Use of Industrial Wastes as Sustainable Nutrient Sources for Bacterial Cellulose (BC) Production: Mechanism, Advances, and Future Perspectives
Abstract
:1. Introduction
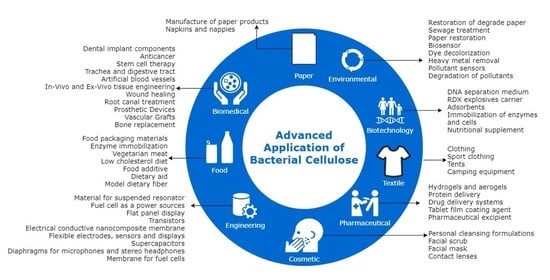
2. Overview of Bacterial Cellulose (BC) and Its Applications

3. Principal Pathways of Cellulose Production
4. Fundamentals of Bacterial Cellulose (BC) Production Process
5. Industrial Waste Streams as Feedstock for the Production of Bacterial Cellulose
5.1. Brewery and Beverages Industries Wastes
5.2. Agro-Industry Waste
5.3. Wastewater Sugar Industries, Pulp Mills and Lignocellulosic Biorefineries Wastes
5.4. Textile Industries Waste
5.5. Biodiesel Industry Waste
5.6. Micro-Algae Biomass Industries
| Microorganism | Production Mode | BC Production | Time | Industrial Waste | Additional Nutrients | References |
|---|---|---|---|---|---|---|
| Beverages/Brewery | ||||||
| Waste as carbon source with additional nutrients | ||||||
| Komagataeibacter xylinus CICC No.10529 | Static | 5.7 g/L | 8 days | Citrus peel and pomace enzymolysis medium | Yeast extract, ethanol and peptone | Fan et al. [216] |
| Gluconacetobacter xylinus NRRL B-42 | Static | 8.00 g/L | 14 days | Grape bagasse | Corn steep liquor | Vazquez et al. [190] |
| Gluconacetobacter xylinus NRRL B-42 | Static | 7.20 g/L | 14 days | Grape bagasse | Diammonium phosphate | |
| Gluconacetobacter xylinus ATCC®® 10788™ | Static | 0.35 g/L | 3 days | Makgeolli sludge filtrate | Modified HS (MHS) medium | Hyun et al. [267] |
| Gluconacetobacter xylinus ATCC®® 10788™ | Static | 1.2 g/L | 3 days | Makgeolli sludge filtrate | Mixed modified HS (MMHS) | |
| Gluconacetobacter xylinus BCRC 12334 | Static | 0.90 g/L | 7 days | Thin stillage (TS) wastewater | 50% TS | Wu & Liu [232] |
| Gluconacetobacter xylinus BCRC 12334 | Static | 6.26 g/L | 7 days | Thin stillage (TS) wastewater | 50/50 TS-HS | |
| Gluconacetobacter oboediens | Shaking | 10.8 g/L | 72 h | Distillery effluent | Sucrose (carbon source) and corn steep liquor (nitrogen source) | Jahan et al. [268] |
| Gluconacetobacter hansenii PJK KCTC 10505BP | Static | 13.95 g/L | 336 h | Untreated WBFB | 1% Glucose | Ha et al. [269] |
| Gluconacetobacter hansenii PJK KCTC 10505BP | Shaking | 1.50 g/L | 168 h | Untreated WBFB | 1% Glucose | |
| Gluconacetobacter hansenii PJK KCTC 10505BP | Static | 7.37 g/L | 336 h | Autolyzed WBFB | Glucose | |
| Gluconacetobacter hansenii PJK KCTC 10505BP | Static | 3.64 g/L | 336 h | Hydrolysed WBFB | 1% Glucose | |
| Waste as a complex medium without any additional nutrients | ||||||
| Komagataeibacter saccharivorans strain BC1 (K. saccharivorans strain BC1) | Static | 1.24 g/L | 8 days | UB breweries limited, Baikampady, Mangalore, India | - | Gayathri et al. [270] |
| Gluconacetobacter xylinus BCRC 12334 | Static | 3.10 g/L | 7 days | Thin stillage (TS) wastewater | - | Wu & Liu [232] |
| Gluconacetobacter xylinus NRRL B-42 | Static | 4.20 g/L | 14 days | Grape bagasse | - | Vazquez et al. [190] |
| Gluconacetobacter xylinus ATCC®® 10788™ | Static | 0.30 g/L | 3 days | Makgeolli sludge filtrate | - | Hyun et al. [267] |
| Gluconacetobacter medellinensis ID13488 | Static | 1.5 g/L | 14 days | Fresh apple peel/ sugar cane ratio (w/w) (1/2.3) | - | Urbina et al. [271] |
| Gluconacetobacter medellinensis ID13488 | Static | 1.4 g/L | 14 days | Apple residue (AR)/ sugar cane (SC) ratio (w/w) (1/2.3) | - | |
| Gluconacetobacter medellinensis ID13488 | Static | 2.0 g/L | 14 days | AR/SC ratio (w/w) (0.5/2.8) | - | |
| Gluconacetobacter medellinensis ID13488 | Static | 1.2 g/L | 14 days | AR/SC ratio (w/w) (2/1.3) | - | |
| Gluconacetobacter medellinensis ID13488 | Static | 2.5 g/L | 14 days | AR/SC ratio (w/w) (1.5/2.3) | - | |
| Gluconoacetobacter xylinum ATCC 23768 | Static | 2.9 g/L | 9 days | Black strap molasses | - | Khattak et al. [272] |
| Gluconoacetobacter xylinum ATCC 23768 | Shaking | 3.05 g/L | 9 days | Black strap molasses | - | |
| Gluconoacetobacter xylinum ATCC 23768 | Static | 1.70 g/L | 9 days | Brewery molasses | - | |
| Gluconoacetobacter xylinum ATCC 23768 | Shaking | 1.75 g/L | 9 days | Brewery molasses | - | |
| Gluconacetobacter oboediens | Shaking | 8.5 g/L | 72 h | Crude effluent | - | Jahan et al. [268,273] |
| Acetobacter xylinum NRRL B-42 | Static | 6.7 g/L | 21 days | Grape pomace extract/corn steep liquor | - | Cerrutti et al. [274] |
| Gluconacetobacter hansenii PJK KCTC 10505BP | Static | 8.46 g/L | 336 h | Untreated Waste from beer fermentation broth (WBFB) | - | Ha et al. [270] |
| Gluconacetobacter hansenii PJK KCTC 10505BP | Static | 2.00 g/L | 336 h | Autolyzed WBFB | - | |
| Gluconacetobacter hansenii PJK KCTC 10505BP | Static | 2.82 g/L | 336 h | Hydrolysed WBFB | - | |
| Gluconacetobacter sucrofermentans B-11267 | Shaking | 2.40 g/L | 3 days | Hestrin and Schramm (HS) medium | - | Revin et al. [230] |
| Gluconacetobacter sucrofermentans B-11267 | Shaking | 6.19 g/L | 3 days | Thin stillage | - | |
| Gluconacetobacter sucrofermentans B-11267 | Shaking | 5.50 g/L | 3 days | Cheese whey | - | |
| Gluconacetobacter sucrofermentans B-11267 | Shaking | 6.19 g/L | 3 days | Thin stillage pH 3.95 | - | |
| Gluconacetobacter sucrofermentans B-11267 | Shaking | 5.40 g/L | 3 days | Thin stillage pH 5 | - | |
| Gluconacetobacter sucrofermentans B-11267 | Shaking | 3.50 g/L | 3 days | Thin stillage pH 6 | - | |
| Gluconacetobacter xylinus | Static | 2.90 g/L | 4 days | Acid hydrolysate of waste oleaginous yeast biomass | - | Luo et al. [275] |
| Gluconacetobacterhansenii CGMCC 3917 | Static | 3.89 g/L | 14 days | Waste beer yeast treated with ultrasonication treatment | - | Lin et al. [237] |
| Gluconacetobacterhansenii CGMCC 3917 | Static | 2.40 g/L | 14 days | Waste beer yeast treated with NaOH treatment | - | |
| Gluconacetobacterhansenii CGMCC 3917 | Static | 2.00 g/L | 14 days | Waste beer yeast treated with high speed homogenizer treatment | - | |
| Gluconacetobacterhansenii CGMCC 3917 | Static | 1.50 g/L | 14 days | Waste beer yeast treated with microwaves treatment | - | |
| Gluconacetobacterhansenii CGMCC 3917 | Static | 1.20 g/L | 14 days | Waste beer yeast treated with untreatment | - | |
| Gluconacetobacterxylinus BC-11 K. | Static | 1.18 g/L | 10 days | Wastewater after pullulan polysaccharide fermentation | - | Zhao et al. [236] |
| Agro industrial waste | ||||||
| Waste as nitrogen source | ||||||
| Gluconacetobacter swingsii | Static | 2.8 g/L | 13 days | Pineapple peel juice | Glucose, fructose and sucrose | Castro et al. [235] |
| Waste as carbon source with additional nutrients | ||||||
| Gluconacetobacter swingsii | Static | - | 13 days | Sugar cane juice | Glucose, fructose and sucrose | Castro et al. [235] |
| Gluconacetobacter xylinum bacterium (ATCC 700178) | Shaking | 10.6 g/L | 7 days | Wheat straw | Corn steep liquor (CSL) | Goyat [266] |
| Gluconacetobacterxylinus | Static | 1.8 g/L | 9 days | Carob and haricot bean (CHb) medium | Citric acid | Bilgi et al. [276,277] |
| Komagataeibacter rhaeticus | Static | 6.0 g/L | 7 days | HS medium and Cashew tree exudates (HSCTE) | HS medium | Pacheco et al. [278] |
| Komagataeibacter rhaeticus | Static | 6.0 g/L | 7 days | HS medium and Cashew tree exudates (HSCG) | HS medium | |
| Acetobacter aceti ATCC 23770 | Shaking and static | 2.12 g/L | 8 days | Cheap agricultural product konjac powder | Yeast extract and tryptone | Hong & Qiu [279] |
| Gluconacetobacter hansenii UAC09 | Static | 8.2 g/L | 14 days | Coffee cherry husk (CCH) | 8% corn steep liquor (CSL) | Rani & Appaiah [242] |
| Gluconacetobacter hansenii UAC09 | Static | 6.5 g/L | 14 days | Coffee cherry husk (CCH) | 0.2% Urea | |
| Gluconacetobacter hansenii UAC09 | Static | 6.9 g/L | 14 days | Coffee cherry husk (CCH) | Ethyl alcohol (EA) + Acetic acid (AA) | |
| Gluconacetobacter hansenii UAC09 | Static | 7.5 g/L | 14 days | Coffee cherry husk (CCH) | 8% CSL + EA + AA | |
| Gluconacetobacter hansenii UAC09 | Static | 6.6 g/L | 14 days | Coffee cherry husk (CCH) | 0.2% urea + EA + AA | |
| Acetobacter xylinus ATCC 23770 | Static | 8.3 g/L | 7 days | Enzymatic hydrolysate of wheat straw | Other components are same as of HS medium | Chen et al. [280] |
| Acetobacter xylinum 0416 MARDI | Static | 4.0 g/L | 8 days | Extracted date syrup (DSH-2%) | Other components are same as of HS medium | Lotfiman et al. [247] |
| Acetobacter xylinum 0416 MARDI | Static | 5.8 g/L | 8 days | Extracted date syrup (DSH-3%) | Other components are same as of HS medium | |
| Acetobacter xylinum 0416 MARDI | Static | 4.5 g/L | 8 days | Extracted date syrup (DSH-5%) | Other components are same as of HS medium | |
| Gluconacetobacter sacchari | Static | 0.1 g/L | 96 h | Grape skins aqueous extract, cheese whey, crude glycerol and sulfite pulping liquor | Organic or inorganic nitrogen | Carreira et al. [281] |
| Acinetobacter sp. BAN1 | Static | 0.3 g/L | 15 days | Pineapple juice medium (PIJM) | Other components are same as that of HS medium | Adebayo-Tayo et al. [282] |
| Acinetobacter sp. BAN1 | Static | 6.4 g/L | 15 days | Pawpaw juice medium (PAJM) | Other components are same as that of HS medium | |
| Acinetobacter sp. BAN1 | Static | 0.6 g/L | 15 days | Watermelon juice medium (WMJM) | Other components are same as that of HS medium | |
| Acetobacter pasteurianus PW1 | Static | 0.1 g/L | 15 days | Pineapple juice medium (PIJM) | Other components are same as that of HS medium | |
| Acetobacter pasteurianus PW1 | Static | 7.7 g/L | 15 days | Pawpaw juice medium (PAJM) | Other components are same as that of HS medium | |
| Acetobacter pasteurianus PW1 | Static | 0.4 g/L | 15 days | Watermelon juice medium (WMJM) | Other components are same as that of HS medium | |
| Gluconoacetobacter xylinus BCRC 12334 | Static | 3.40 g/L | 8 days | Orange peel fluid and orange peel hydrolysate | Acetate buffer, peptone and yeast extract | Kuo et al. [214] |
| Beijerinkia fluminensis WAUPM53 | Static | 0.47 g/L | 14 days | Sago byproduct | Other components are same as of HS medium | Voon et al. [226] |
| Gluconacetobacter xylinus 0416 | Static | 1.55 g/L | 14 days | Sago byproduct | Other components are same as of HS medium | |
| Acetobacterxylinum NBRC 13693 | Static | 4.1 g/L | 14 days | Pineapple | Disodium hydrogen phosphate buffer | Kurosumi et al. [283] |
| Acetobacterxylinum NBRC 13693 | Static | 3.95 g/L | 14 days | Apple | Disodium hydrogen phosphate buffer | |
| Acetobacterxylinum NBRC 13693 | Static | 5.9 g/L | 14 days | Orange | Disodium hydrogen phosphate buffer | |
| Acetobacterxylinum NBRC 13693 | Static | 3.5 g/L | 14 days | Japanese pear | Disodium hydrogen phosphate buffer | |
| Acetobacterxylinum NBRC 13693 | Static | 1.8 g/L | 14 days | Grape | Disodium hydrogen phosphate buffer | |
| Acetobacterxylinum NBRC 13693 | Static | 0.5 g/L | 14 days | Pineapple | Sugar reagent (glucose, fructose and sucrose) | |
| Acetobacterxylinum NBRC 13693 | Static | 0.2 g/L | 14 days | Apple | Sugar reagent (glucose, fructose and sucrose) | |
| Acetobacterxylinum NBRC 13693 | Static | 1.85 g/L | 14 days | Orange | Sugar reagent (glucose, fructose and sucrose) | |
| Acetobacterxylinum NBRC 13693 | Static | 0.5 g/L | 14 days | Japanese pear | Sugar reagent (glucose, fructose and sucrose) | |
| Acetobacterxylinum NBRC 13693 | Static | 0.4 g/L | 14 days | Grape | Sugar reagent (glucose, fructose and sucrose) | |
| Gluconacetobacter sacchari | Static | 1.7 g/L | 96 h | Dry olive mill residue (DOR100) Water extraction at 100 °C | Nitrogen | Gomes et al. [248] |
| Gluconacetobacter sacchari | Static | 1.4 g/L | 96 h | Dry olive mill residue (DOR100) Water extraction at 100 °C | Phosphorus | |
| Komagataeibacter hansenii MCM B-967 | Static | 125 g/L | 7 days | Pineapple and watermelon peels | Sucrose, ammonium sulfate and cycloheximide | Kumbhar et al. [284] |
| Acetobacter xylinum DSMZ2004 | Static | 8.6 g/L | 48 h | Poor quality apple residues in combination with glycerol | Apple glucose equivalents, glycerol, ammonium sulfate and citric acid | Casarica et al. [285] |
| Acetobacter xylinum BCRC 14182 (purchased) | Static | - | 3–7 days | Coconut-water | Sugar | Lin et al. [286] |
| Waste as complex medium without any additional nutrients | ||||||
| Komagataeibacter hansenii GA2016 | Static | 2.06 BC/100 g peel | 21 days | Lemon peels (LBC) | - | Güzel & Akpınar [287] |
| Komagataeibacter hansenii GA2016 | Static | 3.92 BC/100 g peel | 21 days | Mandarin peels (MBC) | - | |
| Komagataeibacter hansenii GA2016 | Static | 2.33 BC/100 g peel | 21 days | Orange peels (OBC) | - | |
