1. Introduction
Since the discovery of graphene, significant progress has been achieved in the synthesis, research, and practical application of nanographene structures. Thus, graphene, which possesses unique properties required for commercial applications, such as high heat conductivity and electron conductivity, mechanical strength, chemical stability, and optical transparency, may be the most promising material for advanced nanoelectronics and optoelectronics [
1,
2,
3,
4]. The prospects for widespread application of graphene and graphene-based composites as photodetectors [
5], solar cells [
6], super capacitors [
7,
8], optoelectronic devices [
9,
10], biosensors [
11,
12], SERS substrates [
13,
14], and nanoliquids [
15,
16] have been demonstrated.
It should be noted that the properties of graphene itself and its derivatives essentially depend on the synthesis technique used, the dimensions and number of the graphene’s atomic layers, and the concentration of vacancy, edge and other structural defects, which impact the device’s performance considerably. The basic properties of 2D graphene, multilayered graphene, and 3D graphene-based composites are essentially different; therefore, studying the physical and chemical properties of graphenic materials creates a firm basis of scientific knowledge for achieving controlled changes in the wide spectrum of parameters of nanostructured carbonic materials.
Along with graphene-based composites filled with single- or double-layer particles, the functional properties of materials with multilayered graphene nanoparticles (MLGs) and their prospective applications have been studied intensively. In multilayer particles, the weak interaction of neighboring monoatomic graphene layers leads to a weak three-dimensionalization of physical properties. Electron scattering increases, while both heat and electrical conductivities decrease. Some parameters of polymer composites filled with highly purified thermally expanded graphite can exceed those of graphene-based composites [
17]; however, this circumstance does not restrict the scope of applications for MLG-filled polymer composites.
MLGs can possess basal surfaces of large size, whereas their lateral surface is of nano-sized thickness. The number of graphene layers can exceed one hundred. In MLG-filled polymer composites, the contacts between the neighboring particles significantly distort both the physical and chemical properties. Therefore, in order to prevent the aggregation of particles and their coming into contact within the host polymer matrix, it is most efficient to use particles with a basal plane size of no more than several tens of μm as a filler [
4,
18]. Due to the particles’ size and surface reactivity, their spatial distribution and orientation in the host polymer matrix contact between particles has an impact on the polymer’s molecular structure and, accordingly, on the mechanical, thermic, and dielectric properties of the composites; therefore, the experimental results obtained in different laboratories are ambiguous [
19,
20,
21,
22]. Nevertheless, in the range of fillings
> 1% the mechanical parameters (compressive and tensile strength) of polymer composites filled with different graphene types are characterized by close values, albeit far from the theoretically predicted values [
23,
24,
25,
26,
27,
28]. However, in the interval of mass concentration
≤ 1% the loading effect of MLGs on mechanical parameters of polymer composites is less studied [
23,
29,
30].
MLGs, along with high mechanical strength, possess a basic surface with high reactivity as well as a nanoscale lateral one, making them promising for applications as fillers in polymer composites to improve their thermal stability [
18,
19,
26,
27,
31,
32]. As a rule, the heat resistance of MLG-filled polymer composites increases with increasing
. Moreover, MLGs are capable of changing the mechanism underlying the thermal destruction of polymer macromolecular chains. In particular, the appearance of an additional, thermal-oxidative destruction and the transformation of the two-stage matrix decomposition process into a one-stage one after loading a polymer matrix with graphene particles was reported in [
32].
The atomic composition of the thermal destruction products of epoxy composites at low MLG loadings has been determined previously using thermal desorption mass spectrometry. It was established that loading polymer with MLGs has no effect on the atomic composition of volatile destruction products, but it changes both the desorption intensity and the outputs of the products [
33].
