013, S5, S374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eijkelkamp, N.; Quick, K.; Wood, J.N. Transient Receptor Potential Channels and Mechanosensation. Annu. Rev. Neurosci. 2013, 36, 519–546. [Google Scholar] [CrossRef] [PubMed]
- Parate, D.; Franco-Obregón, A.; Fröhlich, J.; Beyer, C.; Abbas, A.A.; Kamarul, T.; Hui, J.H.P.; Yang, Z. Enhancement of mesenchymal stem cell chondrogenesis with short-term low intensity pulsed electromagnetic fields. Sci. Rep. 2017, 7, 9421. [Google Scholar] [CrossRef] [PubMed]
- Viti, F.; Landini, M.; Mezzelani, A.; Petecchia, L.; Milanesi, L.; Scaglione, S. Osteogenic Differentiation of MSC through Calcium Signaling Activation: Transcriptomics and Functional Analysis. PLoS ONE 2016, 11, e0148173. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.W.; Choi, B.H.; Park, S.-H.; Pai, K.S.; Li, T.Z.; Min, B.-H.; Park, S.R. Mechanical Stimulation by Ultrasound Enhances Chondrogenic Differentiation of Mesenchymal Stem Cells in a Fibrin-Hyaluronic Acid Hydrogel. Artif. Organs 2013, 37, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Jing, W.; Hu, X.; Huang, Z.; Cai, Q.; Ao, Y.; Yang, X. Time-dependent effect of electrical stimulation on osteogenic differentiation of bone mesenchymal stromal cells cultured on conductive nanofibers. J. Biomed. Mater. Res. Part A 2017, 105, 3369–3383. [Google Scholar] [CrossRef]
- Leppik, L.; Zhihua, H.; Mobini, S.; Thottakkattumana Parameswaran, V.; Eischen-Loges, M.; Slavici, A.; Helbing, J.; Pindur, L.; Oliveira, K.M.C.; Bhavsar, M.B.; et al. Combining electrical stimulation and tissue engineering to treat large bone defects in a rat model. Sci. Rep. 2018, 8, 6307. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Choi, T.G.; Park, S.; Yun, H.R.; Nguyen, N.N.Y.; Jo, Y.H.; Jang, M.; Kim, J.; Kim., J.; Kang, I.; et al. Mitochondrial ROS-derived PTEN oxidation activates PI3K pathway for mTOR-induced myogenic autophagy. Cell Death Differ. 2018, 25, 1921–1937. [Google Scholar] [CrossRef] [PubMed]
- Aisenbrey, E.A.; Bilousova, G.; Payne, K.; Bryant, S.J. Dynamic mechanical loading and growth factors influence chondrogenesis of induced pluripotent mesenchymal progenitor cells in a cartilage-mimetic hydrogel. Biomater. Sci. 2019, 7, 5388–5403. [Google Scholar] [CrossRef]
- Vaca-González, J.J.; Clara-Trujillo, S.; Guillot-Ferriols, M.; Ródenas-Rochina, J.; Sanchis, M.J.; Ribelles, J.L.G.; Garzón-Alvarado, D.A.; Ferrer, G.G. Effect of electrical stimulation on chondrogenic differentiation of mesenchymal stem cells cultured in hyaluronic acid–Gelatin injectable hydrogels. Bioelectrochemistry 2020, 134, 107536. [Google Scholar] [CrossRef] [PubMed]
- Hess, R.; Jaeschke, A.; Neubert, H.; Hintze, V.; Moeller, S.; Schnabelrauch, M.; Wiesmann, H.-P.; Hart, D.A.; Scharnweber, D. Synergistic effect of defined artificial extracellular matrices and pulsed electric fields on osteogenic differentiation of human MSCs. Biomaterials 2012, 33, 8975–8985. [Google Scholar] [CrossRef]
- Maxwell, J.T.; Wagner, M.B.; Davis, M.E. Electrically induced calcium handling in cardiac progenitor cells. Stem Cells Int. 2016, 2016, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Ma, R.; Liang, J.; Huang, W.; Guo, L.; Cai, W.; Wang, L.; Paul, C.; Yang, H.-T.; Kim, H.W.; Wang, Y. Electrical Stimulation Enhances Cardiac Differentiation of Human Induced Pluripotent Stem Cells for Myocardial Infarction Therapy. Antioxid. Redox Signal. 2018, 28, 371–384. [Google Scholar] [CrossRef]
- Spitzer, N.C. Electrical activity in early neuronal development. Nature 2006, 444, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Wang, S.; Yu, X.; Qiu, J.; Li, J.; Tang, W.; Li, Z.; Mou, X.; Liu, H.; Wang, Z. Construction of a 3D rGO–collagen hybrid scaffold for enhancement of the neural differentiation of mesenchymal stem cells. Nanoscale 2016, 8, 1897–1904. [Google Scholar] [CrossRef]