| Komagataeibacter hansenii GA2016 | Static | 2.68 BC/100 g peel | 21 days | Grapefruit peels (GBC) | - | |
| Komagataeibacter xylinus | Static | 2.90 g/L | 10 days | Discarded waste durian shell | - | Luo, Huang et al. [275] |
| Gluconacetobacter xylinus CH001 | Static | 2.67 g/L | 10 days | Discarded waste durian shell | - | Luo, Huang, et al.[288] |
| Gluconacetobacter medellinensis | Static | 3.24 g/L | 7 days | Sugar cane juice and pineapple residues | - | Algar et al. [289] |
| Gluconacetobacter medellinensis | Dynamic | 0.82 g/L | 7 days | Sugar cane juice and pineapple residues | - | |
| Acinetobacter sp. BAN1 | Static | 0.4–0.6 g/L | 15 days | Pineapple waste medium (PIWAM) | - | Adebayo-Tayo et al. [290] |
| Acinetobacter sp. BAN1 | Static | 0.2–1.1 g/L | 15 days | Pawpaw waste medium (PAWAM) | - | |
| Acetobacterpasteurianus PW1 | Static | 0.2–1.0 g/L | 15 days | Pawpaw waste medium (PAWAM) | - | |
| Acetobacterpasteurianus PW1 | Static | 0.1–3.9 g/L | 15 days | Pineapple waste medium (PIWAM) | - | |
| Komagataeibacter rhaeticus iGEM | Static | – | 10 days | Fermented tea | - | Florea et al. [291] |
| Gluconacetobactersacchari | - | 1.28 g/L | - | Industrial residues from olive oil production | - | Gomes et al. [248] |
| Gluconacetobacterpersimmonis GH-2 | Static | 5.75 g/L | 14 days | Watermelon + HS medium | - | Hungund et al. [292] |
| Gluconacetobacterpersimmonis GH-2 | Static | 5.98 g/L | 14 days | Orange juice + HS medium | - | |
| Gluconacetobacterpersimmonis GH-2 | Static | 6.18 g/L | 14 days | Muskmelon + HS medium | - | |
| Gluconacetobacterpersimmonis GH-2 | Static | 8.08 g/L | 14 days | Coconut water +HS medium | - | |
| Acetobacterxylinum | Static | 19.46 g/L | 15 days | Banana peel | - | Hungund et al. [245] |
| Gluconacetobacterxylinus ATCC 53582 | Static | 60 g/L | 96 h | Rotten fruit culture | - | Jozala et al. [293] |
| Gluconacetobacterxylinus CGMCC 2955 | Static | 2.25 g/L | 114 h | Waste water of candied jujube hydrolysate | - | Li et al. [252] |
| Acetobacterxylinum 0416 | Rotary disc reactor | 28.30 g/L | 4 days | Pineapple waste medium | - | Zahan et al. [197] |
| Komagataeibacter rhaeticus | Static | 2.8 g/L | 7 days | Cashew tree exudates (CTE) | - | Pacheco et al. [278] |
| Komagataeibacter rhaeticus | Static | 2.3 g/L | 7 days | Cashew gum (CG) | - | |
| Gluconacetobacter hansenii UAC09 | Static | 5.6 g/L | 14 days | Coffee cherry husk (CCH) | - | Rani & Appaiah [242] |
| Gluconacetobacter sacchari | Static | 0.81 g/L | 96 h | Dry olive mill residue (DOR40) Water extraction at 40 °C | - | Gomes et al. [248] |
| Gluconacetobacter sacchari | Static | 0.85 g/L | 96 h | Dry olive mill residue (DOR100) Water extraction at 100 °C | - | |
| Sugar industries, pulp mills and lignocellulosic biorefineries wastes | ||||||
| Waste as carbon source with additional nutrients | ||||||
| Komagatacibacter xylinus PTCC 1734 | Static | 7.02 g/L | 10 days | Vinasse | Other components are same as of HS medium | Barshan et al. [294] |
| Acetobacter xylinum BPR2001 | Rotary shaker | 3.01 g/L | 70 h | Molasses | Corn steep liquor | Bae & Shoda [187] |
| Acetobacter xylinum BPR2001 | Rotary shaker | 5.30 g/L | 70 h | H2SO4 heat treated molasses | Corn steep liquor | |
| Gluconacetobacter xylinus | Static | 5.9 g/L | 14 days | Cane molasses | Corn steep liquor and diammonium phosphate | Vazquez et al. [190] |
| Acetobacter sp. V6 | Agitated | 3.12 g/L | 168 h | Molasses and corn steep liquor | Acetic acid | Jung et al. [204] |
| Acetobacter xylinum ATCC 10245 | Static | 223% as compared to 100% in HS medium | 7 days | Sugar cane molasses | Carbohydrates, minerals, vitamins and amino acids | Premjet et al. [295] |
| Komagataeibacter rhaeticus | Static | 3.90 g/L | 120 h | Sugarcane molasses (SCM) 10 g/L of SCM | 40 g/L of glucose | Machado et al. [296] |
| Komagataeibacter rhaeticus | Static | 4.01 g/L | 120 h | 20 g/L of SCM | 30 g/L of glucose | |
| Komagataeibacter rhaeticus | Static | 3.7 g/L | 120 h | 30 g/L of SCM | 20 g/L of glucose | |
| Komagataeibacter rhaeticus | Static | 3.50 g/L | 120 h | 40 g/L of SCM | 10 g/L of glucose | |
| Gluconacetobacter xylinus ATCC 23770 | Static | 11 g/L | 7 days | Waste fiber sludge sulfate | Yeast extract and tryptone | Cavka et al. [241] |
| Gluconacetobacter xylinus ATCC 23770 | Static | 10 g/L | 7 days | Waste fiber sludge sulfite | Yeast extract and tryptone | |
| Acetobacter xylinum ATCC 10245 | Static | 20.6 % | 7 days | Softwood purified water-soluble (SPWS) | Other components are same as of HS medium | Uraki et al. [297] |
| Acetobacter xylinum ATCC 10245 | Static | 33 % | 7 days | Hardwood purified water-soluble (HPWS) | Other components are same as of HS medium | |
| Acetobacter xylinum ATCC 53582 | Static | 5.4 % | 7 days | Softwood purified water-soluble (SPWS) | Other components are same as of HS medium | |
| Acetobacter xylinum ATCC 53582 | Static | 8.9 % | 7 days | Hardwood purified water-soluble (HPWS) | Other components are same as of HS medium | |
| Waste as carbon source without any additional nutrients | ||||||
| Acetobacter xylinus 23769 | 0.15 g/L | Hot water extract | - | Erbas Kiziltas et al. [298] | ||
| Gluconoacetobacter xylinum ATCC 23768 | Shaking | 2.51 g/L | 10 days | Scum of sugarcane jaggery or gur (JS) | - | Khattak, Khan, Ul-Islam, Wahid, et al. [299] |
| Gluconoacetobacter xylinum ATCC 23768 | Static | 2.13 g/L | 10 days | Scum of sugarcane jaggery or gur (JS) | - | |
| Komagataeibacter europaeus SGP37 | Static | 6.30 g/L | 16 days | Sweet lime pulp waste | - | Dubey et al. [300] |
| G. persimmonis GH-2 | Static | 5.75 g/L | 14 days | Molasses + HS medium | - | Hungund et al. [292] |
| G. intermedius SNT-1 | Static | 12.6 g/L | 10 days | Molasses pretreated with hea | - | Tyagi et al. [301] |
| Gluconacetobacter xylinus (PTCC, 1734) | Static | 4.35 g/L | 336 h | Date syrup | - | Moosavi-Nasab [246] |
| Komagataeibacter rhaeticus | Static | 1.90 g/L | 120 h | 50 g/L of SCM | - | Machado et al. [296] |
| Gluconaceterxylinus CH001 | Static | 0.66 g/L | 5 days | Lipid fermentation wastewater | - | Huang et al. [302] |
| Gluconaceterxylinus | Static | 1·34 g/L | 7 days | Acetone-butanol-ethanol(ABE) fermentation wastewater | - | Huang et al. [303] |
| Gluconaceterxylinus BC-11 | Static | 1.177 g/L | 10 days | Wastewater after pullulan polysaccharide fermentation | - | Zhao et al. [236] |
| Acetobacterxylinum 23769 | Static | 0.15 g/L | 672 h | Wood hot water extract | - | Erbas Kiziltas et al. [298] |
| Textile mills | ||||||
| Waste as carbon source with additional nutrients | ||||||
| Gluconacetobacter xylinus ATCC 23770 | Static | 10.8 | 14 days | Cotton-based waste textiles | Glucose, yeast extract and peptone | Hong et al. [215] |
| Gluconacetobacter xylinus | Static | 14.2 g/L | 10 days | Waste dyed cotton fabrics hydrolysate - Purple bed sheet (PBS) | Peptone and yeast extract | Guo et al. [257] |
| Gluconacetobacter xylinus | Static | 13.7 g/L | 10 days | Waste dyed cotton fabrics hydrolysate- rose -Red bed sheet (RRBS) | Peptone and yeast extract | |
| Gluconacetobacter xylinus | Static | 14.1 g/L | 10 days | Waste dyed cotton fabrics hydrolysate- green bed sheet (GBS) | Peptone and yeast extract | |
| Gluconacetobacter xylinus | Static | 1.59 g/L | 7 days | Coloured hydrolysate | Peptone and yeast extract | Kuo et al. [256] |
| Gluconacetobacter xylinus | Static | 1.88 g/L | 7 days | Discoloured hydrolysate | Peptone and yeast extract | Kuo et al. [256] |
| Biodiesel industry | ||||||
| Waste as carbon source with additional nutrients | ||||||
| Gluconaceter xylinus BNKC19 | Static | 12.31 g/L | 7 days | Non-detoxified crude glycerol | Pineapple and in combination with HS medium components | Soemphol et al. [264] |
| Gluconacetobacter xylinus DSM 46604 | Agitated | 2.87 g/L | 5 days | 20 g/L glycerol | Yeast extract, ammonium sulphate, potassium hydrogen orthophosphate and magnesium sulphate | Adnan [304] |
| Gluconacetobacter xylinus DSM 46604 | Agitated | 2.87 g/L | 5 days | 50 g/L glucose | Yeast extract, ammonium sulphate, potassium hydrogen orthophosphate and magnesium sulphate | Adnan [304] |
| Gluconacetobacter xylinus | Static | 10 g/L | 14 days | Glycerol from biodiesel | Diammonium phosphate and corn steep liquor | Vazquez et al. [190] |
| Gluconacetobacter intermedius NEDO-01 | Static | 3.4 g/L | 4 days | Waste glycerol | Carboxymethyl Cellulose | Kose et al. [305] |
| Komagataeibacter sucrofermentans DSM 15973 | Shaking | 3.2 g/L | 15 days | Crude glycerol from biodiesel | Yeast extract and peptone | Tsouko et al. [259] |
| Komagataeibacter sucrofermentans DSM 15973 | Shaking | 13.3 g/L | 15 days | Crude glycerol from biodiesel | Sunflower meal hydrolysates | Tsouko et al. [259] |
| Komagataeibacter sucrofermentans DSM 15973 | Shaking | 13 g/L | 15 days | Crude glycerol from biodiesel | Flour-rich hydrolysates | Tsouko et al. [259] |
| Waste as carbon source without additional nutrients | ||||||
| Gluconacetobacter xylinus | Static | 3.5 g/L | 14 days | Glycerol from biodiesel | - | Vazquez et al. [190] |
| Micro-algae industry | ||||||
| Waste as carbon source with additional nutrients | ||||||
| Gluconacetobacter xylinum bacterium (ATCC 700178) | Shaking | 4.86 g/L | 7 days | Algae | Corn steep liquor (CSL) | Goyat [266] |
| Gluconacetobacter xylinus (ATCC #700178) | Static | 77% | 7 days | Chlorella vulgaris | Glucose/yeast extract | Chen et al. [306] |
| Gluconacetobacter xylinus (ATCC #700178) | Static | 94% | 7 days | Scenedesmus obliqnus | Glucose/yeast extract | |
| Gluconacetobacter xylinus (ATCC #700178) | Static | 85% | 7 days | Chlamydomonas reinhardtii | Glucose/yeast extract | |
| Komagataeibacter hansenii DSMZ | Static | 1.104 g/L | 7 days | Algae (Chlorella vulgaris) algae based glucose | Meat extract, peptone, NaCl and ethanol | Uzyol & Saçan [177] |
| Waste as carbon source without additional nutrients | ||||||
| Komagataeibacter saccharivorans | Static | 85.1% | 14 days | Algae (Chlamydomonas debaryana) (BEA0067) | - | Nóbrega et al. [307] |
6. Future Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Abral, H.; Pratama, A.B.; Handayani, D.; Mahardika, M.; Aminah, I.; Sandrawati, N.; Sugiarti, E.; Muslimin, A.N.; Sapuan, S.M.; Ilyas, R.A. Antimicrobial Edible Film Prepared from Bacterial Cellulose Nanofibers/Starch/Chitosan for a Food Packaging Alternative. Int. J. Polym. Sci. 2021, 2021, 1–11. [Google Scholar] [CrossRef]
- Abral, H.; Chairani, M.K.; Rizki, M.D.; Mahardika, M.; Handayani, D.; Sugiarti, E.; Muslimin, A.N.; Sapuan, S.M.; Ilyas, R.A. Characterization of compressed bacterial cellulose nanopaper film after exposure to dry and humid conditions. J. Mater. Res. Technol. 2021, 11, 896–904. [Google Scholar] [CrossRef]
- Cazón, P.; Velazquez, G.; Vázquez, M. Characterization of mechanical and barrier properties of bacterial cellulose, glycerol and polyvinyl alcohol (PVOH) composite films with eco-friendly UV-protective properties. Food Hydrocoll. 2020, 99, 105323. [Google Scholar] [CrossRef]
- Lin, C.-M.; Chang, Y.-C.; Cheng, L.-C.; Liu, C.-H.; Chang, S.C.; Hsien, T.-Y.; Wang, D.-M.; Hsieh, H.-J. Preparation of graphene-embedded hydroxypropyl cellulose/chitosan/polyethylene oxide nanofiber membranes as wound dressings with enhanced antibacterial properties. Cellulose 2020, 27, 2651–2667. [Google Scholar] [CrossRef]
- Kamiński, K.; Jarosz, M.; Grudzień, J.; Pawlik, J.; Zastawnik, F.; Pandyra, P.; Kołodziejczyk, A.M. Hydrogel bacterial cellulose: A path to improved materials for new eco-friendly textiles. Cellulose 2020, 27, 5353–5365. [Google Scholar] [CrossRef] [Green Version]
- Galdino, C.J.S.; Maia, A.D.; Meira, H.M.; Souza, T.C.; Amorim, J.D.P.; Almeida, F.C.G.; Costa, A.F.S.; Sarubbo, L.A. Use of a bacterial cellulose filter for the removal of oil from wastewater. Process Biochem. 2020, 91, 288–296. [Google Scholar] [CrossRef]
- Pang, M.; Huang, Y.; Meng, F.; Zhuang, Y.; Liu, H.; Du, M.; Ma, Q.; Wang, Q.; Chen, Z.; Chen, L.; et al. Application of bacterial cellulose in skin and bone tissue engineering. Eur. Polym. J. 2020, 122, 109365. [Google Scholar] [CrossRef]
- Norizan, M.N.; Harussani, M.M.; Demon, S.Z.N.; Halim, N.A.; Samsuri, A.; Mohamad, I.S.; Knight, V.F.; Abdullah, N. Carbon nanotubes: Functionalisation and their application in chemical sensors. RSC Adv. 2020, 43704–43732. [Google Scholar] [CrossRef]
- Esa, F.; Tasirin, S.M.; Rahman, N.A. Overview of Bacterial Cellulose Production and Application. Agric. Agric. Sci. Procedia 2014, 2, 113–119. [Google Scholar] [CrossRef] [Green Version]