It should be noted that both the mechanical parameters and the thermal stability of polymer composites are dependent on the surface reactivity of the filling particles, their spatial ordering in a host polymer matrix, and peculiarities of the interphase interaction. Hence, a character of the interface interaction and the molecular structure are key factors determining the performance of various polymer composites. The influence of the interface interaction on the polymer molecular structure has been studied using dielectric spectroscopy in many works. The frequency and concentration dependences of the dielectric permittivity (
) and the dielectric loss factor (
) for various graphenic composites filled with single-layer [
34], double-layered [
1,
35], and multilayered (more than three layers) [
36,
37,
38] graphene particles and their mixtures [
39,
40,
41,
42] have been investigated. It has been discovered that at prethreshold concentrations of conductive and dielectrical nanoparticles, the polymer matrix may demonstrate frequency and concentration behavior of
inherent to both dielectrical and metallic systems [
43,
44,
45,
46,
47]. The special behavior of dielectric permittivity has been attributed to the specific molecular structure within the interphase area of the nanocomposites studied. In particular, some models have been proposed to describe the interphase area structure. These include: multicore and dual layer models [
48,
49], a model involving the concept of quasiconductive layers or filaments among graphene particles in the polymer matrix [
50], some models involving charge exchange among neighboring graphene particles, dielectric filler particles or atomic groups in polymer chains [
44,
47], or among separate segments of polymer chains (so-called micro-capacity model) [
51,
52,
53,
54], and also a cluster model or model of flip-flop transitions [
55,
56].
The wide diversity of proposed theoretical models for polymer matrix structure evidences the debatable status of the problem of establishing the interface interaction mechanisms and peculiarities of the influence of the interphase area on the matrix’s molecular structure and thus on the composite’s overall properties. Therefore, the aim of this work was to investigate the interphase interaction and its effect on the molecular structure of epoxy resin nanocomposites filled with nonoxidized MLGs and, as a consequence, to construct a polymer structure model capable of describing the behavior of nanocomposites under mechanical or thermal loading, under loading by either a low-intensity alternating electro-magnetic field or a positron beam.
Epoxy composites filled with nonoxidized multilayered graphene particles with lateral dimensions of no more than 5 × 5 μm and a thickness of about 50 nm were studied. In the composites, the mass values were no more than 5% and did not exceed the threshold value. Such values excluded the formation of conducting graphene clusters. The studies were carried out using several experimental methods, including Raman scattering, static mechanical loading, thermal desorption mass spectrometry, broadband dielectric spectroscopy, and lifetime positron spectroscopy.
3. Results and Discussion
3.1. Mechanical Loading Data
The static compression-loading stress (
)–strain (
) curves for the neat epoxy resin and its MLG composites are shown in
Figure 4. The curves demonstrate two loading intervals that are characterized by different physical mechanisms of deformation. In the deformation range of
3, where strain increases linearly with ε, the deformation is elastic. The conformational plastic deformation occurs at
2.5, when
dependence remains practically unchanged, but irreversible configuration alterations take place in the polymer macromolecular structure. The configuration alterations are unavoidably accompanied by relevant alteration in the free volume of the composites.
The concentration dependences of the compressive strength (
) and the Young’s modulus (
) for MLG composites exhibit a similar nonmonotonous behavior with increasing
. Namely, both
and
values increase in the extremely narrow loading interval of
≤ 0.01%, whereas they both decrease with
within the interval 0.01
1% (
Figure 5).
The conformational deformation is inherent to the neat epoxy (
Figure 4a). Its quantitative measure is
, where
and
correspond the onset and ending of the plastic deformation in
curves. In the MLG composites, Δ
ε varies nonmonotonously with increasing
. At
0.01%, both
and correspondent free volume (f.v.), where the conformational deformation takes place, increase (
Figure 4b). However, in the concentration interval of 0.01
1%,
and f.v. decrease gradually with increasing
(
Figure 4c–e). Thus, the volume of conformational deformations reveals its nonmonotonic concentration dependence in the region
1%.
The behavior of the f.v. versus
is correlated with one of the parameters
and
(
Figure 5). Loading with graphene particles changes the polymeric structure chains, the free volume of the composites, and their mechanical parameters in a similar way. Increasing f.v. at
0.01% leads to growth of
and
. In addition, vice versa, decreasing f.v. at
0.01% is accompanied by decrements in both
and
.