- Shin, J.; Choi, E.J.; Cho, J.H.; Cho, A.-N.; Jin, Y.; Yang, K.; Song, C.; Cho, S.-W. Three-Dimensional Electroconductive Hyaluronic Acid Hydrogels Incorporated with Carbon Nanotubes and Polypyrrole by Catechol-Mediated Dispersion Enhance Neurogenesis of Human Neural Stem Cells. Biomacromolecules 2017, 18, 3060–3072. [Google Scholar] [CrossRef] [PubMed]
- Mostafavi, E.; Medina-Cruz, D.; Kalantari, K.; Taymoori, A.; Soltantabar, P.; Webster, T.J. Electroconductive Nanobiomaterials for Tissue Engineering and Regenerative Medicine. Bioelectricity 2020, 2, 120–149. [Google Scholar] [CrossRef]
- Yi, J.; Choe, G.; Park, J.; Lee, J.Y. Graphene oxide-incorporated hydrogels for biomedical applications. Polym. J. 2020, 52, 823–837. [Google Scholar] [CrossRef]
- Chen, X.; Ranjan, V.D.; Liu, S.; Liang, Y.N.; Lim, J.S.K.; Chen, H.; Hu, X.; Zhang, Y. In Situ Formation of 3D Conductive and Cell-Laden Graphene Hydrogel for Electrically Regulating Cellular Behavior. Macromol. Biosci. 2021, 21, 2000374. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Miller, A.L.; Park, S.; Waletzki, B.E.; Zhou, Z.; Terzic, A.; Lu, L. Functionalized Carbon Nanotube and Graphene Oxide Embedded Electrically Conductive Hydrogel Synergistically Stimulates Nerve Cell Differentiation. ACS Appl. Mater. Interfaces 2017, 9, 14677–14690. [Google Scholar] [CrossRef]
- Bello, A.B.; Kim, D.; Kim, D.; Park, H.; Lee, S.-H. Engineering and Functionalization of Gelatin Biomaterials: From Cell Culture to Medical Applications. Tissue Eng. Part B Rev. 2020, 26, 164–180. [Google Scholar] [CrossRef] [Green Version]
- Heo, D.N.; Ko, W.-K.; Bae, M.S.; Lee, J.B.; Lee, D.-W.; Byun, W.; Lee, C.H.; Kim, E.-C.; Jung, B.-Y.; Kwon, I.K. Enhanced bone regeneration with a gold nanoparticle–hydrogel complex. J. Mater. Chem. B 2014, 2, 1584–1593. [Google Scholar] [CrossRef]
- Dayem, A.A.; Kim, B.; Gurunathan, S.; Choi, H.Y.; Yang, G.; Saha, S.K.; Han, D.; Han, J.; Kim, K.; Kim, J.-H.; et al. Biologically synthesized silver nanoparticles induce neuronal differentiation of SH-SY5Y cells via modulation of reactive oxygen species, phosphatases, and kinase signaling pathways. Biotechnol. J. 2014, 9, 934–943. [Google Scholar] [CrossRef]
- Kim, K.S.; Choi, H.W.; Yoon, H.E.; Kim, I.Y. Reactive Oxygen Species Generated by NADPH Oxidase 2 and 4 Are Required for Chondrogenic Differentiation. J. Biol. Chem. 2010, 285, 40294–40302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hino, K.; Horigome, K.; Nishio, M.; Komura, S.; Nagata, S.; Zhao, C.; Jin, Y.; Kawakami, K.; Yamada, Y.; Ohta, A.; et al. Activin-A enhances mTOR signaling to promote aberrant chondrogenesis in fibrodysplasia ossificans progressiva. J. Clin. Investig. 2017, 127, 3339–3352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Pabon, L.; Murry, C.E. Engineering Adolescence. Circ. Res. 2014, 114, 511–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roshanbinfar, K.; Mohammadi, Z.; Sheikh-Mahdi Mesgar, A.; Dehghan, M.M.; Oommen, O.P.; Hilborn, J.; Engel, F.B. Carbon nanotube doped pericardial matrix derived electroconductive biohybrid hydrogel for cardiac tissue engineering. Biomater. Sci. 2019, 7, 3906–3917. [Google Scholar] [CrossRef]
- Ronaldson-Bouchard, K.; Yeager, K.; Teles, D.; Chen, T.; Ma, S.; Song, L.; Morikawa, K.; Wobma, H.M.; Vasciaveo, A.; Ruiz, E.C. Engineering of human cardiac muscle electromechanically matured to an adult-like phenotype. Nat. Protoc. 2019, 14, 2781–2817. [Google Scholar] [CrossRef]