- Andriani, D.; Apriyana, A.Y.; Karina, M. The optimization of bacterial cellulose production and its applications: A review. Cellulose 2020, 27, 6747–6766. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, S.M.; Harussani, M.M.; Atikah, M.S.N.; Ibrahim, R.; Asyraf, M.R.M.; Radzi, A.M.; Nadlene, R.; Kian, L.K.; Mali, S. Development and characterization of roselle nanocellulose and its potential in reinforced nanocomposites. In Roselle; Elsevier: Amsterdam, The Netherlands, 2021; pp. 285–317. [Google Scholar]
- Omran, A.A.B.; Mohammed, A.A.B.A.; Sapuan, S.M.; Ilyas, R.A.; Asyraf, M.R.M.; Koloor, S.S.R.; Petrů, M. Micro- and Nanocellulose in Polymer Composite Materials: A Review. Polymers 2021, 13, 231. [Google Scholar] [CrossRef] [PubMed]
- Sari, N.H.; Pruncu, C.I.; Sapuan, S.M.; Ilyas, R.A.; Catur, A.D.; Suteja, S.; Sutaryono, Y.A.; Pullen, G. The effect of water immersion and fibre content on properties of corn husk fibres reinforced thermoset polyester composite. Polym. Test. 2020, 91, 106751. [Google Scholar] [CrossRef]
- Picheth, G.F.; Pirich, C.L.; Sierakowski, M.R.; Woehl, M.A.; Sakakibara, C.N.; de Souza, C.F.; Martin, A.A.; da Silva, R.; de Freitas, R.A. Bacterial cellulose in biomedical applications: A review. Int. J. Biol. Macromol. 2017, 104, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Aisyah, H.A.; Paridah, M.T.; Sapuan, S.M.; Ilyas, R.A.; Khalina, A.; Nurazzi, N.M.; Lee, S.H.; Lee, C.H. A Comprehensive Review on Advanced Sustainable Woven Natural Fibre Polymer Composites. Polymers 2021, 13, 471. [Google Scholar] [CrossRef] [PubMed]
- Norizan, M.N.; Moklis, M.H.; Alias, A.H.; Rushdan, A.I.; Norrrahim, M.N.F.; Abdan, K.; Abdullah, N. Treatments of Natural Fibre as Reinforcement in Polymer Composites-Short Review. Funct. Compos. Struct. 2021, 3, 024002. [Google Scholar] [CrossRef]
- Norrrahim, M.N.F.; Huzaifah, M.R.M.; Farid, M.A.A.; Shazleen, S.S.; Misenan, M.S.M.; Yasim-Anuar, T.A.T.; Naveen, J.; Nurazzi, N.M.; Rani, M.S.A.; Hakimi, M.I.; et al. Greener Pretreatment Approaches for the Valorisation of Natural Fibre Biomass into Bioproducts. Polymers 2021, 13, 2971. [Google Scholar] [CrossRef] [PubMed]
- Azman, M.A.; Asyraf, M.R.M.; Khalina, A.; Petrů, M.; Ruzaidi, C.M.; Sapuan, S.M.; Wan Nik, W.B.; Ishak, M.R.; Ilyas, R.A.; Suriani, M.J. Natural Fiber Reinforced Composite Material for Product Design: A Short Review. Polymers 2021, 13, 1917. [Google Scholar] [CrossRef]
- Nurazzi, N.M.; Asyraf, M.R.M.; Khalina, A.; Abdullah, N.; Aisyah, H.A.; Rafiqah, S.A.; Sabaruddin, F.A.; Kamarudin, S.H.; Norrrahim, M.N.F.; Ilyas, R.A.; et al. A Review on Natural Fiber Reinforced Polymer Composite for Bullet Proof and Ballistic Applications. Polymers 2021, 13, 646. [Google Scholar] [CrossRef]
- Alsubari, S.; Zuhri, M.Y.M.; Sapuan, S.M.; Ishak, M.R.; Ilyas, R.A.; Asyraf, M.R.M. Potential of natural fiber reinforced polymer composites in sandwich structures: A review on its mechanical properties. Polymers 2021, 13, 423. [Google Scholar] [CrossRef] [PubMed]
- Ayu, R.S.; Khalina, A.; Harmaen, A.S.; Zaman, K.; Isma, T.; Liu, Q.; Ilyas, R.A.; Lee, C.H. Characterization Study of Empty Fruit Bunch (EFB) Fibers Reinforcement in Poly(Butylene) Succinate (PBS)/Starch/Glycerol Composite Sheet. Polymers 2020, 12, 1571. [Google Scholar] [CrossRef]
- Ullah, H.; Santos, H.A.; Khan, T. Applications of bacterial cellulose in food, cosmetics and drug delivery. Cellulose 2016, 23, 2291–2314. [Google Scholar] [CrossRef]
- Rebelo, A.R.; Archer, A.J.; Chen, X.; Liu, C.; Yang, G.; Liu, Y. Dehydration of bacterial cellulose and the water content effects on its viscoelastic and electrochemical properties. Sci. Technol. Adv. Mater. 2018, 19, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.-C.; Liu, S.-X.; Li, C.-F.; Li, J.; Liu, L.-X.; Deng, J.; Yang, Y.-C. Morphology and structure characterization of bacterial celluloses produced by different strains in agitated culture. J. Appl. Microbiol. 2014, 117, 1305–1311. [Google Scholar] [CrossRef] [PubMed]
- Evans, B.R.; O’Neill, H.M. Effect of surface attachment on synthesis of bacterial cellulose. Appl. Biochem. Biotechnol. Part A Enzym. Eng. Biotechnol. 2005, 121, 439–450. [Google Scholar] [CrossRef]
- Costa, A.F.S.; Almeida, F.C.G.; Vinhas, G.M.; Sarubbo, L.A. Production of bacterial cellulose by Gluconacetobacter hansenii using corn steep liquor as nutrient sources. Front. Microbiol. 2017, 8, 1–12. [Google Scholar] [CrossRef]
- Torres, F.G.; Commeaux, S.; Troncoso, O.P. Biocompatibility of bacterial cellulose based biomaterials. J. Funct. Biomater. 2012, 3, 864–878. [Google Scholar] [CrossRef] [Green Version]
- Boisset, C.; Fraschini, C.; Schülein, M.; Henrissat, B.; Chanzy, H. Imaging the enzymatic digestion of bacterial cellulose ribbons reveals the endo character of the cellobiohydrolase Cel6A from Humicola insolens and its mode of synergy with cellobiohydrolase Cel7A. Appl. Environ. Microbiol. 2000, 66, 1444–1452. [Google Scholar] [CrossRef] [Green Version]
- Basta, A.H.; El-Saied, H. Performance of improved bacterial cellulose application in the production of functional paper. J. Appl. Microbiol. 2009, 107, 2098–2107. [Google Scholar] [CrossRef]
- El-Saied, H.; Basta, A.H.; Gobran, R.H. Research Progress in Friendly Environmental Technology for the Production of Cellulose Products (Bacterial Cellulose and Its Application). Polym. Plast. Technol. Eng. 2004, 43, 797–820. [Google Scholar] [CrossRef]
- Tahara, N.; Tabuchi, M.; Watanabe, K.; Yano, H.; Morinaga, Y.; Yoshinaga, F. Degree of Polymerization of Cellulose from Acetobacter xylinum BPR2001 Decreased by Cellulase Produced by the Strain. Biosci. Biotechnol. Biochem. 1997, 61, 1862–1865. [Google Scholar] [CrossRef]
- Grande, C.J.; Torres, F.G.; Gomez, C.M.; Troncoso, O.P.; Canet-Ferrer, J.; Martínez-Pastor, J. Development of self-assembled bacterial cellulose-starch nanocomposites. Mater. Sci. Eng. C 2009, 29, 1098–1104. [Google Scholar] [CrossRef]
- Gorgieva, S.; Trček, J. Bacterial cellulose: Production, modification and perspectives in biomedical applications. Nanomaterials 2019, 9, 1352. [Google Scholar] [CrossRef] [Green Version]
- Lou, Z.C. A better design is needed for clinical studies of chronic tympanic membrane perforations using biological materials. Eur. Arch. Oto Rhino-Laryngol. 2016, 273, 4045–4046. [Google Scholar] [CrossRef]
- Biskin, S.; Damar, M.; Oktem, S.N.; Sakalli, E.; Erdem, D.; Pakir, O. A new graft material for myringoplasty: Bacterial cellulose. Eur. Arch. Oto-Rhino-Laryngol. 2016, 273, 3561–3565. [Google Scholar] [CrossRef]
- Lang, N.; Merkel, E.; Fuchs, F.; Schumann, D.; Klemm, D.; Kramer, F.; Mayer-Wagner, S.; Schroeder, C.; Freudenthal, F.; Netz, H.; et al. Bacterial nanocellulose as a new patch material for closure of ventricular septal defects in a pig model. Eur. J. Cardio-Thorac. Surg. 2014, 47, 1013–1021. [Google Scholar] [CrossRef] [Green Version]
- Azeredo, H.M.C.; Rosa, M.F.; Mattoso, L.H.C. Nanocellulose in bio-based food packaging applications. Ind. Crops Prod. 2017, 97, 664–671. [Google Scholar] [CrossRef]
- Gellner, P.E.L.; Dang, A.E.; Jay, H. Acoustic Diaphragm and Method for Producing Same. US Patent US005274199A, 28 December 1993. [Google Scholar]
- Hioki, N.; Hori, Y.; Watanabe, K.; Morinaga, Y.; Yoshinaga, F.; Hibino, Y.; Ogura, T. Bacterial cellulose; as a new material for papermaking. Jpn. TAPPI J. 1995, 49, 718–723. [Google Scholar] [CrossRef]
- Yang, J.; Yu, J.; Fan, J.; Sun, D.; Tang, W.; Yang, X. Biotemplated preparation of CdS nanoparticles/bacterial cellulose hybrid nanofibers for photocatalysis application. J. Hazard. Mater. 2011, 189, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Benito-González, I.; López-Rubio, A.; Gómez-Mascaraque, L.G.; Martínez-Sanz, M. PLA coating improves the performance of renewable adsorbent pads based on cellulosic aerogels from aquatic waste biomass. Chem. Eng. J. 2020, 390, 124607. [Google Scholar] [CrossRef]
- Morales-Narváez, E.; Golmohammadi, H.; Naghdi, T.; Yousefi, H.; Kostiv, U.; Horák, D.; Pourreza, N.; Merkoçi, A. Nanopaper as an Optical Sensing Platform. ACS Nano 2015, 9, 7296–7305. [Google Scholar] [CrossRef]
- Yuen, J.D.; Shriver-Lake, L.C.; Walper, S.A.; Zabetakis, D.; Breger, J.C.; Stenger, D.A. Microbial nanocellulose printed circuit boards for medical sensing. Sensors 2020, 20, 2047. [Google Scholar] [CrossRef] [Green Version]
- Portela, R.; Leal, C.R.; Almeida, P.L.; Sobral, R.G. Bacterial cellulose: A versatile biopolymer for wound dressing applications. Microb. Biotechnol. 2019, 12, 586–610. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, X.; Zhang, S.W. Biodegradable medical polymers. In Science and Principles of Biodegradable and Bioresorbable Medical Polymers; Zhang, X., Ed.; Elsevier: Duxford, UK, 2017; pp. 1–33. [Google Scholar]
- Czaja, W.; Krystynowicz, A.; Kawecki, M.; Wysota, K.; Sakiel, S.; Wróblewski, P.; Glik, J.; Nowak, M.; Bielecki, S. Biomedical Applications of Microbial Cellulose in Burn Wound Recovery. In Cellulose: Molecular and Structural Biology; Springer: Dordrecht, The Netherlands, 2007; pp. 307–321. [Google Scholar] [CrossRef]
- Sulaeva, I.; Hettegger, H.; Bergen, A.; Rohrer, C.; Kostic, M.; Konnerth, J.; Rosenau, T.; Potthast, A. Fabrication of bacterial cellulose-based wound dressings with improved performance by impregnation with alginate. Mater. Sci. Eng. C 2020, 110, 110619. [Google Scholar] [CrossRef]
- Blanco Parte, F.G.; Santoso, S.P.; Chou, C.C.; Verma, V.; Wang, H.T.; Ismadji, S.; Cheng, K.C. Current progress on the production, modification, and applications of bacterial cellulose. Crit. Rev. Biotechnol. 2020, 40, 397–414. [Google Scholar] [CrossRef] [Green Version]
- Żur, J.; Piński, A.; Michalska, J.; Hupert-Kocurek, K.; Nowak, A.; Wojcieszyńska, D.; Guzik, U. A whole-cell immobilization system on bacterial cellulose for the paracetamol-degrading Pseudomonas moorei KB4 strain. Int. Biodeterior. Biodegrad. 2020, 149, 104919. [Google Scholar] [CrossRef]
- Zhang, S.; He, H.; Guan, S.; Cai, B.; Li, Q.; Rong, S. Bacterial cellulose-alginate composite beads as yarrowia lipolytica cell carriers for lactone production. Molecules 2020, 25, 928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feil, G.; Horres, R.; Schulte, J.; Mack, A.F.; Petzoldt, S.; Arnold, C.; Meng, C.; Jost, L.; Boxleitner, J.; Kiessling-Wolf, N.; et al. Bacterial cellulose shifts transcriptome and proteome of cultured endothelial cells towards native differentiation. Mol. Cell. Proteom. 2017, 16, 1563–1577. [Google Scholar] [CrossRef] [Green Version]
- Chan, J.X.; Wong, J.F.; Petrů, M.; Hassan, A.; Nirmal, U.; Othman, N.; Ilyas, R.A. Effect of Nanofillers on Tribological Properties of Polymer Nanocomposites: A Review on Recent Development. Polymers 2021, 13, 2867. [Google Scholar] [CrossRef] [PubMed]
- Punia Bangar, S.; Nehra, M.; Siroha, A.K.; Petrů, M.; Ilyas, R.A.; Devi, U.; Devi, P. Development and Characterization of Physical Modified Pearl Millet Starch-Based Films. Foods 2021, 10, 1609. [Google Scholar] [CrossRef] [PubMed]
- Kumari, N.; Bangar, S.P.; Petrů, M.; Ilyas, R.A.; Singh, A.; Kumar, P. Development and Characterization of Fenugreek Protein-Based Edible Film. Foods 2021, 10, 1976. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, R.A.; Sapuan, S.M.; Harussani, M.M.; Hakimi, M.Y.A.Y.; Haziq, M.Z.M.; Atikah, M.S.N.; Asyraf, M.R.M.; Ishak, M.R.; Razman, M.R.; Nurazzi, N.M.; et al. Polylactic Acid (PLA) Biocomposite: Processing, Additive Manufacturing and Advanced Applications. Polymers 2021, 13, 1326. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, R.A.; Sapuan, S.M. Biopolymers and Biocomposites: Chemistry and Technology. Curr. Anal. Chem. 2020, 16, 500–503. [Google Scholar] [CrossRef]
- Abral, H.; Atmajaya, A.; Mahardika, M.; Hafizulhaq, F.; Kadriadi; Handayani, D.; Sapuan, S.M.; Ilyas, R.A. Effect of ultrasonication duration of polyvinyl alcohol (PVA) gel on characterizations of PVA film. J. Mater. Res. Technol. 2020, 9, 2477–2486. [Google Scholar] [CrossRef]
- Sapuan, S.M.; Aulia, H.S.; Ilyas, R.A.; Atiqah, A.; Dele-Afolabi, T.T.; Nurazzi, M.N.; Supian, A.B.M.; Atikah, M.S.N. Mechanical properties of longitudinal basalt/woven-glass-fiber-reinforced unsaturated polyester-resin hybrid composites. Polymers 2020, 12, 2211. [Google Scholar] [CrossRef] [PubMed]
- Pötzinger, Y.; Kralisch, D.; Fischer, D. Bacterial nanocellulose: The future of controlled drug delivery? Ther. Deliv. 2017, 8, 753–761. [Google Scholar] [CrossRef]