It should be noted that the alteration of the macromolecular structure and the corresponding variations in f.v., and occurring at 0.01% can be attributed to the fastening of unbound moieties of polymer chains with graphene’s active surface sites (ASSs). The fastening process decreases the faultiness of both epoxy and crosslinks. On the other hand, a frame of MLGs restricts the chain’s mobility. Numerical estimations show that there are 42 MLG particles per 1 of 0.01% nanocomposite (provided that the particles are uniformly distributed over the bulk). Thus, these two effects lead to a more perfect and elastic molecular structure which is characterized by increased free volume and enhanced mechanical parameters at 0.01%.
In the concentration interval of 0.01 1%, the disordering of the macromolecular structure is increased due to the nonuniform spatial distribution of sites and the formation of interphase layers around MLGs. The free volume and mechanical parameters are decreased.
3.2. T. hermal Destruction Data
3.2.1. Destruction’s Volatile Products
Figure 6 shows the mass spectra of atomic fragments of thermal destruction of the neat resin and its MLG composite filled with 1% MLGs. The mass spectra were obtained at temperatures corresponding to the maximal intensity of the lines. It can be seen that the rather strong lines correspond to the following
moieties: 14–18, 28–31, 40–45, 56–59, 65–67, 94. The majority of lines observed in the spectra can easily be identified by looking at the epoxy’s structural formula [
57]. Among these lines, hydroxyl- and oxygen-containing fragments of
= 17 (OH), 28 (CO), 43 (CH
2COH), and 94 (C
6H
5OH) originate from epoxy chains.
The embedding of MLGs into epoxy does not vary the total number of lines. However, the interaction of polymer atoms with the graphene’s ASSs significantly affects the thermal decomposition intensity of the epoxy matrix and its thermal stability. The loading of epoxy with MLGs enhances the thermal stability of the composites. The latter is clearly manifested upon
1%, when the line’s intensity decreases by ~3–7 times (
Figure 6).
Peculiarities of the MLG–epoxy interaction are clearly visible in the thermal desorption curves (
Figure 7) calculated from the mass-spectra. Unbound moieties reveal themselves in the desorption curves at temperatures
≤ 250 °C (
Figure 7a,b curve 1). In the composites, desorption peaks corresponding to unbound OH- and O-containing fragments vanish (
Figure 7a,b curves 2, 3 and 4). Simultaneously, the thermal destruction intensity of the built-in-network epoxy’s chains and crosslinks is significantly decreased, which occurs in the temperature range of 250–450 °C (
Figure 7a,b curves 2, 3 and 4).
Normalized concentration dependences
for thermal desorption peaks
are plotted in
Figure 7e.
Thus, embedding MLGs into the host epoxy matrix significantly changes the epoxy’s molecular structure due to the interaction of ASSs with both unbound moieties and those incorporated into the macromolecular network. The decreased intensity of the thermal desorption lines indicates an increase in the thermal stability of the polymeric structure and is a consequence of the stabilization effect, which takes place in composites at low filler loading [
65,
67]. A mechanism for stabilizing polymer structures is the fastening of both unbound moieties and those incorporated into the macromolecular network to ASSs. It should be noted that the main contribution to the improvement in thermal stability comes from the fastened moieties incorporating the hydroxyl group and oxygen, such as OH, CO, CH
2COH and C
6H
5COH (
Figure 6).
It may be concluded from examination of
Figure 7 that the fastening process of unbound moieties seems to be saturated at
1%. Further MLG loading has a slight effect on the desorption intensity of stabilized polymeric structure. Indeed, there is a slight nonmonotonic increase in the destruction intensity, with a maximum at
= 2% (
Figure 7a,b curves 2, 3 and 4). Hence, the stabilization process is mainly fueled by the fastening of unbound moieties in the concentration range
1%, whereas fastening of network-incorporated moieties apparently dominates at
1%.