- Wittkowske, C.; Reilly, G.C.; Lacroix, D.; Perrault, C.M. In Vitro Bone Cell Models: Impact of Fluid Shear Stress on Bone Formation. Front. Bioeng. Biotechnol. 2016, 4, 87. [Google Scholar] [CrossRef] [Green Version]
- Goldfracht, I.; Efraim, Y.; Shinnawi, R.; Kovalev, E.; Huber, I.; Gepstein, A.; Arbel, G.; Shaheen, N.; Tiburcy, M.; Zimmermann, W.H.; et al. Engineered heart tissue models from hiPSC-derived cardiomyocytes and cardiac ECM for disease modeling and drug testing applications. Acta Biomater. 2019, 92, 145–159. [Google Scholar] [CrossRef]
- Meinert, C.; Schrobback, K.; Hutmacher, D.W.; Klein, T.J. A novel bioreactor system for biaxial mechanical loading enhances the properties of tissue-engineered human cartilage. Sci. Rep. 2017, 7, 16997. [Google Scholar] [CrossRef] [Green Version]
- Vazquez, M.; Evans, B.A.J.; Riccardi, D.; Evans, S.L.; Ralphs, J.R.; Dillingham, C.M.; Mason, D.J. A New Method to Investigate How Mechanical Loading of Osteocytes Controls Osteoblasts. Front. Endocrinol. 2014, 5, 208. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Yang, Z.; Zhang, H.; Chen, W.; Chen, M.; Zhu, Z. Low-intensity pulsed ultrasound regulates proliferation and differentiation of osteoblasts through osteocytes. Biochem. Biophys. Res. Commun. 2012, 418, 296–300. [Google Scholar] [CrossRef]
- Kuo, T.-R.; Chen, C.-H. Bone biomarker for the clinical assessment of osteoporosis: Recent developments and future perspectives. Biomark. Res. 2017, 5, 18. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.-C.; Shen, M.-Y.; Chen, H.-H.; Lin, S.-C.; Chiang, W.-H.; Wu, P.-H.; Chang, C.-W.; Chiang, C.-S.; Chiu, H.-C. Monocytic delivery of therapeutic oxygen bubbles for dual-modality treatment of tumor hypoxia. J. Control. Release 2015, 220, 738–750. [Google Scholar] [CrossRef]
- Niculescu, A.-G.; Grumezescu, A.M. Photodynamic Therapy—An Up-to-Date Review. Appl. Sci. 2021, 11, 3626. [Google Scholar] [CrossRef]
- Highley, C.B.; Kim, M.; Lee, D.; Burdick, J.A. Near-infrared light triggered release of molecules from supramolecular hydrogel-nanorod composites. Nanomedicine 2016, 11, 1579–1590. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Shen, J.; Banerjee, P.; Zhou, S. Core–shell hybrid nanogels for integration of optical temperature-sensing, targeted tumor cell imaging, and combined chemo-photothermal treatment. Biomaterials 2010, 31, 7555–7566. [Google Scholar] [CrossRef] [PubMed]
- Beharry, A.A.; Woolley, G.A. Azobenzene photoswitches for biomolecules. Chem. Soc. Rev. 2011, 40, 4422. [Google Scholar] [CrossRef] [PubMed]
- Rosales, A.M.; Rodell, C.B.; Chen, M.H.; Morrow, M.G.; Anseth, K.S.; Burdick, A. Reversible Control of Network Properties in Azobenzene-Containing Hyaluronic Acid-Based Hydrogels. Bioconjug. Chem. 2018, 29, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Jiang, H.; Wu, X.; Xu, Z.; Yao, C.; Wang, J.; Qin, M.; Jiang, Q.; Wang, W.; Shi, D.; et al. Strong dual-crosslinked hydrogels for ultrasound-triggered drug delivery. Nano Res. 2019, 12, 115–119. [Google Scholar] [CrossRef]
- Ravichandran, R.; Astrand, C.; Patra, H.K.; Turner, A.P.F.; Chotteau, V.; Phopase, J. Intelligent ECM mimetic injectable scaffolds based on functional collagen building blocks for tissue engineering and biomedical applications. RSC Adv. 2017, 7, 21068–21078. [Google Scholar] [CrossRef] [Green Version]
- Shoukat, H.; Buksh, K.; Noreen, S.; Pervaiz, F.; Maqbool, I. Hydrogels as potential drug-delivery systems: Network design and applications. Ther. Deliv. 2021, 12, 375–396. [Google Scholar] [CrossRef]