- Unal, S.; Gunduz, O.; Uzun, M. Tissue Engineering Applications of Bacterial Cellulose Based Nanofibers. In Green Nanomaterials. Advanced Structured Materials; Ahmed, S., Ali, W., Eds.; Springer: Singapore, 2020; pp. 319–346. [Google Scholar]
- Wang, K.; Ma, Q.; Zhang, Y.M.; Han, G.T.; Qu, C.X.; Wang, S.D. Preparation of bacterial cellulose/silk fibroin double-network hydrogel with high mechanical strength and biocompatibility for artificial cartilage. Cellulose 2020, 27, 1845–1852. [Google Scholar] [CrossRef]
- Klinthoopthamrong, N.; Chaikiawkeaw, D.; Phoolcharoen, W.; Rattanapisit, K.; Kaewpungsup, P.; Pavasant, P.; Hoven, V.P. Bacterial cellulose membrane conjugated with plant-derived osteopontin: Preparation and its potential for bone tissue regeneration. Int. J. Biol. Macromol. 2020, 149, 51–59. [Google Scholar] [CrossRef]
- Junka, A.; Bartoszewicz, M.; Dziadas, M.; Szymczyk, P.; Dydak, K.; Żywicka, A.; Owczarek, A.; Bil-Lula, I.; Czajkowska, J.; Fijałkowski, K. Application of bacterial cellulose experimental dressings saturated with gentamycin for management of bone biofilm in vitro and ex vivo. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 30–37. [Google Scholar] [CrossRef]
- Svensson, A.; Nicklasson, E.; Harrah, T.; Panilaitis, B.; Kaplan, D.L.; Brittberg, M.; Gatenholm, P. Bacterial cellulose as a potential scaffold for tissue engineering of cartilage. Biomaterials 2005, 26, 419–431. [Google Scholar] [CrossRef]
- Codreanu, A.; Balta, C.; Herman, H.; Cotoraci, C.; Mihali, C.V.; Zurbau, N.; Zaharia, C.; Rapa, M.; Stanescu, P.; Radu, I.C.; et al. Bacterial cellulose-modified polyhydroxyalkanoates scaffolds promotes bone formation in critical size calvarial defects in mice. Materials 2020, 13, 1433. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Wang, X.C.; Li, X.Y.; Zhang, L.L.; Jiang, F. A 3D porous microsphere with multistage structure and component based on bacterial cellulose and collagen for bone tissue engineering. Carbohydr. Polym. 2020, 236, 116043. [Google Scholar] [CrossRef] [PubMed]
- Lina, F.; Chandra, P.; Adrianna, M.; Wankei, W. Bacterial cellulose production using a novel microbe. Front. Bioeng. Biotechnol. 2016, 4, 1–2. [Google Scholar] [CrossRef]
- Bodea, I.M.; Cătunescu, G.M.; Stroe, T.F.; Dîrlea, S.A.; Beteg, F.I. Applications of bacterial-synthesized cellulose in veterinary medicine—A review. Acta Vet. Brno 2019, 88, 451–471. [Google Scholar] [CrossRef]
- Lin, N.; Dufresne, A. Nanocellulose in biomedicine: Current status and future prospect. Eur. Polym. J. 2014, 59, 302–325. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.-K.; Lee, S.-C.; Cho, Y.-Y.; Oh, H.-J.; Ko, Y.H. Isolation of Cellulolytic Bacillus subtilis Strains from Agricultural Environments. ISRN Microbiol. 2012, 2012, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Inoue, B.S.; Streit, S.; dos Santos Schneider, A.L.; Meier, M.M. Bioactive bacterial cellulose membrane with prolonged release of chlorhexidine for dental medical application. Int. J. Biol. Macromol. 2020, 148, 1098–1108. [Google Scholar] [CrossRef]
- Li, N.; Yang, L.; Pan, C.; Saw, P.E.; Ren, M.; Lan, B.; Wu, J.; Wang, X.; Zeng, T.; Zhou, L.; et al. Naturally-occurring bacterial cellulose-hyperbranched cationic polysaccharide derivative/MMP-9 siRNA composite dressing for wound healing enhancement in diabetic rats. Acta Biomater. 2020, 102, 298–314. [Google Scholar] [CrossRef]
- Stumpf, T.R.; Tang, L.; Kirkwood, K.; Yang, X.; Zhang, J.; Cao, X. Production and evaluation of biosynthesized cellulose tubes as promising nerve guides for spinal cord injury treatment. J. Biomed. Mater. Res.-Part A 2020, 108, 1380–1389. [Google Scholar] [CrossRef]
- Czaja, W.K.; Young, D.J.; Kawecki, M.; Brown, R.M. The Future Prospects of Microbial Cellulose in Biomedical Applications. Biomacromolecules 2007, 8, 1–12. [Google Scholar] [CrossRef]
- Schaffner, M.; Rühs, P.A.; Coulter, F.; Kilcher, S.; Studart, A.R. 3D printing of bacteria into functional complex materials. Sci. Adv. 2017, 3, eaao6804. [Google Scholar] [CrossRef] [Green Version]
- Okahisa, Y.; Yoshida, A.; Miyaguchi, S.; Yano, H. Optically transparent wood-cellulose nanocomposite as a base substrate for flexible organic light-emitting diode displays. Compos. Sci. Technol. 2009, 69, 1958–1961. [Google Scholar] [CrossRef]
- Gomes, N.O.; Carrilho, E.; Antonio, S.; Machado, S.; Sgobbi, F. Bacterial cellulose-based electrochemical sensing platform: A smart material for miniaturized biosensors. Electrochim. Acta 2020, 349, 136341. [Google Scholar] [CrossRef]
- Buruaga-Ramiro, C.; Valenzuela, S.V.; Valls, C.; Roncero, M.B.; Pastor, F.I.J.; Díaz, P.; Martínez, J. Bacterial cellulose matrices to develop enzymatically active paper. Cellulose 2020, 27, 3413–3426. [Google Scholar] [CrossRef]
- Park, S.; Park, J.; Jo, I.; Cho, S.P.; Sung, D.; Ryu, S.; Park, M.; Min, K.A.; Kim, J.; Hong, S.; et al. In situ hybridization of carbon nanotubes with bacterial cellulose for three-dimensional hybrid bioscaffolds. Biomaterials 2015, 58, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Czaja, W.; Krystynowicz, A.; Bielecki, S.; Brown, J.R.M. Microbial cellulose—The natural power to heal wounds. Biomaterials 2006, 27, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Markstedt, K.; Mantas, A.; Tournier, I.; Martínez Ávila, H.; Hägg, D.; Gatenholm, P. 3D Bioprinting Human Chondrocytes with Nanocellulose–Alginate Bioink for Cartilage Tissue Engineering Applications. Biomacromolecules 2015, 16, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, D.; Yang, L.; Zhou, L.; You, T. Self-assembled three-dimensional graphene-based materials for dye adsorption and catalysis. J. Mater. Chem. A 2015, 3, 10031–10037. [Google Scholar] [CrossRef]
- Naseri-Nosar, M.; Salehi, M.; Hojjati-Emami, S. Cellulose acetate/poly lactic acid coaxial wet-electrospun scaffold containing citalopram-loaded gelatin nanocarriers for neural tissue engineering applications. Int. J. Biol. Macromol. 2017, 103, 701–708. [Google Scholar] [CrossRef]
- Mathew, A.P.; Oksman, K.; Pierron, D.; Harmand, M.F. Biocompatible Fibrous Networks of Cellulose Nanofibres and Collagen Crosslinked Using Genipin: Potential as Artificial Ligament/Tendons. Macromol. Biosci. 2013, 13, 289–298. [Google Scholar] [CrossRef]
- Sämfors, S.; Karlsson, K.; Sundberg, J.; Markstedt, K.; Gatenholm, P. Biofabrication of bacterial nanocellulose scaffolds with complex vascular structure. Biofabrication 2019, 11, 45010. [Google Scholar] [CrossRef]
- Hussain, Z.; Sajjad, W.; Khan, T.; Wahid, F. Production of bacterial cellulose from industrial wastes: A review. Cellulose 2019, 26, 2895–2911. [Google Scholar] [CrossRef]
- Vandamme, E.J.; De Baets, S.; Vanbaelen, A.; Joris, K.; De Wulf, P. Improved production of bacterial cellulose and its application potential. Polym. Degrad. Stab. 1998, 59, 93–99. [Google Scholar] [CrossRef]
- Bajpai, P. Biobased Polymers: Properties and Applications in Packaging, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128184042. [Google Scholar]
- Torgbo, S.; Sukyai, P. Bacterial cellulose-based scaffold materials for bone tissue engineering. Appl. Mater. Today 2018, 11, 34–49. [Google Scholar] [CrossRef]
- Mohd Nurazzi, N.; Asyraf, M.R.M.; Khalina, A.; Abdullah, N.; Sabaruddin, F.A.; Kamarudin, S.H.; Ahmad, S.; Mahat, A.M.; Lee, C.L.; Aisyah, H.A.; et al. Fabrication, Functionalization, and Application of Carbon Nanotube-Reinforced Polymer Composite: An Overview. Polymers 2021, 13, 1047. [Google Scholar] [CrossRef] [PubMed]
- Stumpf, T.R.; Yang, X.; Zhang, J.; Cao, X. In situ and ex situ modifications of bacterial cellulose for applications in tissue engineering. Mater. Sci. Eng. C 2018, 82, 372–383. [Google Scholar] [CrossRef]
- Asyraf, M.R.M.; Ishak, M.R.; Sapuan, S.M.; Yidris, N.; Ilyas, R.A.; Rafidah, M.; Razman, M.R. Potential Application of Green Composites for Cross Arm Component in Transmission Tower: A Brief Review. Int. J. Polym. Sci. 2020, 2020, 1–15. [Google Scholar] [CrossRef]
- Asyraf, M.R.M.; Rafidah, M.; Ishak, M.R.; Sapuan, S.M.; Ilyas, R.A.; Razman, M.R. Integration of TRIZ, Morphological Chart and ANP method for development of FRP composite portable fire extinguisher. Polym. Compos. 2020, 41, 2917–2932. [Google Scholar] [CrossRef]
- Nazrin, A.; Sapuan, S.M.; Zuhri, M.Y.M.; Tawakkal, I.S.M.A.; Ilyas, R.A. Water barrier and mechanical properties of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch (TPS)/poly(lactic acid) (PLA) blend bionanocomposites. Nanotechnol. Rev. 2021, 10, 431–442. [Google Scholar] [CrossRef]
- Tarique, J.; Sapuan, S.M.; Khalina, A.; Sherwani, S.F.K.; Yusuf, J.; Ilyas, R.A. Recent developments in sustainable arrowroot (Maranta arundinacea Linn) starch biopolymers, fibres, biopolymer composites and their potential industrial applications: A review. J. Mater. Res. Technol. 2021, 13, 1191–1219. [Google Scholar] [CrossRef]
- Zaborowska, M.; Bodin, A.; Bäckdahl, H.; Popp, J.; Goldstein, A.; Gatenholm, P. Microporous bacterial cellulose as a potential scaffold for bone regeneration. Acta Biomater. 2010, 6, 2540–2547. [Google Scholar] [CrossRef]
- Gonçalves-Pimentel, C.; Moreno, G.M.M.; Trindade, B.S.; Isaac, A.R.; Rodrigues, C.G.; Savariradjane, M.; de Albuquerque, A.V.; de Andrade Aguiar, J.L.; Andrade-da-Costa, B.L.d.S. Cellulose exopolysaccharide from sugarcane molasses as a suitable substrate for 2D and 3D neuron and astrocyte primary cultures. J. Mater. Sci. Mater. Med. 2018, 29, 139. [Google Scholar] [CrossRef] [PubMed]
- Padra, J.; Silva, P.; Sencadas, V. Bacterial Cellulose as a Support for the Growth of Retinal Pigment Epithelium. Biomacromolecules 2015, 16, 1341–1351. [Google Scholar] [CrossRef] [Green Version]
- Seoane, I.T.; Manfredi, L.B.; Cyras, V.P.; Torre, L.; Fortunati, E.; Puglia, D. Effect of Cellulose Nanocrystals and Bacterial Cellulose on disintegrability in composting conditions of Plasticized PHB Nanocomposites. Polymers 2017, 9, 561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Zheng, S.; Hu, Z.; Zhong, L.; Wang, Y.; Zhang, X.; Xue, J. Preparation of Polyvinyl Alcohol/Bacterial-Cellulose-Coated Biochar–Nanosilver Antibacterial Composite Membranes. Appl. Sci. 2020, 10, 752. [Google Scholar] [CrossRef] [Green Version]
- Hamedi, S.; Shojaosadati, S.A.; Najafi, V.; Alizadeh, V. A novel double-network antibacterial hydrogel based on aminated bacterial cellulose and schizophyllan. Carbohydr. Polym. 2020, 229, 115383. [Google Scholar] [CrossRef]
- Sukhavattanakul, P.; Manuspiya, H. Fabrication of hybrid thin film based on bacterial cellulose nanocrystals and metal nanoparticles with hydrogen sulfide gas sensor ability. Carbohydr. Polym. 2020, 230, 115566. [Google Scholar] [CrossRef]
- Gupta, A.; Briffa, S.M.; Swingler, S.; Gibson, H.; Kannappan, V.; Adamus, G.; Kowalczuk, M.; Martin, C.; Radecka, I. Synthesis of Silver Nanoparticles Using Curcumin-Cyclodextrins Loaded into Bacterial Cellulose-Based Hydrogels for Wound Dressing Applications. Biomacromolecules 2020, 21, 1802–1811. [Google Scholar] [CrossRef]
- Di Pasquale, G.; Graziani, S.; Pollicino, A.; Trigona, C. Performance characterization of a biodegradable deformation sensor based on bacterial cellulose. IEEE Trans. Instrum. Meas. 2019, 69, 2561–2569. [Google Scholar] [CrossRef]
- Wang, L.; Mao, L.; Qi, F.; Li, X.; Ullah, M.W.; Zhao, M.; Shi, Z.; Yang, G. Synergistic effect of highly aligned bacterial cellulose/gelatin membranes and electrical stimulation on directional cell migration for accelerated wound healing. Chem. Eng. J. 2021, 424, 130563. [Google Scholar] [CrossRef]
- Rebelo, A.; Liu, Y.; Liu, C.; Schäfer, K.-H.; Saumer, M.; Yang, G. Poly (4-vinylaniline)/polyaniline bilayer functionalized bacterial cellulose membranes as bioelectronics interfaces. Carbohydr. Polym. 2019, 204, 190–201. [Google Scholar] [CrossRef] [Green Version]
- Saxena, I.M.; Brown, R.M. A Perspective on the Assembly of Cellulose-Synthesizing Complexes: Possible Role of KORRIGAN and Microtubules in Cellulose Synthesis in Plants. In Cellulose: Molecular and Structural Biology; Springer: Dordrecht, The Netherlands, 2007; pp. 169–181. [Google Scholar]
- Putra, A.; Kakugo, A.; Furukawa, H.; Gong, J.P.; Osada, Y. Tubular bacterial cellulose gel with oriented fibrils on the curved surface. Polymer 2008, 49, 1885–1891. [Google Scholar] [CrossRef]
- Nimeskern, L.; Martínez Ávila, H.; Sundberg, J.; Gatenholm, P.; Müller, R.; Stok, K.S. Mechanical evaluation of bacterial nanocellulose as an implant material for ear cartilage replacement. J. Mech. Behav. Biomed. Mater. 2013, 22, 12–21. [Google Scholar] [CrossRef] [Green Version]
