The Synthesis of a Covalent Organic Framework from Thiophene Armed Triazine and EDOT and Its Application as Anode Material in Lithium-Ion Battery
Abstract
:1. Introduction
2. Experimental
2.1. Synthesis of the Monomer TBYT
2.2. The Synthesis of PTT-O and PTT-O@C
2.3. The Detailed Information on Materials and Characterization
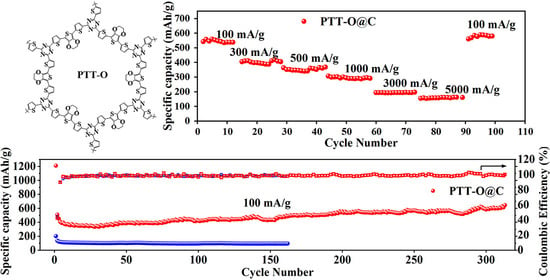
3. Results and Discussion
3.1. Materials Characterization
3.2. Electrochemical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bi, X.; Bai, P.; Yang, T.; Zhang, J.Y.; Li, H.; Chai, Z.L.; Wang, X.J. In-situ synthesis of Ta2O5@few-layered rGO core-shell nanospere with abundant oxygen vacancies for highly stable lithium-ion battery. J. Solid State Electrochem. 2020, 24, 1567–1575. [Google Scholar] [CrossRef]
- William, J.J.; Babu, I.M.; Muralidharan, G. Nichel bismuth oxide as negative electrode for battery-type asymmetric supercapacitor. Chem. Eng. J. 2021, 422, 130058. [Google Scholar] [CrossRef]
- Chai, W.W.; Yin, W.H.; Wang, K.; Ye, W.K.; Tang, B.; Rui, Y. Carbon-coated bismuth nanospheres derived from Bi-BTC as a promising anode material for lithium storage. Electrochim. Acta 2019, 325, 134927. [Google Scholar] [CrossRef]
- Zhao, T.; Ji, R.; Meng, Y. Foamed porous structure Fe-Mn oxides/C composites as novel anode materials of lithium-ion batteries. J. Alloy. Compd. 2021, 882, 160643. [Google Scholar] [CrossRef]
- Zu, Y.F.; Xu, Y.; Ma, L.J.; Kang, Q.; Yao, H.F.; Hou, J.H. Carbonyl bridge based p−π conjugated polymers as high performance electrodes of organic lithium-ion batteries. ACS Appl. Mater. Interfaces 2020, 12, 18457–18464. [Google Scholar] [CrossRef]
- Wu, I.S.; Rui, X.H.; Wang, C.Y.; Pei, W.B.; Lau, R.; Yan, Q.Y.; Zhang, Q.C. Nanostructured conjugated ladder polymers for stable and fast lithium storage anodes with high capacity. Adv. Energy Mater. 2015, 6, 1402189. [Google Scholar] [CrossRef]
- Buyukcakir, N.; Ryu, J.; Joo, S.H.; Kang, J.; Yuksel, R.; Lee, J.; Jiang, Y.; Choi, S.; Lee, S.H.; Kwak, S.K.; et al. Lithium accommodation in a redox-active covalent triazine framwork for high areal capacity and fast-charging lithium-ion batteries. Adv. Funct. Mater. 2020, 30, 2003761. [Google Scholar] [CrossRef]
- Cui, H.X.; Hu, P.D.; Zhang, Y.; Huang, W.W.; Li, A. Researcch progress of high performance organic material pyrene-4,5,9,10-tetraone in secondary batteries. ChemElectroChem 2021, 8, 352–359. [Google Scholar] [CrossRef]
- Lu, Y.; Hou, X.S.; Miao, L.C.; Li, L.; Shi, R.J.; Liu, L.J.; Chen, J. Cyclohexanehexone with ultrahigh capacity as cathode materials for lithium ion batteries. Angew. Chem. Int. Ed. 2019, 58, 7020–7024. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yao, C.J.; Nie, H.J.; Wang, K.Z.; Zhong, Y.W.; Chen, P.W.; Mei, S.L.; Zhang, Q.C. Recent progress in carbonyl-based organic polymers as promising electrode materials for lithium-ion batteries. J. Mater. Chem. A 2020, 8, 11906–11922. [Google Scholar] [CrossRef]