On the other hand, a nonmonotonous increase of the thermal destruction intensity with increasing
at
1% (see
Figure 7e) may evidence a nonregular spatial alteration of polymeric structure due to a random distribution of fastening sites at the MLG–epoxy interface. Indeed, since the basal and lateral surfaces of graphene particles have a complicated morphology that is characterized by bends, pores, waviness, roughness and edge defects (see
Figure 1), the ASSs locate in an arbitrary manner over the surface and thus result in a random distribution of sites of fastening. As a result, the fastening is accompanied by the formation of interphase layers around MLG particles. The interphase layers are characterized with a nonuniform distribution in both mass and bound-charge densities. Therefore, the dielectric parameters of the interface regions are essentially assumed to be increased with respect to those of the remote regions (as was discussed above, in 3.2.1).
The degree of this structural disordering is higher than that in remote regions, and it varies nonmonotonously with increasing . It may be assumed that terminal and lateral O- and OH-containing epoxy-chain segments bearing relatively high dipole moments will “catalyze” the process of the chain’s destruction in the interphase regions. This process is capable of deteriorating the composite’s overall properties at high values.
The concentration dependences for the thermodesorbed fragment’s outputs
are shown in
Figure 8. The
values were calculated using the following expression:
where
= 250 °C and
= 550 °C are the bounds for the temperature interval of the thermal desorption peak of intensity
for the
ith thermally desorbed fragment of the nanocomposite of a given MLG’s concentration
.
One can see that the curves descend steeply in the region of 0 1% and then decrease nonmonotonously, with a slight maximum at = 2%, when is increased. A value of averaged over the fragments can be used as a quantitative measure of the material’s thermal stability. The lower the averaged value, the higher the thermal stability.
3.2.2. Activation Energy of Thermal Destruction
Nonmonotonous variations in with increasing can be explained by the corresponding variations in the desorption activation energies of thermodesorbed fragments.
The
values were calculated by using the Polanyi–Wigner formula [
68]:
where
is the universal gas constant,
−
is the temperature width of the
ith desorption peak width as measured at the half-height of the
curves,
,
are the areas under the desorption curves that correspond to the amount of the
th fragment remaining after reaching
and
, respectively.
Calculations of the activation energy for neat epoxy polymer provide
values in the range of 55–132 kJ/mol. The
concentration dependences are presented in
Figure 9.
It can be seen from
Figure 9 that the
values for all volatile fragments decrease noticeably at
1%. The
values increase slightly and nonmonotonously with increasing
, revealing their local maxima at
2%, which are essentially lower than
values. It should be noted that previously, the authors of [
69,
70] noted the possibility for the destruction activation energy to decrease due to the incorporation of residual solvent molecules or impurities into polymer chains during the curing process. It is obvious when comparing
Figure 8 and
Figure 9 that the
dependences resemble those of
. However, the
dependences do not correlate with the
dependences. The nonmonotonic variations in
with
evidence the above-mentioned nonregular spatial alteration of the macromolecular structure of the epoxy in nanocomposites filled with MLGs. In other words, the random spatial interaction between ASSs of MLG nanoparticles with both unbound moieties and atoms of polymer’s chains and crosslinks leads to the formation of interphase regions with decreased destruction energy.
3.2.3. Heat Transport
It is known [
68] that the stabilization effect in a polymer’s molecular structure leads to an increase in polymer’s heat resistance (HR). The effect of increasing the thermal stability in the MLG–epoxy nanocomposites described above can also be regarded as increasing their HR. Besides the stabilization effect, an additional mechanism responsible for increasing the HR of MLG composites could be related to the participation of graphene’s electron subsystem in heat transport within the nanocomposite. Namely, when the various moieties are fastened on ASSs, the phonon heat flux propagating through macromolecular epoxy chains is redistributed between the phonon and electron subsystems of MLGs at the MLG–epoxy interface. As a result, the graphene’s phonon subsystem has a lower temperature at the interface than that of the epoxy’s phonon subsystem. In other words, the local decrement in the vibrational energy of surface atoms of fastened moieties takes place. This process reveals itself as a local weakening of destruction for moieties bonded with ASSs.