- Bodin, A.; Concaro, S.; Brittberg, M.; Gatenholm, P. Bacterial cellulose as a potential meniscus implant. J. Tissue Eng. Regen. Med. 2007, 1, 406–408. [Google Scholar] [CrossRef]
- Charpentier, P.A.; Maguire, A.; Wan, W. Surface modification of polyester to produce a bacterial cellulose-based vascular prosthetic device. Appl. Surf. Sci. 2006, 252, 6360–6367. [Google Scholar] [CrossRef]
- Klemm, D.; Schumann, D.; Udhardt, U.; Marsch, S. Bacterial synthesized cellulose—Artificial blood vessels for microsurgery. Prog. Polym. Sci. 2001, 26, 1561–1603. [Google Scholar] [CrossRef]
- Sepúlveda, R.V.; Valente, F.L.; Reis, E.C.C.; Araújo, F.R.; Eleotério, R.B.; Queiroz, P.V.S.; Borges, A.P.B. Bacterial cellulose and bacterial cellulose/polycaprolactone composite as tissue substitutes in rabbits’ cornea. Pesqui. Veterinária Bras. 2016, 36, 986–992. [Google Scholar] [CrossRef] [Green Version]
- Barud, H.S.; Ribeiro, S.J.L.; Carone, C.L.P.; Ligabue, R.; Einloft, S.; Queiroz, P.V.S.; Borges, A.P.B.; Jahno, V.D. Optically transparent membrane based on bacterial cellulose/polycaprolactone. Polimeros 2013, 23, 135–138. [Google Scholar] [CrossRef]
- Caiut, J.M.A.; Barud, H.S.; Santos, M.V.; Oliveira, U.L.; Menezes, J.F.S.; Messaddeq, Y.; Ribeiro, S.J.L. Luminescent multifunctional biocellulose membranes. In Proceedings of the Nanostructured Thin Films IV, San Diego, CA, USA, 21–25 August 2011; Volume 8104, p. 81040. [Google Scholar] [CrossRef]
- Kim, J.; Cai, Z.; Lee, H.S.; Choi, G.S.; Lee, D.H.; Jo, C. Preparation and characterization of a Bacterial cellulose/Chitosan composite for potential biomedical application. J. Polym. Res. 2011, 18, 739–744. [Google Scholar] [CrossRef]
- Legeza, V.I.; Galenko-Yaroshevskii, V.P.; Zinov’ev, E.V.; Paramonov, B.A.; Kreichman, G.S.; Turkovskii, I.I.; Gumenyuk, E.S.; Karnovich, A.G.; Khripunov, A.K. Effects of new wound dressings on healing of thermal burns of the skin in acute radiation disease. Bull. Exp. Biol. Med. 2004, 138, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Messaddeq, Y.; Ribeiro, S.J.L.; Thomazini, W. Contact Lens for Therapy, Method and Apparatus for Their Production and Use. Brazil Patent BR PI0603704-6, 2008. [Google Scholar]
- Osorio, M.; Velásquez-Cock, J.; Restrepo, L.M.; Zuluaga, R.; Gañán, P.; Rojas, O.J.; Ortiz-Trujillo, I.; Castro, C. Bioactive 3D-Shaped Wound Dressings Synthesized from Bacterial Cellulose: Effect on Cell Adhesion of Polyvinyl Alcohol Integrated In Situ. Int. J. Polym. Sci. 2017, 2017, 1–10. [Google Scholar] [CrossRef]
- Zhu, H.; Jia, S.; Yang, H.; Jia, Y.; Yan, L.; Li, J. Preparation and Application of Bacterial Cellulose Sphere: A Novel Biomaterial. Biotechnol. Biotechnol. Equip. 2011, 25, 2233–2236. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.-C.; Li, M.-H. Production of bacterial cellulose membranes in a modified airlift bioreactor by Gluconacetobacter xylinus. J. Biosci. Bioeng. 2015, 120, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Catchmark, J.M.; Vogler, E.A. Factors Impacting the Formation of Sphere-Like Bacterial Cellulose Particles and Their Biocompatibility for Human Osteoblast Growth. Biomacromolecules 2013, 14, 3444–3452. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Catchmark, J.M. Formation and Characterization of Spherelike Bacterial Cellulose Particles Produced by Acetobacter xylinum JCM 9730 Strain. Biomacromolecules 2010, 11, 1727–1734. [Google Scholar] [CrossRef] [PubMed]
- Czaja, W.; Romanovicz, D.; malcolm Brown, R. Structural investigations of microbial cellulose produced in stationary and agitated culture. Cellulose 2004, 11, 403–411. [Google Scholar] [CrossRef]
- Cai, Q.; Hu, C.; Yang, N.; Wang, Q.; Wang, J.; Pan, H.; Hu, Y.; Ruan, C. Enhanced activity and stability of industrial lipases immobilized onto spherelike bacterial cellulose. Int. J. Biol. Macromol. 2018, 109, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Catchmark, J.M. Impact of hemicelluloses and pectin on sphere-like bacterial cellulose assembly. Carbohydr. Polym. 2012, 88, 547–557. [Google Scholar] [CrossRef]
- Yan, Z.; Chen, S.; Wang, H.; Wang, B.; Jiang, J. Biosynthesis of bacterial cellulose/multi-walled carbon nanotubes in agitated culture. Carbohydr. Polym. 2008, 74, 659–665. [Google Scholar] [CrossRef]
- Zhu, H.; Jia, S.; Wan, T.; Jia, Y.; Yang, H.; Li, J.; Yan, L.; Zhong, C. Biosynthesis of spherical Fe3O4/bacterial cellulose nanocomposites as adsorbents for heavy metal ions. Carbohydr. Polym. 2011, 86, 1558–1564. [Google Scholar] [CrossRef]
- Hu, D. Study on Structural Modulation and Compounding with Graphene of Bacterial Cellulose for Adsorption of Organics. Master’s Thesis, Tianjin University, Tianjin, China, 2014. [Google Scholar]
- Ilyas, R.A.; Sapuan, S.M.; Ishak, M.R.; Zainudin, E.S. Development and characterization of sugar palm nanocrystalline cellulose reinforced sugar palm starch bionanocomposites. Carbohydr. Polym. 2018, 202, 186–202. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, S.M.; Ishak, M.R.; Zainudin, E.S. Sugar palm nanofibrillated cellulose (Arenga pinnata (Wurmb.) Merr): Effect of cycles on their yield, physic-chemical, morphological and thermal behavior. Int. J. Biol. Macromol. 2019, 123, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, R.A.; Sapuan, S.M.; Ibrahim, R.; Abral, H.; Ishak, M.R.; Zainudin, E.S.; Atikah, M.S.N.; Mohd Nurazzi, N.; Atiqah, A.; Ansari, M.N.M.; et al. Effect of sugar palm nanofibrillated celluloseconcentrations on morphological, mechanical andphysical properties of biodegradable films basedon agro-waste sugar palm (Arenga pinnata (Wurmb.) Merr) starch. J. Mater. Res. Technol. 2019, 8, 4819–4830. [Google Scholar] [CrossRef]
- Hazrol, M.D.; Sapuan, S.M.; Ilyas, R.A.; Othman, M.L.; Sherwani, S.F.K. Electrical properties of sugar palm nanocrystalline cellulose reinforced sugar palm starch nanocomposites. Polimery 2020, 65, 363–370. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, S.M.; Ibrahim, R.; Abral, H.; Ishak, M.R.; Zainudin, E.S.; Asrofi, M.; Atikah, M.S.N.; Huzaifah, M.R.M.; Radzi, A.M.; et al. Sugar palm (Arenga pinnata (Wurmb.) Merr) cellulosic fibre hierarchy: A comprehensive approach from macro to nano scale. J. Mater. Res. Technol. 2019, 8, 2753–2766. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, S.M.; Atiqah, A.; Ibrahim, R.; Abral, H.; Ishak, M.R.; Zainudin, E.S.; Nurazzi, N.M.; Atikah, M.S.N.; Ansari, M.N.M.; et al. Sugar palm (Arenga pinnata [Wurmb.] Merr) starch films containing sugar palm nanofibrillated cellulose as reinforcement: Water barrier properties. Polym. Compos. 2019, 41, 459–467. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, S.M.; Atikah, M.S.N.; Asyraf, M.R.M.; Rafiqah, S.A.; Aisyah, H.A.; Nurazzi, N.M.; Norrrahim, M.N.F. Effect of hydrolysis time on the morphological, physical, chemical, and thermal behavior of sugar palm nanocrystalline cellulose (Arenga pinnata (Wurmb.) Merr). Text. Res. J. 2021, 91, 152–167. [Google Scholar] [CrossRef]
- Rozilah, A.; Jaafar, C.N.A.; Sapuan, S.M.; Zainol, I.; Ilyas, R.A. The Effects of Silver Nanoparticles Compositions on the Mechanical, Physiochemical, Antibacterial, and Morphology Properties of Sugar Palm Starch Biocomposites for Antibacterial Coating. Polymers 2020, 12, 2605. [Google Scholar] [CrossRef]
- Syafiq, R.; Sapuan, S.M.; Zuhri, M.Y.M.; Ilyas, R.A.; Nazrin, A.; Sherwani, S.F.K.; Khalina, A. Antimicrobial Activities of Starch-Based Biopolymers and Biocomposites Incorporated with Plant Essential Oils: A Review. Polymers 2020, 12, 2403. [Google Scholar] [CrossRef] [PubMed]
- Syafri, E.; Sudirman; Mashadi; Yulianti, E.; Deswita; Asrofi, M.; Abral, H.; Sapuan, S.M.; Ilyas, R.A.; Fudholi, A. Effect of sonication time on the thermal stability, moisture absorption, and biodegradation of water hyacinth (Eichhornia crassipes) nanocellulose-filled bengkuang (Pachyrhizus erosus) starch biocomposites. J. Mater. Res. Technol. 2019, 8, 6223–6231. [Google Scholar] [CrossRef]
- Abral, H.; Ariksa, J.; Mahardika, M.; Handayani, D.; Aminah, I.; Sandrawati, N.; Pratama, A.B.; Fajri, N.; Sapuan, S.M.; Ilyas, R.A. Transparent and antimicrobial cellulose film from ginger nanofiber. Food Hydrocoll. 2020, 98, 105266. [Google Scholar] [CrossRef]
- Abral, H.; Ariksa, J.; Mahardika, M.; Handayani, D.; Aminah, I.; Sandrawati, N.; Sapuan, S.M.; Ilyas, R.A. Highly transparent and antimicrobial PVA based bionanocomposites reinforced by ginger nanofiber. Polym. Test. 2019, 81, 106186. [Google Scholar] [CrossRef]
- Sabaruddin, F.A.; Paridah, M.T.; Sapuan, S.M.; Ilyas, R.A.; Lee, S.H.; Abdan, K.; Mazlan, N.; Roseley, A.S.M.; Abdul Khalil, H.P.S. The effects of unbleached and bleached nanocellulose on the thermal and flammability of polypropylene-reinforced kenaf core hybrid polymer bionanocomposites. Polymers 2020, 13, 116. [Google Scholar] [CrossRef]
- Asrofi, M.; Sapuan, S.M.; Ilyas, R.A.; Ramesh, M. Characteristic of composite bioplastics from tapioca starch and sugarcane bagasse fiber: Effect of time duration of ultrasonication (Bath-Type). Mater. Today Proc. 2020, 46, 1626–1630. [Google Scholar] [CrossRef]
- Asrofi, M.; Sujito; Syafri, E.; Sapuan, S.M.; Ilyas, R.A. Improvement of Biocomposite Properties Based Tapioca Starch and Sugarcane Bagasse Cellulose Nanofibers. Key Eng. Mater. 2020, 849, 96–101. [Google Scholar] [CrossRef]
- Kamaruddin, Z.H.; Jumaidin, R.; Selamat, M.Z.; Ilyas, R.A. Characteristics and Properties of Lemongrass (Cymbopogan Citratus): A Comprehensive Review. J. Nat. Fibers 2021, 1–18. [Google Scholar] [CrossRef]
- Wahab, M.; Sapuan, S.M.; Harussani, M.M.; Zuhri, M.Y.M.; Saleh, A.A. Conceptual Design of Glass/Renewable Natural Fibre-Reinforced Polymer Hybrid Composite Motorcycle Side Cover. J. Renew. Mater. 2021, 9, 1973–1989. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, S.M.; Sanyang, M.L.; Ishak, M.R.; Zainudin, E.S. Nanocrystalline cellulose as reinforcement for polymeric matrix nanocomposites and its potential applications: A Review. Curr. Anal. Chem. 2018, 14, 203–225. [Google Scholar] [CrossRef]
- Harussani, M.M.; Sapuan, S.M.; Rashid, U.; Khalina, A. Development and Characterization of Polypropylene Waste from Personal Protective Equipment (PPE)-Derived Char-Filled Sugar Palm Starch Biocomposite Briquettes. Polymers 2021, 13, 1707. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.I.; Jeong, J.H.; Lee, O.M.; Park, G.T.; Kim, K.K.; Park, H.C.; Lee, S.M.; Kim, Y.G.; Son, H.J. Influence of glycerol on production and structural-physical properties of cellulose from Acetobacter sp. V6 cultured in shake flasks. Bioresour. Technol. 2010, 101, 3602–3608. [Google Scholar] [CrossRef]
- Rangaswamy, B.E.; Vanitha, K.P.; Hungund, B.S. Microbial Cellulose Production from Bacteria Isolated from Rotten Fruit. Int. J. Polym. Sci. 2015, 2015, 280784. [Google Scholar] [CrossRef] [Green Version]
- Singh, O.; Panesar, P.S.; Chopra, H.K. Response surface optimization for cellulose production from agro industrial waste by using new bacterial isolate Gluconacetobacter xylinus C18. Food Sci. Biotechnol. 2017, 26, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Xu, X.; Song, L.; Guan, W.; Li, J.; Liu, B.; Shi, P.; Zhang, W. The use of Agrobacterium-mediated insertional mutagenesis sequencing to identify novel genes of Humicola insolens involved in cellulase production. 3 Biotech 2018, 8, 153. [Google Scholar] [CrossRef]
- Molina-Ramírez, C.; Castro, M.; Osorio, M.; Torres-Taborda, M.; Gómez, B.; Zuluaga, R.; Gómez, C.; Gañán, P.; Rojas, O.J.; Castro, C. Effect of different carbon sources on bacterial nanocellulose production and structure using the low pH resistant strain Komagataeibacter medellinensis. Materials 2017, 10, 639. [Google Scholar] [CrossRef]
- Reese, C. Characterization of WssF; a Putative Acetyltransferase from Achromobacter insuavis and Pseudomonas fluorescens. Master’s Thesis, Wilfrid Laurier University, Waterloo, ON, Canada, 2019. [Google Scholar]
- Ahmed, S.A.; Kazim, A.R.; Hassan, H.M. Increasing Cellulose Production from Rhizobium leguminosarum bv. viciae. J. Al-Nahrain Univ. 2017, 20, 120–125. [Google Scholar] [CrossRef]
- Anusuya, R.S.; Anandham, R.; Kumutha, K.; Gayathry, G.; Mageshwaran, V. Characterization and optimization of bacterial cellulose produced by Acetobacter spp. J. Environ. Biol. 2020, 41, 207–215. [Google Scholar] [CrossRef]
- Sun, B.; Zi, Q.; Chen, C.; Zhang, H.; Gu, Y.; Liang, G.; Sun, D. Study of specific metabolic pattern of Acetobacter xylinum NuST4.2 and bacterial cellulose production improvement. Cellul. Chem. Technol. 2018, 52, 795–801. [Google Scholar]
- Canale-Parola, E.; Wolfe, R.S. Synthesis of cellulose by Sarcina ventriculi. Biochim. Biophys. Acta-Gen. Subj. 1964, 82, 403–405. [Google Scholar] [CrossRef]