- Traverse, J.H.; Henry, T.D.; Dib, N.; Patel, A.N.; Pepine, C.; Schaer, G.L.; DeQuach, J.A.; Kinsey, A.M.; Chamberlin, P.; Christman, K.L. First-in-Man Study of a Cardiac Extracellular Matrix Hydrogel in Early and Late Myocardial Infarction Patients. JACC Basic Transl. Sci. 2019, 4, 659–669. [Google Scholar] [CrossRef]
- Noor, N.; Shapira, A.; Edri, R.; Gal, I.; Wertheim, L.; Dvir, T. 3D Printing of Personalized Thick and Perfusable Cardiac Patches and Hearts. Adv. Sci. 2019, 6, 1900344. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Stimulus | Stimulation Parameters | Stimulus Enhancer | Hydrogel | Embedded Cells/Cargo | Effect | Ref. |
|---|---|---|---|---|---|---|
| Light | UV light (365 nm) at 18 W/cm2 intensity for ‘several’ seconds. | Irgacure 2959 | Porcine liver dECM, thiolated-HA, thiolated-gelatin, PEG-acrylate, and PEG-alkyne | Primary human hepatocytes, primary human stellate cells, and primary human Kupffer cells spheroids. | Bioink stiffness increases by more than one order of magnitude upon light stimulus. | [34] |
| White light exposure for 5 min post-bioprinting. | Eosin Y | Cardiac dECM-GelMA | Neonatal human cardiac progenitor cells | Improved mechanical stiffness upon photocrosslinking while maintaining cell viability above 75%. | [35] | |
| Blue-light exposure (405 nm) with 1.5 W/cm2 intensity for 40 s. | Riboflavin phosphate (RFP) | Thiol- and methacryloyl-modified HA | Primary rabbit corneal fibroblasts | Improved hydrogel crosslinking and mechanical stiffness in an RFP-dependent manner. Crosslinking degree dictated bovine serum albumin release profiles and hydrogel degradation. | [36] | |
| NIR laser excitation (808 nm) at 5.6 and 8.3 mW/cm2 power densities for 3 min every other day | Carbon dot nanoparticles | Type I collagen | Bone marrow-derived stem cells (BMSCs) | Increased proliferation and chondrogenic differentiation of BMSCs as a result of non-lethal doses of ROS produced with photodynamic therapy. | [37] | |
| UV exposure (365 nm) for 120 s. | Irgacure 2959 | Methacryloyl-modified kidney dECM, HA, gelatin, and glycerol | Human primary kidney cells | Almost 2-fold increase in storage modulus after photocrosslinking when compared to unmodified kidney dECM-based hydrogels. | [38] | |
| Blue light exposure (405 nm) at 20 W/cm2 for 15, 30, and 45 s. | LAP | Methacryloyl-modified bone dECM | Human dental pulp stem cells, hMSCs, and HUVECs | Storage modulus increase in a dose-dependent manner. | [39] | |
| Blue light exposure (405 nm) at 62 W/cm2 for 1 min | Riboflavin | Methacryloyl-modified SIS dECM | Adipose-derived MSCs | Two-fold increase in storage modulus upon photocrosslinking. | [40] | |
| Blue light exposure (405 nm) at 30 mW/cm2. | Ruthenium/sodium persulfate | Corneal and heart dECM | Human bone marrow- and turbunate-derived MSCs, hiPSC-derived cardiomyocytes | A 2.55- and 3.79-fold increase in compressive and storage modulus, respectively, upon photocrosslinking. High shape fidelity allowed bioprinting of multi-layered and complex anatomical structures. | [41] | |
| Blue light exposure (laser at 488 and LED at 460 nm). | tris (2,2′-bipyridyl) dichlororuthenium (II) hexahydrate/sodium persulfate. | Fibrin | Normal human dermal fibroblasts | The stiffness of fibrin hydrogels was patterned at the micron scale by spatio-selective irradiation with a blue light laser. | [42] | |
| NIR laser irradiation (635 nm) at 169.85 mW/cm2 for 10 min. | meso-Tetra (N-methyl-4-pyridyl) porphine tetrachloride (photosensitive drug) and gold nanoparticles | Type I collagen | meso-Tetra (N-methyl-4-pyridyl) porphine tetrachloride | After a single injection, but multiple treatments with NIR, the combined photothermal and photodynamic therapies achieved complete tumor eradication on a breast tumor xenograft mice model. | [14] | |