A phenomenological model of heat-transfer processes at the interface is presented in the following. The heat flux
entering into the unfilled polymer from an external source is transported by propagation of the phonon flux
through the polymer chains and crosslinks into the epoxy’s bulk. Neglecting heat dissipation on the surface, defects and phonons, we can write:
In MLG–epoxy composites, the heat is transported through chains attached to ASSs. The phonon heat flux
is governed by the Fourier equation:
where
is the thermal conductivity coefficient of the epoxy’s chains,
is the temperature gradient in the chains. At points of fastening, the heat is transferred from local phonons to the electronic and phonon subsystems of MLGs, and the flux obeys laws of both energy and momentum conservation. In the local approximation, we can write
where
is the heat transfer efficiency at the interface,
and
are the phonon and electron heat fluxes, respectively.
At high temperatures
close to chain’s destruction temperatures, the heat transfer occurs in the anharmonic mode of phonon generation, when the appearance of backscattered local phonons at the interface is unlikely and the transfer efficiency is close to unity:
1. The heat obtained by the electronic subsystem of graphene does not return back into the epoxy’s phonon subsystem due to the absence of electron transport in the epoxy. In graphene, electron gas dissipates the received heat into the phonon subsystem due to electron–phonon interaction during the relaxation time
at the distance
from the interface:
where
is the velocity of the heated electrons. In the local approximation supposed above, the interfacial temperatures of epoxy’s phonons
, graphene’s phonons
and graphene’s electrons
obey the simple relation:
Hence, there is a temperature decrement
at the interphase boundary for the temperature
as compared to
, which is given by
As a result, the vibration energy of the vibrations of the surface site’s atoms is decreased by . Therefore, the destruction probability for every ith moiety fixed on ASSs decreases, and its decomposition intensity also decreases.
Since different th moieties fastened to ASSs have different masses, their vibration frequencies are different too. In addition, at the interface, the velocities and impulses of local phonons in fastened moieties are also different. Therefore, both momentum and energy values transferred from the epoxy’s phonon subsystem into the graphene electronic and phonon subsystems, as well as the temperature decrement , depend on the masses of bound atoms at the interface. The heat transfer from polymer chain to graphene takes place through local surface site.
The
values can be estimated from the desorption curves of fragments with
17, 43 and 94 for neat epoxy resin and its MLG composite of
2% in the temperature region
. Namely, the
value is the difference between the two temperatures
and
determined from the desorption curves
and
, respectively:
Here, the temperature
corresponds to maximal intensity
:
. When
has been determined, one can find
from the
curves using the criteria:
. The
values are shown in
Figure 7. Estimations give
34, 28.5, and 17 K for the fragments of
17, 43, and 94, respectively (
Figure 7). One can see that
decreases with increasing
, and thus the amount of heat transferred into the graphene’s electronic subsystem decreases too.
Thus, the thermal destruction data evidence that loading epoxy resin with multilayered graphene nanoparticles results in an increase in the composite’s thermal stability in the mass-concentration range of 1%, and the thermal stability decreases slightly with increasing . Along with the stabilization of molecular composite’s structure at 1%, the thermal destruction activation energy decreases.
3.3. Dielectric Parameter Data
3.3.1. Dielectric Permittivity
The frequency dependences of the real part (
) of the complex dielectric permittivity for the neat (unfilled) resin and its composites filled with MLG, measured at low (95 K) and room (300 K) temperature are depicted in
Figure 10a,b, respectively. At low temperatures,
of the neat resin remains unchanged over the entire frequency region studied and is equal to about 3.7. Embedding MLG into the epoxy stimulates
to increase. However, the concentration dependence
reveals a nonmonotonous variation with increasing
. Thus,
rises to 4.25–4.3 when
is increased to 1%, then it grows slowly to 4.45 when
is increased to 2%. It increases further when
is increased to 5%.
Thus, at 2%, one can see a similarity in the loading behavior of the dielectric permittivity and the thermophysical characteristics, namely and . This may reveal a certain structural peculiarity of the nanocomposites.
The identical frequency behavior of in the neat resin and its MLG nanocomposites evidence that the dielectric response is mainly caused by the epoxy’s dipolar molecular structure in all of the materials. With respect to the influence of MLG’s electron subsystem on the dependences, it is negligible. Hence, the concentration effect of MLG on the dielectric permittivity is indirect.