- Rastogi, A.; Banerjee, R. Production and characterization of cellulose from Leifsonia sp. Process Biochem. 2019, 85, 35–42. [Google Scholar] [CrossRef]
- Sreena, C.P.; Sebastian, D. Augmented cellulase production by Bacillus subtilis strain MU S1 using different statistical experimental designs. J. Genet. Eng. Biotechnol. 2018, 16, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Toor, Y.; Ilyas, U. Optimization of cellulase production by Aspergillus ornatus by the solid state fermentation of Cicer arietinum. Am. J. Res. 2014, 2, 125–141. [Google Scholar]
- Picart, P.; Diaz, P.; Pastor, F.I.J. Cellulases from two Penicillium sp. strains isolated from subtropical forest soil: Production and characterization. Lett. Appl. Microbiol. 2007, 45, 108–113. [Google Scholar] [CrossRef]
- Prasanna, H.N.; Ramanjaneyulu, G.; Rajasekhar Reddy, B. Optimization of cellulase production by Penicillium sp. 3 Biotech 2016, 6, 162. [Google Scholar] [CrossRef] [Green Version]
- Sohail, M.; Ahmad, A.; Khan, S.A. Production of cellulase from Aspergillus terreus MS105 on crude and commercially purified substrates. 3 Biotech 2016, 6, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, J.; Weng, H.; Zhu, D.; Yuan, M.; Guan, F.; Xi, Y. Production and characterization of cellulolytic enzymes from the thermoacidophilic fungal Aspergillus terreus M11 under solid-state cultivation of corn stover. Bioresour. Technol. 2008, 99, 7623–7629. [Google Scholar] [CrossRef] [PubMed]
- Santos, T.C.D.; Abreu Filho, G.; Brito, A.R.D.; Pires, A.J.V.; Bonomo, R.C.F.; Franco, M. Production and characterization of cellulolytic enzymes by Aspergillus niger and Rhizopus sp. by solid state fermentation of prickly pear. Rev. Caatinga 2016, 29, 222–233. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.K.; Darah, I.; Ibrahim, C.O. Production and Optimization of Cellulase Enzyme Using Aspergillus niger USM AI 1 and Comparison with Trichoderma reesei via Solid State Fermentation System. Biotechnol. Res. Int. 2011, 2011, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Qurat-Ul-Ain; Baig, S.; Saleem, M. Production and characterization of cellulases of Aspergillus niger by using rice husk and saw dust as substrates. Pak. J. Bot. 2012, 44, 377–382. [Google Scholar]
- Pachauri, P.; Aranganathan, V.; More, S.; Sullia, S.B.; Deshmukh, S. Purification and characterization of cellulase from a novel isolate of Trichoderma longibrachiatum. Biofuels 2020, 11, 85–91. [Google Scholar] [CrossRef]
- Petlamul, W.; Sripornngam, T.; Buakwan, N.; Buakaew, S.; Mahamad, K. The Capability of Beauveria Bassiana for Cellulase Enzyme Production. In Proceedings of the 7th International Conference on Bioscience, Biochemistry and Bioinformatics–ICBBB ’17, Bangkok Thailand, 21–23 January 2017; ACM Press: New York, NY, USA, 2017; pp. 62–66. [Google Scholar]
- Hernández, C.; Milagres, A.M.F.; Vázquez-Marrufo, G.; Muñoz-Páez, K.M.; García-Pérez, J.A.; Alarcón, E. An ascomycota coculture in batch bioreactor is better than polycultures for cellulase production. Folia Microbiol. 2018, 63, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Schuerg, T.; Gabriel, R.; Baecker, N.; Baker, S.E.; Singer, S.W. Thermoascus aurantiacus is an Intriguing Host for the Industrial Production of Cellulases. Curr. Biotechnol. 2017, 6, 89–97. [Google Scholar] [CrossRef]
- Baldrian, P.; Valášková, V. Degradation of cellulose by basidiomycetous fungi. FEMS Microbiol. Rev. 2008, 32, 501–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.W.; Lee, H.V.; Juan, J.C.; Phang, S.-M. Production of new cellulose nanomaterial from red algae marine biomass Gelidium elegans. Carbohydr. Polym. 2016, 151, 1210–1219. [Google Scholar] [CrossRef] [PubMed]
- Tarchoun, A.F.; Trache, D.; Klapötke, T.M. Microcrystalline cellulose from Posidonia oceanica brown algae: Extraction and characterization. Int. J. Biol. Macromol. 2019, 138, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Sebeia, N.; Jabli, M.; Ghith, A.; Elghoul, Y.; Alminderej, F.M. Production of cellulose from Aegagropila Linnaei macro-algae: Chemical modification, characterization and application for the bio-sorptionof cationic and anionic dyes from water. Int. J. Biol. Macromol. 2019, 135, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Uzyol, H.K.; Saçan, M.T. Bacterial cellulose production by Komagataeibacter hansenii using algae-based glucose. Environ. Sci. Pollut. Res. 2017, 24, 11154–11162. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Gao, W.; Chen, L.; Lan, W.; Zhu, J.Y.; Runge, T. A comparison of cellulose nanofibrils produced from Cladophora glomerata algae and bleached eucalyptus pulp. Cellulose 2016, 23, 493–503. [Google Scholar] [CrossRef]
- Kobayashi, S.; Kashiwa, K.; Shimada, J.; Kawasaki, T.; Shoda, S. Enzymatic polymerization: The first in vitro synthesis of cellulose via nonbiosynthetic path catalyzed by cellulase. Makromol. Chem. Macromol. Symp. 55. [CrossRef]
- Kobayashi, S.; Kashiwa, K.; Kawasaki, T.; Shoda, S. Novel method for polysaccharide synthesis using an enzyme: The first in vitro synthesis of cellulose via a nonbiosynthetic path utilizing cellulase as catalyst. J. Am. Chem. Soc. 1991, 113, 3079–3084. [Google Scholar] [CrossRef]
- Nakatsubo, F.; Kamitakahara, H.; Hori, M. Cationic Ring-Opening Polymerization of 3,6-Di-O-benzyl-α-d-glucose 1,2,4-Orthopivalate and the First Chemical Synthesis of Cellulose. J. Am. Chem. Soc. 1996, 118, 1677–1681. [Google Scholar] [CrossRef]
- Wu, H.; Williams, G.R.; Wu, J.; Wu, J.; Niu, S.; Li, H.; Wang, H.; Zhu, L. Regenerated chitin fibers reinforced with bacterial cellulose nanocrystals as suture biomaterials. Carbohydr. Polym. 2018, 180, 304–313. [Google Scholar] [CrossRef]
- Keshk, S.M. Bacterial Cellulose Production and its Industrial Applications. J. Bioprocess. Biotech. 2014, 4, 2. [Google Scholar] [CrossRef]
- Abol-Fotouh, D.; Hassan, M.A.; Shokry, H.; Roig, A.; Azab, M.S.; Kashyout, A.E.-H.B. Bacterial nanocellulose from agro-industrial wastes: Low-cost and enhanced production by Komagataeibacter saccharivorans MD1. Sci. Rep. 2020, 10, 1–14. [Google Scholar]
- Bae, S.O.; Sugano, Y.; Ohi, K.; Shoda, M. Features of bacterial cellulose synthesis in a mutant generated by disruption of the diguanylate cyclase 1 gene of Acetobacter xylinum BPR 2001. Appl. Microbiol. Biotechnol. 2004, 65, 315–322. [Google Scholar] [CrossRef]
- Cakar, F.; Özer, I.; Aytekin, A.Ö.; Şahin, F. Improvement production of bacterial cellulose by semi-continuous process in molasses medium. Carbohydr. Polym. 2014, 106, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.O.; Shoda, M. Production of bacterial cellulose by Acetobacter xylinum BPR2001 using molasses medium in a jar fermentor. Appl. Microbiol. Biotechnol. 2005, 67, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Poddar, M.K.; Dikshit, P.K. Recent development in bacterial cellulose production and synthesis of cellulose based conductive polymer nanocomposites. Nano Sel. 2021, 2, 1605–1628. [Google Scholar] [CrossRef]
- Shi, Z.; Li, Y.; Chen, X.; Han, H.; Yang, G. Double network bacterial cellulose hydrogel to build a biology-device interface. Nanoscale 2014, 6, 970–977. [Google Scholar] [CrossRef]
- Vazquez, A.; Foresti, M.L.; Cerrutti, P.; Galvagno, M. Bacterial Cellulose from Simple and Low Cost Production Media by Gluconacetobacter xylinus. J. Polym. Environ. 2013, 21, 545–554. [Google Scholar] [CrossRef]
- Wang, J.; Tavakoli, J.; Tang, Y. Bacterial cellulose production, properties and applications with different culture methods—A review. Carbohydr. Polym. 2019, 219, 63–76. [Google Scholar] [CrossRef] [Green Version]
- Choi, C.N.; Song, H.J.; Kim, M.J.; Chang, M.H.; Kim, S.J. Properties of bacterial cellulose produced in a pilot-scale spherical type bubble column bioreactor. Korean J. Chem. Eng. 2009, 26, 136–140. [Google Scholar] [CrossRef]
- Chao, Y.P.; Sugano, Y.; Kouda, T.; Yoshinaga, F.; Shoda, M. Production of bacterial cellulose by Acetobacter xylinum with an air-lift reactor. Biotechnol. Tech. 1997, 11, 829–832. [Google Scholar] [CrossRef]
- Chao, Y.; Sugano, Y.; Shoda, M. Bacterial cellulose production under oxygen-enriched air at different fructose concentrations in a 50-L, internal-loop airlift reactor. Appl. Microbiol. Biotechnol. 2001, 55, 673–679. [Google Scholar] [CrossRef]
- Chao, Y.; Ishida, T.; Sugano, Y.; Shoda, M. Bacterial cellulose production by Acetobacter xylinum in a 50-L internal-loop airlift reactor. Biotechnol. Bioeng. 2000, 68, 345–352. [Google Scholar] [CrossRef]
- Mormino, R.; Bungay, H. Composites of bacterial cellulose and paper made with a rotating disk bioreactor. Appl. Microbiol. Biotechnol. 2003, 62, 503–506. [Google Scholar] [CrossRef]
- Zahan, K.A.; Pa’e, N.; Muhamad, I.I. An evaluation of fermentation period and discs rotation speed of rotary discs reactor for bacterial cellulose production. Sains Malays. 2016, 45, 393–400. [Google Scholar]
- Lin, S.-P.; Hsieh, S.-C.; Chen, K.-I.; Demirci, A.; Cheng, K.-C. Semi-continuous bacterial cellulose production in a rotating disk bioreactor and its materials properties analysis. Cellulose 2014, 21, 835–844. [Google Scholar] [CrossRef]
- Lin, S.-P.; Liu, C.-T.; Hsu, K.-D.; Hung, Y.-T.; Shih, T.-Y.; Cheng, K.-C. Production of bacterial cellulose with various additives in a PCS rotating disk bioreactor and its material property analysis. Cellulose 2016, 23, 367–377. [Google Scholar] [CrossRef]
- Fijałkowski, K.; Żywicka, A.; Drozd, R.; Junka, A.F.; Peitler, D.; Kordas, M.; Konopacki, M.; Szymczyk, P.; Rakoczy, R. Increased water content in bacterial cellulose synthesized under rotating magnetic fields. Electromagn. Biol. Med. 2017, 36, 192–201. [Google Scholar] [CrossRef]
- Fija, K.; Anna, Ż.; Junka, A.F.; Kordas, M.; Rakoczy, R.; Fijałkowski, K.; Drozd, R.; Żywicka, A.; Junka, A.F.; Kordas, M.; et al. Biochemical and cellular properties of Gluconacetobacter xylinus cultures exposed to different modes of rotating magnetic field. Pol. J. Chem. Technol. 2017, 19, 107–114. [Google Scholar] [CrossRef] [Green Version]
- Fijałkowski, K.; Rakoczy, R.; Żywicka, A.; Drozd, R.; Zielińska, B.; Wenelska, K.; Cendrowski, K.; Peitler, D.; Kordas, M.; Konopacki, M.; et al. Time Dependent Influence of Rotating Magnetic Field on Bacterial Cellulose. Int. J. Polym. Sci. 2016, 2016, 7536397. [Google Scholar] [CrossRef] [Green Version]
- Fijałkowski, K.; Żywicka, A.; Drozd, R.; Niemczyk, A.; Junka, A.F.; Peitler, D.; Kordas, M.; Konopacki, M.; Szymczyk, P.; El Fray, M.; et al. Modification of bacterial cellulose through exposure to the rotating magnetic field. Carbohydr. Polym. 2015, 133, 52–60. [Google Scholar] [CrossRef]
- Jung, J.Y.; Khan, T.; Park, J.K.; Chang, H.N. Production of bacterial cellulose by Gluconacetobacter hansenii using a novel bioreactor equipped with a spin filter. Korean J. Chem. Eng. 2007, 24, 265–271. [Google Scholar] [CrossRef]
- Hornung, M.; Ludwig, M.; Schmauder, H.P. Optimizing the Production of Bacterial Cellulose in Surface Culture: A Novel Aerosol Bioreactor Working on a Fed Batch Principle (Part 3). Eng. Life Sci. 2007, 7, 35–41. [Google Scholar] [CrossRef]
- Cheng, K.-C.; Catchmark, J.M.; Demirci, A. Enhanced production of bacterial cellulose by using a biofilm reactor and its material property analysis. J. Biol. Eng. 2009, 3, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, K.-C.; Catchmark, J.M.; Demirci, A. Effects of CMC Addition on Bacterial Cellulose Production in a Biofilm Reactor and Its Paper Sheets Analysis. Biomacromolecules 2011, 12, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Velásquez-Riaño, M.; Bojacá, V. Production of bacterial cellulose from alternative low-cost substrates. Cellulose 2017, 24, 2677–2698. [Google Scholar] [CrossRef]
- Harussani, M.M.; Salit, M.S.; Rashid, U.; Abdan, K. Plastic Waste Conversion into Electrical, Thermal and Fuel Energy via Incinerations and Pyrolysis amidst COVID-19 Pandemic. In Proceedings of the AIUE Proceedings of the 2nd Energy and Human Habitat Conference, Cape Town, South Africa, 26–27 July 2021. [Google Scholar]
- Harussani, M.M.; Sapuan, S.M.; Khalina, A.; Rashid, U.; Tarique, J. Slow pyrolysis of disinfected COVID-19 non-woven polypropylene (PP) waste. In Proceedings of the International Symposium on Applied Sciences and Engineering ISASE2021, Erzurum, Turkey, 7–9 April 2021; Office of International Affairs, Atatürk University: Erzurum, Turkey, 2021; pp. 310–312. [Google Scholar]