| NIR laser irradiation (633 nm) at 50 mW/cm2 for 10 min. | Protophorphyrin IX (PpIX) | Adipic dihydrazide-modified hyaluronic acid conjugated with PpIX, and dialdehyde-functionalized thioketal containing a ROS-cleavable thioketal linker | Doxorubicin (DOX) | Combined photodynamic therapy and chemotherapy that nearly suppressed tumor growth on a tumor xenograft mice model. | [43] | |
| NIR laser irradiation (760 nm) at 0.47 W/cm2 for 3 min. | Indocyanine green | Type I collagen, poly (gamma-glutamic acid) | DOX or granulocyte macrophage colony-stimulating factor | Upon local temperature increase induced by NIR irradiation, the hydrogel network was disrupted, and payload release was increased up to 3-fold in vitro. | [44] | |
| NIR laser irradiation at 660 nm (0.5 W/cm2) for 20 min and, three days later, at 915 nm (0.5 W/cm2) for 10 min. | Perylene diimide zwitterionic polymer | Benzoxaborole-modified HA, fructose-based glycopolymer | DOX and photothermal polymeric nanoparticles | In a 4T1 tumor bearing mice model, tumors were almost eradicated with a combined chemo- and photothermal therapy. First, NIR irradiation at 660 nm led to enzymatic degradation of the hydrogel, thus releasing the DOX and the photothermal nanoparticles. Then, after 3 days, a second NIR irradiation, but at 915 nm, led to localized hyperthermia for killing tumor cells. | [45] | |
| Electric | Biphasic waveforms with 50 ms pulses of 3–5 V/cm at 0.5, 1, 2, and 3 Hz. | rGO | GelMA | Primary neonatal rat cardiomyocytes | Increased contractility of rGO-GelMA constructs with respect to GelMA. | [46] |
| (i) Dielectrophoresis before crosslinking: sinusoidal electric field of 1 MHz and 20 V for 10 s. (ii) Biphasic waveforms with 10 ms pulses of 3 V at 1 Hz applied continuously from day 2 to day 4. | MW-CNTs | GelMA | 129/SVE-derived mouse stem cells (embryoid bodies) | Enhanced electrical conductivity, mechanical stiffness, and cardiac differentiation in aligned CNT-GelMA constructs when compared to unaligned CNT-GelMA and pristine GelMA. | [47] | |
| Rectangular electrical pulses of 2 ms at 1, 2, and 3 Hz applied continuously from days 3 to day 7. | Dopamine-rGO | GelMA | Cardiomyocytes | Improved orientational order of sarcomeres, propagation of intercellular pacing signals, and calcium handling. | [48] | |
| Square-wave pulses of 5 V at 1.5 Hz. | rGO | Myocardial dECM | hiPSC-derived cardiomyocytes | Improved electrophysiological function (calcium handling, action potential duration, and conduction velocity) and physiologically relevant drug responses in rGO-GelMA. | [49] | |
| Constant electric field of 100 mV/cm applied 20 min daily for 1 week. | Polydopamine-modified black phosphorus (PDA-BP) nanosheets | GelMA | Rat bone marrow derived MSCs | Enhanced neural differentiation of MSCs in stimulated PDA-BP@GelMA hydrogels when compared to unstimulated PDA-BP@GelMA and GelMA. | [50] | |
| External electric field of 5 V AC pulses at 1 Hz applied continuously for 21 days. | GNWs | Type I collagen | C2C12 myoblasts | Aligned GNWs in electric field direction favored myoblast alignment and enhanced myotube formation. | [51] | |
| DC stimulation at 50 mV/mm applied continuously for 8 hrs. | SW-CNTs | Matrigel-type I collagen | Neonatal rat dorsal root ganglia cells | Enhanced neurite outgrowth on stimulated SWCNT-loaded hydrogels when compared to unstimulated SWCNT-loaded hydrogels and SWCNT-free hydrogels. | [52] | |
| Electric current below 1 mA generated with an electric potential of 1 V. Applied for 15, 30, 45, and 60 min. | Ag nanowires | GelMA-Collagen | Fluorescein isothiocyanate (FITC)-dextran | Ion currents during electrical stimulation created osmotic gradients that caused periodic hydrogel contractions and facilitated drug release. | [53] | |