Figure 11 shows the temperature dependences of
for the neat resin and its MLG composites measured at fixed frequencies of 1.0262 and 212.73 kHz. The dependences exhibit typical “ascending steps” pertaining to relaxation processes (see Figures 2-36b in [
71]).
The concentration effect, which is clearly visible in both the frequency and temperature dependences of , seems to be related to a disordering in the polymer structure owing to the random attachment of both epoxy chains and crosslinks to the graphene’s active surface sites, which, in turn, are located randomly within the bulk epoxy. The structure disordering increases nonmonotonously with increasing . The disordering effect prevails at 0.01% and leads to both increasing and decreasing , and .
The concentration behavior of the composite dielectric permittivity can be described by the following relation:
where
and
are the dielectric permittivity of the initial polymer structure and the disordered structure in the interface regions formed around MLGs, respectively;
is a structural factor. The variations observed in
can be explained by decreasing
, which is accompanied by increasing
with increasing
due to structural rearrangement of polymer chains and crosslinks in the interface regions as well as the occupied interfacial volume, resulting in a more disordered and rigid structure of the polymer chains with enhanced dielectric response. However, Relation (10) fails to describe
in the vicinity of 2%.
3.3.2. Dielectric Losses
Frequency Dependences of the Dielectric Loss Factor
Frequency dependences of the dielectric loss factor
are presented in
Figure 12. There are three frequency intervals where the various physical mechanisms contribute to the losses. In the narrow range of low frequencies 4–12 Hz, a sharp descending trend in
can be attributed to the term
associated with residual dc conductivity (
) of the epoxy. On the other hand, several sharp, δ-shaped peaks are imposed on the trend. These may originate from either low mobile macromolecular atomic segments and the absorbed water molecules and/or hydroxyl groups localized on the polymer chains. However, the latter reason appears unlikely due to the fact that the loading with MLGs leads to the essential reduction in the quantity of water molecules and OH groups captured in the epoxy structure [
33]. Additionally, the low-frequency losses increase with increasing
.
In the wide frequency range of 12
10
6 Hz,
increases weakly with
at low temperatures. From the other hand,
decreases linearly with increasing frequency up to
10
6 Hz. It is remarkable that
. A further increase of
occurs when
is increased from 2% to 5%. (
Figure 12b). On the whole, within the frequency interval of 12
10
6 Hz, the frequency behavior of
is caused by the orientational relaxational losses of vibrating molecular dipoles of various masses and located mainly in the polymer chains due to their interaction with the dipole environment [
71,
72].
Another peculiarity of the dielectric losses is the minimum of
at
10
6 Hz, which is followed by a steep growth with increasing frequency in the range of
10
6 Hz for all the materials studied. Additionally, the
dependences are practically identical for the unfilled resin and its MLG composites at
10
6 Hz. The identical behavior of the
curves at high frequencies suggests the mechanism for the change in the dielectric loss, namely, that the dielectric losses are related to the energy dissipation of the lateral atomic dipoles of the macromolecular chains [
71,
72,
73].
Temperature Dependences of
As temperature increases, several broad relaxation peaks become visible in the temperature dependences of
measured at fixed frequencies for both the neat resin and their nanocomposites. The sets of
curves measured at fixed frequencies are depicted in
Figure 13. It is generally accepted that the relaxation mechanism responsible for the appearance of these peaks is that referred to as “the dipole-group relaxation”, which originates mainly from local vibration modes of various ensembles of dipolar moieties [
71].
At low temperatures −130
50 K, several
curves contain two slight peaks within the frequency range of
500 Hz (
Figure 13a,b). Both the peaks can be ascribed to
relaxators [
52,
74,
75]. Both the peaks decrease and narrow with increasing
and become invisible at
= 5% (see
Figure 13d).
The
peaks can be attributed to low mobile unreacted molecular fragments of polymer structure and long dipole segments in the polymer chains [
74,
75]. The suppression of
peaks with increasing
is caused by the decreased free volume reduction in the matrix and the fastening of chain segments on the active surface sites of the MLGs.