- Miah, J.H.; Griffiths, A.; McNeill, R.; Halvorson, S.; Schenker, U.; Espinoza-Orias, N.D.; Morse, S.; Yang, A.; Sadhukhan, J. Environmental management of confectionery products: Life cycle impacts and improvement strategies. J. Clean. Prod. 2018, 177, 732–751. [Google Scholar] [CrossRef]
- Kongruang, S. Bacterial cellulose production by Acetobacter xylinum strains from agricultural waste products. In Biotechnology for Fuels and Chemicals; Springer: Berlin/Heidelberg, Germany, 2007; pp. 763–774. [Google Scholar]
- Goelzer, F.D.E.; Faria-Tischer, P.C.S.; Vitorino, J.C.; Sierakowski, M.-R.; Tischer, C.A. Production and characterization of nanospheres of bacterial cellulose from Acetobacter xylinum from processed rice bark. Mater. Sci. Eng. C 2009, 29, 546–551. [Google Scholar] [CrossRef]
- Kuo, C.-H.H.; Huang, C.-Y.Y.; Shieh, C.-J.J.; Wang, H.-M.M.D.; Tseng, C.-Y.Y. Hydrolysis of Orange Peel with Cellulase and Pectinase to Produce Bacterial Cellulose using Gluconacetobacter xylinus. Waste Biomass Valorization 2019, 10, 85–93. [Google Scholar] [CrossRef]
- Hong, F.; Guo, X.; Zhang, S.; Han, S.F.; Yang, G.; Jönsson, L.J. Bacterial cellulose production from cotton-based waste textiles: Enzymatic saccharification enhanced by ionic liquid pretreatment. Bioresour. Technol. 2012, 104, 503–508. [Google Scholar] [CrossRef]
- Fan, X.; Gao, Y.; He, W.; Hu, H.; Tian, M.; Wang, K.; Pan, S. Production of nano bacterial cellulose from beverage industrial waste of citrus peel and pomace using Komagataeibacter xylinus. Carbohydr. Polym. 2016, 151, 1068–1072. [Google Scholar] [CrossRef] [PubMed]
- Keshk, S.; Sameshima, K. The utilization of sugar cane molasses with/without the presence of lignosulfonate for the production of bacterial cellulose. Appl. Microbiol. Biotechnol. 2006, 72, 291–296. [Google Scholar] [CrossRef]
- Qi, G.; Luo, M.; Huang, C.; Guo, H.; Chen, X.; Xiong, L.; Wang, B.; Lin, X.; Peng, F.; Chen, X. Comparison of bacterial cellulose production by Gluconacetobacter xylinus on bagasse acid and enzymatic hydrolysates. J. Appl. Polym. Sci. 2017, 134, 45066. [Google Scholar] [CrossRef]
- Pensupa, N.; Leu, S.-Y.; Hu, Y.; Du, C.; Liu, H.; Jing, H.; Wang, H.; Lin, C.S.K. Recent trends in sustainable textile waste recycling methods: Current situation and future prospects. In Chemistry and Chemical Technologies in Waste Valorization; Springer: Berlin/Heidelberg, Germany, 2017; pp. 189–228. [Google Scholar]
- Wang, Y. Fiber and textile waste utilization. Waste Biomass Valorization 2010, 1, 135–143. [Google Scholar] [CrossRef]
- Gao, X.; Shi, Z.; Liu, C.; Yang, G.; Sevostianov, I.; Silberschmidt, V.V. Inelastic behaviour of bacterial cellulose hydrogel: In aqua cyclic tests. Polym. Test. 2015, 44, 82–92. [Google Scholar] [CrossRef] [Green Version]
- Almeida, L.R.; Martins, A.R.; Fernandes, E.M.; Oliveira, M.B.; Correlo, V.M.; Pashkuleva, I.; Marques, A.P.; Ribeiro, A.S.; Durães, N.F.; Silva, C.J.; et al. New biotextiles for tissue engineering: Development, characterization and in vitro cellular viability. Acta Biomater. 2013, 9, 8167–8181. [Google Scholar] [CrossRef] [PubMed]
- Kowalska-Ludwicka, K.; Cala, J.; Grobelski, B.; Sygut, D.; Jesionek-Kupnicka, D.; Kolodziejczyk, M.; Bielecki, S.; Pasieka, Z. Modified bacterial cellulose tubes for regeneration of damaged peripheral nerves. Arch. Med. Sci. 2013, 9, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhu, C.; Yang, J.; Nie, Y.; Chen, C.; Sun, D. Recent advances in bacterial cellulose. Cellulose 2014, 21, 1–30. [Google Scholar] [CrossRef]
- Bıyık, H.; Çoban, E.P. Evaluation of different carbon, nitrogen sources and industrial wastes for bacterial cellulose production. Eur. J. Biotechnol. Biosci. 2017, 5, 74–80. [Google Scholar] [CrossRef]
- Voon, W.W.Y.; Muhialdin, B.J.; Yusof, N.L.; Rukayadi, Y.; Meor Hussin, A.S. Bio-cellulose Production by Beijerinckia fluminensis WAUPM53 and Gluconacetobacter xylinus 0416 in Sago By-product Medium. Appl. Biochem. Biotechnol. 2019, 187, 211–220. [Google Scholar] [CrossRef]
- Uzuner, S.; Cekmecelioglu, D. Enzymes in the beverage industry. In Enzymes in Food Biotechnology; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128132807. [Google Scholar]
- Chandrasekaran, M. Enzymes in Food and Beverage Processing; CRC Press: Boca Raton, FL, USA, 2015; pp. 1–518. [Google Scholar] [CrossRef]
- Jain, S. Development of Low Cost Nutritional Beverage from Whey. IOSR J. Environ. Sci. Toxicol. Food Technol. 2013, 5, 73–88. [Google Scholar] [CrossRef]
- Revin, V.; Liyaskina, E.; Nazarkina, M.; Bogatyreva, A.; Shchankin, M. Cost-effective production of bacterial cellulose using acidic food industry by-products. Braz. J. Microbiol. 2018, 49, 151–159. [Google Scholar] [CrossRef]
- Ratanapariyanuch, K.; Shen, J.; Jia, Y.; Tyler, R.T.; Shim, Y.Y.; Reaney, M.J.T. Rapid NMR method for the quantification of organic compounds in thin stillage. J. Agric. Food Chem. 2011, 59, 10454–10460. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.M.; Liu, R.H. Cost-effective production of bacterial cellulose in static cultures using distillery wastewater. J. Biosci. Bioeng. 2013, 115, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Jozala, A.F.; Pértile, R.A.N.; dos Santos, C.A.; de Carvalho Santos-Ebinuma, V.; Seckler, M.M.; Gama, F.M.; Pessoa, A. Bacterial cellulose production by Gluconacetobacter xylinus by employing alternative culture media. Appl. Microbiol. Biotechnol. 2015, 99, 1181–1190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lima, H.L.S.; Nascimento, E.S.; Andrade, F.K.; Brígida, A.I.S.; Borges, M.F.; Cassales, A.R.; Muniz, C.R.; Souza Filho, M.D.S.M.; Morais, J.P.S.; Rosa, M.D.F. Bacterial cellulose production by Komagataeibacter hansenii ATCC 23769 using sisal juice—An agroindustry waste. Braz. J. Chem. Eng. 2017, 34, 671–680. [Google Scholar] [CrossRef] [Green Version]
- Castro, C.; Zuluaga, R.; Putaux, J.L.; Caro, G.; Mondragon, I.; Gañán, P. Structural characterization of bacterial cellulose produced by Gluconacetobacter swingsii sp. from Colombian agroindustrial wastes. Carbohydr. Polym. 2011, 84, 96–102. [Google Scholar] [CrossRef]
- Zhao, H.; Xia, J.; Wang, J.; Yan, X.; Wang, C.; Lei, T.; Xian, M.; Zhang, H. Production of bacterial cellulose using polysaccharide fermentation wastewater as inexpensive nutrient sources. Biotechnol. Biotechnol. Equip. 2018, 32, 350–356. [Google Scholar] [CrossRef] [Green Version]
- Lin, D.; Lopez-Sanchez, P.; Li, R.; Li, Z. Production of bacterial cellulose by Gluconacetobacter hansenii CGMCC 3917 using only waste beer yeast as nutrient source. Bioresour. Technol. 2014, 151, 113–119. [Google Scholar] [CrossRef]
- Viana, R.M.; Sá, N.M.S.M.; Barros, M.O.; Borges, M.d.F.; Azeredo, H.M.C. Nanofibrillated bacterial cellulose and pectin edible films added with fruit purees. Carbohydr. Polym. 2018, 196, 27–32. [Google Scholar] [CrossRef] [Green Version]
- Manrich, A.; Moreira, F.K.V.; Otoni, C.G.; Lorevice, M.V.; Martins, M.A.; Mattoso, L.H.C. Hydrophobic edible films made up of tomato cutin and pectin. Carbohydr. Polym. 2017, 164, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Azeredo, H.M.C.; Kontou-Vrettou, C.; Moates, G.K.; Wellner, N.; Cross, K.; Pereira, P.H.F.; Waldron, K.W. Wheat straw hemicellulose films as affected by citric acid. Food Hydrocoll. 2015, 50, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Cavka, A.; Guo, X.; Tang, S.-J.J.; Winestrand, S.; Jönsson, L.J.; Hong, F. Production of bacterial cellulose and enzyme from waste fiber sludge. Biotechnol. Biofuels 2013, 6, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Rani, M.U.; Appaiah, K.A.A. Production of bacterial cellulose by Gluconacetobacter hansenii UAC09 using coffee cherry husk. J. Food Sci. Technol. 2013, 50, 755–762. [Google Scholar] [CrossRef]
- Xiao, X.; Hou, Y.; Liu, Y.; Liu, Y.; Zhao, H.; Dong, L.; Du, J.; Wang, Y.; Bai, G.; Luo, G. Classification and analysis of corn steep liquor by UPLC/Q-TOF MS and HPLC. Talanta 2013, 107, 344–348. [Google Scholar] [CrossRef]
- Joshi, S.; Goyal, S.; Reddy, M.S. Corn steep liquor as a nutritional source for biocementation and its impact on concrete structural properties. J. Ind. Microbiol. Biotechnol. 2018, 45, 657–667. [Google Scholar] [CrossRef]
- Khami, S.; Khamwichit, W.; Suwannahong, K.; Sanongraj, W. Characteristics of Bacterial Cellulose Production from Agricultural Wastes. Adv. Mater. Res. 2014, 931-932, 693–697. [Google Scholar] [CrossRef]
- Moosavi-Nasab, M.; Yousefi, A. Biotechnological production of cellulose by Gluconacetobacter xylinus from agricultural waste. Iran. J. Biotechnol. 2011, 9, 94–101. [Google Scholar]
- Lotfiman, S.; Awang Biak, D.R.; Ti, T.B.; Kamarudin, S.; Nikbin, S. Influence of Date Syrup as a Carbon Source on Bacterial Cellulose Production by Acetobacter xylinum 0416. Adv. Polym. Technol. 2018, 37, 1085–1091. [Google Scholar] [CrossRef]
- Gomes, F.P.; Silva, N.H.C.S.; Trovatti, E.; Serafim, L.S.; Duarte, M.F.; Silvestre, A.J.D.; Neto, C.P.; Freire, C.S.R. Production of bacterial cellulose by Gluconacetobacter sacchari using dry olive mill residue. Biomass Bioenergy 2013, 55, 205–211. [Google Scholar] [CrossRef]
- Al-Farsi, M.; Alasalvar, C.; Al-Abid, M.; Al-Shoaily, K.; Al-Amry, M.; Al-Rawahy, F. Compositional and functional characteristics of dates, syrups, and their by-products. Food Chem. 2007, 104, 943–947. [Google Scholar] [CrossRef]
- Lestari, P.; Elfrida, N.; Suryani, A.; Suryadi, Y. Study on the Production of Bacterial Cellulose from Acetobacter xylinum Using Agro-Waste. Jordan J. Biol. Sci. 2014, 7, 75–80. [Google Scholar] [CrossRef] [Green Version]
- Gülsah, K.; Gülnur, K.; Mikhael, B.; Céline, P.-B.; Mualla, Ö. Potential of polyhydroxyalkanoate (PHA) polymers family as substitutes of petroleum based polymers for packaging applications and solutions brought by their composites to form barrier materials. Pure Appl. Chem. 2017, 89, 1841. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Hua, J.; Jia, S.; Zhang, J.; Liu, H. Production of nano bacterial cellulose from waste water of candied jujube-processing industry using Acetobacter xylinum. Carbohydr. Polym. 2015, 120, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.B.; Che, L.L.; Guo, J.; Wang, R.Z. Use of 4-chloro-3, 5-dinitrobenzotrifluoride (CNBF) Derivatization and Ultrahigh-performance Liquid Chromatography Tandem Mass Spectrometry for the Determination of 20 Free Amino Acids in Chinese Jujube Date. Food Anal. Methods 2014, 7, 571–579. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Zhao, G.; Wang, Y. Influence of oxidized starch on the properties of thermoplastic starch. Carbohydr. Polym. 2013, 96, 358–364. [Google Scholar] [CrossRef]
- Estur, G. (Ed.) Cotton Exporter’s Guide, 1st ed.; International Trade Centre UNCTAD/WTO; University of California: Berkeley, CA, USA, 2007. [Google Scholar]
- Kuo, C.H.; Lin, P.J.; Lee, C.K. Enzymatic saccharification of dissolution pretreated waste cellulosic fabrics for bacterial cellulose production by Gluconacetobacter xylinus. J. Chem. Technol. Biotechnol. 2010, 85, 1346–1352. [Google Scholar] [CrossRef]
- Guo, X.; Chen, L.; Tang, J.; Jönsson, L.J.; Hong, F.F. Production of bacterial nanocellulose and enzyme from [AMIM]Cl-pretreated waste cotton fabrics: Effects of dyes on enzymatic saccharification and nanocellulose production. J. Chem. Technol. Biotechnol. 2016, 91, 1413–1421. [Google Scholar] [CrossRef]
- Shao, Y.; Yashiro, T.; Okubo, K.; Fujii, T. Effect of cellulose nano fiber (CNF) on fatigue performance of carbon fiber fabric composites. Compos. Part A Appl. Sci. Manuf. 2015, 76, 244–254. [Google Scholar] [CrossRef]
- Tsouko, E.; Kourmentza, C.; Ladakis, D.; Kopsahelis, N.; Mandala, I.; Papanikolaou, S.; Paloukis, F.; Alves, V.; Koutinas, A. Bacterial cellulose production from industrial waste and by-product streams. Int. J. Mol. Sci. 2015, 16, 14832–14849. [Google Scholar] [CrossRef]
- Schrecker, S.T.; Gostomski, P.A. Determining the water holding capacity of microbial cellulose. Biotechnol. Lett. 2005, 27, 1435–1438. [Google Scholar] [CrossRef] [PubMed]
- Moosavi-nasab, M.; Yousefi, A.R. Investigation of Physicochemical Properties of the Bacterial. World Acad. Sci. Eng. Technol. 2010, 44, 1258–1263. [Google Scholar]
- Gayathry, G.; Gopalaswamy, G. Production and characterisation of microbial cellulosic fibre from Acetobacter xylinum. Indian J. Fibre Text. Res. 2014, 39, 93–96. [Google Scholar]