| Magnetic | 2 mT cylindrical magnet placed beneath the constructs. | Streptavidin-coated IONs | Type I collagen-agarose | Human primary knee articular chondrocytes | Collagen fiber alignment in magnetic field direction, as a result of ION motion inside hydrogel, improved type II collagen secretion by chondrocytes. | [54] |
| 100 mT magnetic field. | Rod-shaped acrylate-modified poly(ethylene oxide-stat-propylene oxide) microgels embedded with IONs | Fibrin | Chicken-derived primary dorsal root ganglions | Unidirectional alignment of rod-shaped microgels in field direction-oriented neurite outgrowth inside hydrogel. | [55] | |
| External alternating magnetic field (1478 Hz, 10 A, 10 min). | IONs | Dopamine-conjugated hyaluronan and IONs | DOX | IONs serve as structural crosslinkers and facilitate hyperthermia treatment and on-demand release of DOX under alternating magnetic fields. | [56] | |
| Permanent magnet placed against the hydrogel immersed in water. | IONs | Collagen | Fluorescein sodium salt | Cargo release was triggered and accurately controlled upon hydrogel deformation induced by external magnetic field. | [57] | |
| Acoustic | 2.5 MHz ultrasound at either 3.3 or 8.8 MPa peak rarefactional pressures with 4.3 or 17.2 MPa peak compressional pressures, respectively. | Perfluorocarbon (PFC) double emulsion droplets | Fibrin | bFGF | Ultrasound stimulus induced vaporization of PFC in the double droplet emulsions, thereby causing the release of encapsulated bFGF. The timely release of this factor promoted angiogenic sprouting of HUVECs within the outer layer of the fibrin hydrogel. | [58] |
| Acoustic standing wave fields of 3.25 MHz at day 0 and of 8.6 MHz at day 4 | Two PFC double emulsion droplets based on perfluoropentane and perfluorohexane | Fibrin | bFGF and PDGF-BB | Sequential payload release induced by vaporization of PFC double droplet emulsions at different pressure thresholds modulated with ultrasound standing wave fields. bFGF was released at day 0 and PDGF-BB at day 4. | [59] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serna, J.A.; Rueda-Gensini, L.; Céspedes-Valenzuela, D.N.; Cifuentes, J.; Cruz, J.C.; Muñoz-Camargo, C. Recent Advances on Stimuli-Responsive Hydrogels Based on Tissue-Derived ECMs and Their Components: Towards Improving Functionality for Tissue Engineering and Controlled Drug Delivery. Polymers 2021, 13, 3263. https://doi.org/10.3390/polym13193263
Serna JA, Rueda-Gensini L, Céspedes-Valenzuela DN, Cifuentes J, Cruz JC, Muñoz-Camargo C. Recent Advances on Stimuli-Responsive Hydrogels Based on Tissue-Derived ECMs and Their Components: Towards Improving Functionality for Tissue Engineering and Controlled Drug Delivery. Polymers. 2021; 13(19):3263. https://doi.org/10.3390/polym13193263
Chicago/Turabian StyleSerna, Julian A., Laura Rueda-Gensini, Daniela N. Céspedes-Valenzuela, Javier Cifuentes, Juan C. Cruz, and Carolina Muñoz-Camargo. 2021. "Recent Advances on Stimuli-Responsive Hydrogels Based on Tissue-Derived ECMs and Their Components: Towards Improving Functionality for Tissue Engineering and Controlled Drug Delivery" Polymers 13, no. 19: 3263. https://doi.org/10.3390/polym13193263
APA StyleSerna, J. A., Rueda-Gensini, L., Céspedes-Valenzuela, D. N., Cifuentes, J., Cruz, J. C., & Muñoz-Camargo, C. (2021). Recent Advances on Stimuli-Responsive Hydrogels Based on Tissue-Derived ECMs and Their Components: Towards Improving Functionality for Tissue Engineering and Controlled Drug Delivery. Polymers, 13(19), 3263. https://doi.org/10.3390/polym13193263