The most intensive relaxation losses in the neat epoxy and its MLG composites occurs at higher temperatures
220 K, within the frequency range 12–10
6 Hz. These
peaks are caused by the
relaxation process, which is fed by various dipolar moieties [
74,
75]. The temperature position of the peaks (
) shifts toward higher temperatures when measuring frequency increases (see
Figure 12), as predicted by the classical Debye theory [
71].
It is remarkable that the dependence of
versus
varies with increasing frequency (
Figure 14). For frequencies
2000 Hz,
decreases when
1%, and increases when
1% (
Figure 14a). In contrast, for frequencies
2000 Hz,
increases over the entire concentration interval (
Figure 14b).
The nonmonotonous
dependence in the frequency range of
2000 Hz and with low MLG content
1% is caused by two competing processes. The first process occurs at low MLG content
0.01% and consists of the spatial fixing of low-movable unbound segments of polymer chains. This process leads to increased free volume of the polymer structure and spatial ordering. Such structural transformation manifests itself in slight variations in the losses and in a low-temperature
shift. The second process dominates at 0.01
1% and consists of the fastening of polymer chains and crosslinks on the active surface sites of the MGLs and the disordering of the polymer structure due to the formation of disordered interface regions. Competition of the two processes results in a decrease in
along with a slight enhancement of dielectric losses (
Figure 14a, curve 2).
Thus, the presence of unbound fragments of polymer chains and their attachment to the active surface sites of MLG particles, as well as the change of MLG content in the polymer matrix are the key factors affecting the degree of disorder in the polymer structure and the energy distribution of molecular dipoles.
Using a set of
temperature dependences measured at different frequencies, the activation energy
of dipole relaxation can be determined as a function of
. In accordance with the classic Debye theory [
71], at the relaxation resonance temperature
the dipole resonance frequency
is related to relaxation time (
) by the following expression [
71]:
where the
value depends on the Boltzmann probability of the energy distribution of vibrating dipoles. The probability term is taken into account as follows:
where
is the frequency-independent time parameter of the
relaxation process,
is the Boltzmann constant. The formula to determine
is
The set of temperature dependences of
show that the relaxation process exhibits about the same activation energy for both the neat resin and its MLG composites (
Figure 15). Namely,
= 0.70 eV and
= 0.72 eV. This means that the atomic environment for the epoxy’s
dipoles undergoes a negligible variation at low loadings
2%.
3.4. Positron Annihilation Lifetime Data
Lifetime positron annihilation spectroscopy was applied to probe molecular structure alterations in the MLG–epoxy nanocomposites. Three positron lifetimes (
) and corresponding annihilation intensities (
) were determined.
,
,
,
and
,
describe the annihilation of the quasi-free positrons, the trapped positrons, and the trapped positronium atoms, respectively, while the same experimental annihilation parameters (
,
) and the calculated ones (namely, the average positron lifetime
, the radius of positronium-capturing traps
, and the free volume
) are presented in
Table 1.
Here,
was calculated on the basis of the following expression [
76]:
decreases smoothly with increasing
(see
Figure 16). Since
is inversely proportional to the electron density
in the annihilation volume, lowering
means increasing
.
Despite increasing , both and take their minimal values at 2%. It is remarkable that dependence is essentially nonmonotonous. Indeed, 0.2 ps, but 8.7 ps. The low value of may be attributed to the influence of two opposite processes of structural alteration. The first process fastens the unbound moieties on ASSs. This leads to decreased disorder in the macromolecular structure, and is accompanied by increased , and thus by a decrease in electron density in the positron’s annihilation volume. Another process is the formation of interphase layers around MLGs due to the nonuniform spatial distribution of ASSs, resulting in enhanced disorder within the polymer structure due to the random process of the chemical binding of atoms in polymer chains and crosslinks with the surface sites of graphene particles. Superposition of the two processes results in decreasing and a corresponding increase in the electron density along epoxy chains and crosslinks in the annihilation volume.
The structural variations at 2% also manifest themselves in local maxima of and . They evidence an increasing number of nanosized pores with radii of about 2.4–2.5 Å, since and are proportional to the densities of the corresponding pores. Again, the local minima of and at 2% can be attributed to the increasing electron density on the walls of the corresponding pores trapping positrons and ortho-positronium atoms, respectively.