- Wang, Y.R.; Liu, Z.; Liu, H.J.; Liu, H.; Li, B.; Guan, S.Y. A novel high capacity anode material derived from aromatic imides for lithium-ion batteries. Small 2018, 14, 1704094. [Google Scholar] [CrossRef] [PubMed]
- Lei, A.A.D.; Yang, Q.S.; Xu, Y.; Guo, S.Y.; Sun, W.W.; Liu, H.; Lv, L.P.; Zhang, Y.; Wang, Y. Boosting lithium storage in covalent organic framework via activation of 14-electron redox chemistry. Nat. Commun. 2018, 9, 576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Q.C.; Xue, Z.H.; Li, M.C.; Qiu, P.M.; Li, C.G.; Wang, S.P.; Yu, J.X.; Nara, H.; Na, J.; Yamauchi, Y. Electrochemical activity of nitrogen-containing groups in organic electrode materials and related improvement strategies. Adv. Energy Mater. 2021, 11, 2002523. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Y.D.; Xu, C.Y.; Cao, M.F.; Dou, H.; Zhang, X.G. Two π-conjugated covalent organic frameworks with long-term cyclability at high current density for lithium ion battery. Chem.-Eur. J. 2019, 25, 15472–15476. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Lee, W.J.; Paek, S.M.; Park, J.K. Covalent organic nanosheets as effective sodium-ion storage materials. ACS. Appl. Mater. Interfaces 2018, 10, 32102–32111. [Google Scholar] [CrossRef]
- Yue, H.G.; Ju, X.P.; Du, Y.C.; Zhang, Y.; Du, H.M.; Zhao, J.S.; Zhang, J.H. Synthesis of the novel thienoisoindigo-based donor-acceptor type conjugated polymers and the stable switching performance of purple-to-transparent as the electrochromic materials. Org. Electron. 2021, 95, 106183. [Google Scholar] [CrossRef]
- Sun, U.; Xie, J.; Guo, W.; Li, D.S.; Zhang, Q.C. Covalent-organic framework: Advanced organic electrode materials for rechargeable batteries. Adv. Energy Mater. 2020, 10, 1904199. [Google Scholar] [CrossRef]
- Yuan, Q.; Li, C.X.; Guo, X.; Zhao, J.S.; Zhang, Y.; Wang, B.; Dong, Y.Y.; Liu, L.X. Electrochemical performance and storage mechanism study of conjugate donor–acceptor organic polymers as anode materials of lithium-ion battery. Energy Rep. 2020, 6, 2094–2105. [Google Scholar] [CrossRef]
- Li, C.X.; Guo, X.; Du, H.M.; Zhao, J.S.; Liu, L.X.; Yuan, Q.; Fu, C.G. The synthesis of the D-A-type polymers containing benzo[1,2-b:6,5-b′]dithiophene-4,5-dione unit, their composites with carbon, and the lithium storage performances as electrode materials. J. Solid State Electr. 2021, 25, 1847–1859. [Google Scholar] [CrossRef]
- Gao, H.; Tian, B.B.; Yang, H.F.; Neale, A.R.; Little, M.A.; Sprick, R.S.; Hardwick, L.J.; Cooper, A.I. Crosslinked polyimide and reduced graphene oxide composites as long cycle life positive electrode for lithium-ion cells. ChemSusChem 2020, 13, 5571–5579. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Luo, J.M.; Kong, L.Q.; Zhao, J.S.; Zhang, Y.; Du, H.M.; Chen, S.; Xie, Y. The synthesis of triazine–thiophene–thiophene conjugated porous polymers and their composites with carbon as anode materials in lithium-ion batteries. RSC Adv. 2021, 11, 10688–10698. [Google Scholar] [CrossRef]
- Liu, Z.J.; Sun, G.Y.; Cai, X.H.; Yang, F.; Ma, C.; Xue, M.; Tao, X.Y. Nanostructured strategies towards boosting organic lithium-ion batteries. J. Energy Chem. 2021, 54, 179–193. [Google Scholar] [CrossRef]
- Wu, J.; Xie, Y.; Ling, Y.; Si, J.C.; Li, X.; Wang, J.L.; Ye, H.; Zhao, J.S.; Li, S.Q.; Zhao, Q.D.; et al. One-step synthesis and Gd3+ decoration of BiOBr microspheres consisting of nanosheets toward improving photocatalytic reduction of CO2 into hydrocarbon fuel. Chem. Eng. J. 2020, 400, 125944. [Google Scholar] [CrossRef]
- Guo, X.; Yuan, Q.; Li, C.X.; Du, H.M.; Zhao, J.S.; Liu, L.X.; Li, Y.W.; Xie, Y.; Vaidya, V. The synthesis of alternating donor–acceptor polymers based on pyrene-4,5,9,10-tetraone and thiophene derivatives, their composites with carbon, and their lithium storage performances as anode materials. RSC Adv. 2021, 11, 15044–15053. [Google Scholar] [CrossRef]
- Wang, I.; Yao, H.Y.; Du, C.Y.; Guan, S.W. Polyimide schiff based as a high-performance anode material for lithium-ion batteries. J. Power Sources 2021, 482, 228931. [Google Scholar] [CrossRef]
- Zhao, H.; Sheng, L.; Wang, L.; Xu, H.; He, X.M. The opportunity of metal organic frameworks and covalent organic frameworks in lithium (ion) batteries and fuel cells. Energy Storage Mater. 2020, 33, 360–381. [Google Scholar] [CrossRef]
- Wang, F.M.; Rick, J. Synergy of Nyquist and Bode electrochemical impedance spectroscopy studies to commercial type lithium ion batteries. Solid State Ion. 2014, 268, 31–34. [Google Scholar] [CrossRef]
- Han, M.C.; Zhang, J.H.; Cui, P.; Yi, T.F.; Li, X.F. Porous ZnTiO3 rods as a novel lithium storage material for Li-ion batteries. Ceram. Int. 2020, 46, 14030–14037. [Google Scholar] [CrossRef]
- Gu, F.L.; Liu, W.B.; Huang, R.; Song, Y.H.; Jia, J.B.; Wang, L. A g-C3N4 self-templated preparation of N-doped carbon nanosheets@Co-Co3O4/Carbon nanotubes as high-rate lithium-ion batteries’ anode materials. J. Colloid Interf. Sci. 2021, 597, 1–8. [Google Scholar] [CrossRef] [PubMed]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Wang, S.; Xue, X.; Zhao, J.; Du, H. The Synthesis of a Covalent Organic Framework from Thiophene Armed Triazine and EDOT and Its Application as Anode Material in Lithium-Ion Battery. Polymers 2021, 13, 3300. https://doi.org/10.3390/polym13193300
Chen S, Wang S, Xue X, Zhao J, Du H. The Synthesis of a Covalent Organic Framework from Thiophene Armed Triazine and EDOT and Its Application as Anode Material in Lithium-Ion Battery. Polymers. 2021; 13(19):3300. https://doi.org/10.3390/polym13193300
Chicago/Turabian StyleChen, Shuang, Shukun Wang, Xin Xue, Jinsheng Zhao, and Hongmei Du. 2021. "The Synthesis of a Covalent Organic Framework from Thiophene Armed Triazine and EDOT and Its Application as Anode Material in Lithium-Ion Battery" Polymers 13, no. 19: 3300. https://doi.org/10.3390/polym13193300
APA StyleChen, S., Wang, S., Xue, X., Zhao, J., & Du, H. (2021). The Synthesis of a Covalent Organic Framework from Thiophene Armed Triazine and EDOT and Its Application as Anode Material in Lithium-Ion Battery. Polymers, 13(19), 3300. https://doi.org/10.3390/polym13193300