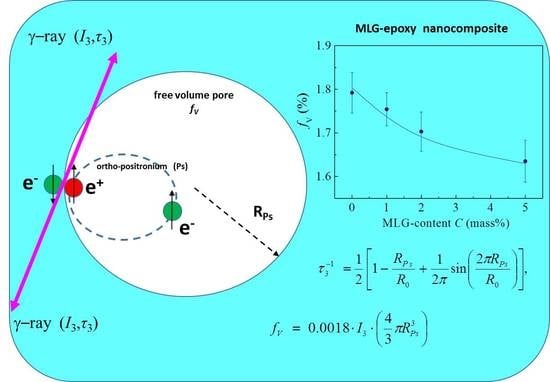
The concentration dependence of free volume
is presented in
Figure 17.
values were calculated using the following well-known formula [
77]:
where
is the radius of positronium-trapping pores (voids), evaluated by solving the following equation [
78]:
where
and
= 1.66 Å is the fitting parameter [
78].
The free volume was found to decrease with increasing
and tend to saturation at
5% (
Figure 17). Such behavior is correlated with a smooth growth of the mean electron densities in polymer chains and on the walls of positron- and positronium-trapping pores. It is remarkable that
and
resemble each other. This confirms the conclusion that MLG filler compacts the macromolecular structure of epoxy smoothly, and the mean electron density (which is proportional to
in the annihilation volume increases as the free volume portion decreases.
However, the structure’s constituents, such as epoxy chains, crosslinks, and nanosized defects (such as pores), are characterized by nonmonotonous alteration with MLG loading. In the concentration range 0 1%, when unbound moieties fasten to ASSs and the macromolecular network structure undergoes slight conformational deformation, PALS-data reveal a weak increase in electron density in the epoxy chains (which is proportional to ), along with an increasing number of positronium-trapping pores (which is proportional to ).
In the concentration range 1
2%, both the free volume (
) and the conformational deformation (
) decrease, and the chain’s electron density (
) decreases too. In addition, the numbers of both positron-trapping pores and positronium trapping pores increase due to increasing
and
, respectively (see
Table 1). The electron densities on the pore walls increase due to decreasing
and
, respectively. It can be easily seen from
Figure 10a that
undergoes negligible variations in the range of 1
2%. This behavior in
is caused by the effect of “breeding” the pores.
In the concentration range 2
5%, the numbers of both positron-trapping pores and positronium-trapping pores decrease and the electron densities on the pore walls decrease too (see
Table 1). The dielectric permittivity begins to increase again, along with a further decrease in the conformational deformation.
Thus, the peculiarity observed in the macromolecular structure of the 2%-nanocomposite, which is related to the intensive formation of nanopores, reveals itself in overall physical characteristics such as thermal stability, destruction activation energy, and dielectric permittivity.
4. Conclusions
The effect of low concentration loading of multilayered graphene particles on thermal stability, compressive strength and Young’s modulus, dielectric permittivity, dielectric losses, and the free volume of the epoxy composites were studied.
At 5%, the MLG loading leads to an essential increase in thermal stability and a decrease in destruction activation energy.
The compressive strength and the Young’s modulus are increased at extremely low loadings of 1% and decrease when is increased to 1%.
At 1%, fastening unbound moieties on ASSs stabilize the composite’s molecular structure, resulting in the enhanced thermal stability of the composite. The enhancement of composite’s thermal stability may occur due to a partial transfer of heat from polymer chains fastened on graphene’s active surface sites into the electron subsystem of the MLGs.
At 1%, the fastening process is accompanied by the formation of interphase areas around the MLG particles, which may be due to the random distribution of fastening sites across the MLG–epoxy interface. In turn, the formation of interphase areas leads to an increase in the dielectric permittivity of the composites.
Structural alteration in the 2% nanocomposite is related to a spontaneous increase in the density of nanovoids, which enhances the dielectric response of the nanocomposites to the external electric field.
At 0% 5%, both the free volume and the mean electron density in the composite’s bulk decrease gradually with increasing .