- Wang, B.; Qi, G.X.; Huang, C.; Yang, X.Y.; Zhang, H.R.; Luo, J.; Chen, X.F.; Xiong, L.; Chen, X.D. Preparation of Bacterial Cellulose/Inorganic Gel of Bentonite Composite by In Situ Modification. Indian J. Microbiol. 2016, 56, 72–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soemphol, W.; Hongsachart, P.; Tanamool, V. Production and characterization of bacterial cellulose produced from agricultural by-product by Gluconacetobacter strains. Mater. Today Proc. 2018, 5, 11159–11168. [Google Scholar] [CrossRef]
- Camacho, F.; Macedo, A.; Malcata, F. Potential industrial applications and commercialization of microalgae in the functional food and feed industries: A short review. Mar. Drugs 2019, 17, 312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goyat, M. Production of Green Bacterial Cellulose Nanofibers by Utilizing Renewable Resources of Algae in Comparison with Agricultural Residue. Ph.D. Thesis, Ryerson University, Toronto, ON, Canada, 2016. [Google Scholar]
- Hyun, J.Y.; Mahanty, B.; Kim, C.G. Utilization of Makgeolli Sludge Filtrate (MSF) as Low-Cost Substrate for Bacterial Cellulose Production by Gluconacetobacter xylinus. Appl. Biochem. Biotechnol. 2014, 172, 3748–3760. [Google Scholar] [CrossRef] [PubMed]
- Jahan, F.; Kumar, V.; Saxena, R.K.K. Distillery effluent as a potential medium for bacterial cellulose production: A biopolymer of great commercial importance. Bioresour. Technol. 2018, 250, 922–926. [Google Scholar] [CrossRef]
- Ha, J.H.; Shehzad, O.; Khan, S.; Lee, S.Y.; Park, J.W.; Khan, T.; Park, J.K. Production of bacterial cellulose by a static cultivation using the waste from beer culture broth. Korean J. Chem. Eng. 2008, 25, 812–815. [Google Scholar] [CrossRef]
- Gayathri, G.; Srinikethan, G. Bacterial Cellulose production by K. saccharivorans BC1 strain using crude distillery effluent as cheap and cost effective nutrient medium. Int. J. Biol. Macromol. 2019, 138, 950–957. [Google Scholar] [CrossRef]
- Urbina, L.; Hernández-Arriaga, A.M.; Eceiza, A.; Gabilondo, N.; Corcuera, M.A.; Prieto, M.A.; Retegi, A. By-products of the cider production: An alternative source of nutrients to produce bacterial cellulose. Cellulose 2017, 24, 2071–2082. [Google Scholar] [CrossRef]
- Khattak, W.A.; Khan, T.; Ul-Islam, M.; Ullah, M.W.; Khan, S.; Wahid, F.; Park, J.K. Production, characterization and biological features of bacterial cellulose from scum obtained during preparation of sugarcane jaggery (gur). J. Food Sci. Technol. 2015, 52, 8343–8349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahan, F.; Kumar, V.; Rawat, G.; Saxena, R.K. Production of microbial cellulose by a bacterium isolated from fruit. Appl. Biochem. Biotechnol. 2012, 167, 1157–1171. [Google Scholar]
- Cerrutti, P.; Roldán, P.; García, R.M.; Galvagno, M.A.; Vázquez, A.; Foresti, M.L. Production of bacterial nanocellulose from wine industry residues: Importance of fermentation time on pellicle characteristics. J. Appl. Polym. Sci. 2016, 133, 1–9. [Google Scholar] [CrossRef]
- Luo, M.-T.; Huang, C.; Chen, X.-F.; Huang, Q.-L.; Qi, G.-X.; Tian, L.-L.; Xiong, L.; Li, H.-L.; Chen, X.-D. Efficient bioconversion from acid hydrolysate of waste oleaginous yeast biomass after microbial oil extraction to bacterial cellulose by Komagataeibacter xylinus. Prep. Biochem. Biotechnol. 2017, 47, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
- Bilgi, E.; Bayir, E.; Sendemir-Urkmez, A.; Hames, E.E. Statistical optimization of bacterial cellulose production by gluconacetobacter xylinus using alternative raw materials. In Proceedings of the International Biomedical Engineering Congress, Toronto, Canada, 7–12 June 2015; p. 49. [Google Scholar]
- Bilgi, E.; Bayir, E.; Sendemir-Urkmez, A.; Hames, E.E. Optimization of bacterial cellulose production by Gluconacetobacter xylinus using carob and haricot bean. Int. J. Biol. Macromol. 2016, 90, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, G.; Nogueira, C.R.; Meneguin, A.B.; Trovatti, E.; Silva, M.C.C.; Machado, R.T.A.; Ribeiro, S.J.L.; da Silva Filho, E.C.; Barud, H.d.S. Development and characterization of bacterial cellulose produced by cashew tree residues as alternative carbon source. Ind. Crops Prod. 2017, 107, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Hong, F.; Qiu, K. An alternative carbon source from konjac powder for enhancing production of bacterial cellulose in static cultures by a model strain Acetobacter aceti subsp. xylinus ATCC 23770. Carbohydr. Polym. 2008, 72, 545–549. [Google Scholar] [CrossRef]
- Chen, L.; Hong, F.; Yang, X.; Han, S. Biotransformation of wheat straw to bacterial cellulose and its mechanism. Bioresour. Technol. 2013, 135, 464–468. [Google Scholar] [CrossRef]
- Carreira, P.; Mendes, J.A.S.; Trovatti, E.; Serafim, L.S.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P. Utilization of residues from agro-forest industries in the production of high value bacterial cellulose. Bioresour. Technol. 2011, 102, 7354–7360. [Google Scholar] [CrossRef]
- Adebayo-Tayo, B.; Akintunde, M.; Sanusi, J. Effect of Different Fruit Juice Media on Bacterial Cellulose Production by Acinetobacter sp. BAN1 and Acetobacter pasteurianus PW1. J. Adv. Biol. Biotechnol. 2017, 14, 1–9. [Google Scholar] [CrossRef]
- Kurosumi, A.; Sasaki, C.; Yamashita, Y.; Nakamura, Y. Utilization of various fruit juices as carbon source for production of bacterial cellulose by Acetobacter xylinum NBRC 13693. Carbohydr. Polym. 2009, 76, 333–335. [Google Scholar] [CrossRef]
- Kumbhar, J.V.; Rajwade, J.M.; Paknikar, K.M. Fruit peels support higher yield and superior quality bacterial cellulose production. Appl. Microbiol. Biotechnol. 2015, 99, 6677–6691. [Google Scholar] [CrossRef] [PubMed]
- Casarica, A.; Campeanu, G.; Moscovici, M.; Ghiorghita, A.; Manea, V. Improvement of bacterial cellulose production by Aceobacter xyilinum dsmz-2004 on poor quality horticultural substrates using the taguchi method for media optimization. Part I. Cellul. Chem. Technol. 2013, 47, 61–68. [Google Scholar]
- Lin, W.C.; Lien, C.C.; Yeh, H.J.; Yu, C.M.; Hsu, S.H. Bacterial cellulose and bacterial cellulose-chitosan membranes for wound dressing applications. Carbohydr. Polym. 2013, 94, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Güzel, M.; Akpınar, Ö. Production and Characterization of Bacterial Cellulose from Citrus Peels. Waste Biomass Valorization 2019, 10, 2165–2175. [Google Scholar] [CrossRef]
- Luo, M.-T.; Zhao, C.; Huang, C.; Chen, X.-F.; Huang, Q.-L.; Qi, G.-X.; Tian, L.-L.; Xiong, L.; Li, H.-L.; Chen, X.-D. Efficient Using Durian Shell Hydrolysate as Low-Cost Substrate for Bacterial Cellulose Production by Gluconacetobacter xylinus. Indian J. Microbiol. 2017, 57, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Algar, I.; Fernandes, S.C.M.; Mondragon, G.; Castro, C.; Garcia-Astrain, C.; Gabilondo, N.; Retegi, A.; Eceiza, A. Pineapple agroindustrial residues for the production of high value bacterial cellulose with different morphologies. J. Appl. Polym. Sci. 2015, 132, 41237. [Google Scholar] [CrossRef]
- Adebayo-Tayo, B.C.; Akintunde, M.O.; Alao, S.O. Comparative effect of agrowastes on bacterial cellulose production by Acinetobacter sp. BAN1 and Acetobacter pasteurianus PW1. Turk. J. Agri. Nat. Sci. 2014, 4, 145–154. [Google Scholar]
- Florea, M.; Hagemann, H.; Santosa, G.; Abbott, J.; Micklem, C.N.; Spencer-Milnes, X.; de Arroyo Garcia, L.; Paschou, D.; Lazenbatt, C.; Kong, D.; et al. Engineering control of bacterial cellulose production using a genetic toolkit and a new cellulose-producing strain. Proc. Natl. Acad. Sci. USA 2016, 113, E3431–E3440. [Google Scholar] [CrossRef] [Green Version]
- Hungund, B.; Prabhu, S.; Shetty, C.; Acharya, S.; Prabhu, V.; Gupta, S.G. Production of bacterial cellulose from Gluconacetobacter persimmonis GH-2 using dual and cheaper carbon sources. J. Microb. Biochem. Technol. 2013, 5, 31–33. [Google Scholar] [CrossRef] [Green Version]
- Jozala, A.F.; de Lencastre-Novaes, L.C.; Lopes, A.M.; de Carvalho Santos-Ebinuma, V.; Mazzola, P.G.; Pessoa, A., Jr.; Grotto, D.; Gerenutti, M.; Chaud, M.V. Bacterial nanocellulose production and application: A 10-year overview. Appl. Microbiol. Biotechnol. 2016, 100, 2063–2072. [Google Scholar] [CrossRef] [Green Version]
- Barshan, S.; Rezazadeh-Bari, M.; Almasi, H.; Amiri, S. Optimization and characterization of bacterial cellulose produced by Komagatacibacter xylinus PTCC 1734 using vinasse as a cheap cultivation medium. Int. J. Biol. Macromol. 2019, 136, 1188–1195. [Google Scholar] [CrossRef]
- Premjet, S.; Premjet, D.; Ohtani, Y. The Effect of Ingredients of Sugar Cane Molasses on Bacterial Cellulose Production by Acetobacter xylinum ATCC 10245. Fiber 2007, 63, 193–199. [Google Scholar] [CrossRef] [Green Version]
- Machado, R.T.A.; Meneguin, A.B.; Sábio, R.M.; Franco, D.F.; Antonio, S.G.; Gutierrez, J.; Tercjak, A.; Berretta, A.A.; Ribeiro, S.J.L.; Lazarini, S.C.; et al. Komagataeibacter rhaeticus grown in sugarcane molasses-supplemented culture medium as a strategy for enhancing bacterial cellulose production. Ind. Crops Prod. 2018, 122, 637–646. [Google Scholar] [CrossRef] [Green Version]
- Uraki, Y.; Morito, M.; Kishimoto, T.; Sano, Y. Bacterial Cellulose Production Using Monosaccharides Derived from Hemicelluloses in Water-Soluble Fraction of Waste Liquor from Atmospheric Acetic Acid Pulping. Holzforschung 2002, 56, 341–347. [Google Scholar] [CrossRef]
- Erbas Kiziltas, E.; Kiziltas, A.; Gardner, D.J. Synthesis of bacterial cellulose using hot water extracted wood sugars. Carbohydr. Polym. 2015, 124, 131–138. [Google Scholar] [CrossRef]
- Khattak, W.A.; Khan, T.; Ul-Islam, M.; Wahid, F.; Park, J.K. Production, Characterization and Physico-mechanical Properties of Bacterial Cellulose from Industrial Wastes. J. Polym. Environ. 2015, 23, 45–53. [Google Scholar] [CrossRef]
- Dubey, S.; Singh, J.; Singh, R.P. Biotransformation of sweet lime pulp waste into high-quality nanocellulose with an excellent productivity using Komagataeibacter europaeus SGP37 under static intermittent fed-batch cultivation. Bioresour. Technol. 2018, 247, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, N.; Suresh, S. Production of cellulose from sugarcane molasses using Gluconacetobacter intermedius SNT-1: Optimization & characterization. J. Clean. Prod. 2016, 112, 71–80. [Google Scholar] [CrossRef]
- Huang, C.; Guo, H.J.; Xiong, L.; Wang, B.; Shi, S.L.; Chen, X.F.; Lin, X.Q.; Wang, C.; Luo, J.; Chen, X.D. Using wastewater after lipid fermentation as substrate for bacterial cellulose production by Gluconacetobacter xylinus. Carbohydr. Polym. 2016, 136, 198–202. [Google Scholar] [CrossRef]
- Huang, C.; Yang, X.-Y.; Xiong, L.; Guo, H.-J.; Luo, J.; Wang, B.; Zhang, H.-R.; Lin, X.-Q.; Chen, X.-D. Evaluating the possibility of using acetone-butanol-ethanol (ABE) fermentation wastewater for bacterial cellulose production by Gluconacetobacter xylinus. Lett. Appl. Microbiol. 2015, 60, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Adnan, A.B. Production of Bacterial Cellulose Using Low-Cost Media. Ph.D. Dissertation, University of Waikato, Hamilton, New Zealand, 2015. [Google Scholar]
- Kose, R.; Sunagawa, N.; Yoshida, M.; Tajima, K. One-step production of nanofibrillated bacterial cellulose (NFBC) from waste glycerol using Gluconacetobacter intermedius NEDO-01. Cellulose 2013, 20, 2971–2979. [Google Scholar] [CrossRef]
- Chen, Q.; Fan, Q.; Zhang, Z.; Mei, Y.; Wang, H. Effective in situ harvest of microalgae with bacterial cellulose produced by Gluconacetobacter xylinus. Algal Res. 2018, 35, 349–354. [Google Scholar] [CrossRef]
- Nóbrega, V.; Faria, M.; Quintana, A.; Kaufmann, M.; Ferreira, A.; Cordeiro, N. From a basic microalga and an acetic acid bacterium cellulose producer to a living symbiotic biofilm. Materials 2019, 12, 2275. [Google Scholar] [CrossRef] [Green Version]










| Bacterial Cellulose and Bacterial Cellulose-Based Biocomposites | Applications | Structure and Properties | References |
|---|---|---|---|
| Fabrication of BC and BC-based composites under static culture methods | |||
| BC | BC mask | Fast healing, high moisture donation, and high conformability | Saxena et al. [107] |
| Blood vessel; Vascular grafts | Excellent mechanical properties, thin layers, dense | Putra et al. [108] | |
| Implant material for auricular cartilage regeneration and for ear cartilage replacement | Compatible mechanical strength and patient-specific shapes | Nimeskern et al. [109] | |
| Potential meniscus implant | High compression strain and mechanical strength | Bodin et al. [110] | |
| Replacement of blood vessels and diseased arteries | High water holding capacity and mechanical strength | Charpentier et al. [111] | |
| Artificial blood vessels for microsurgery | The smooth inner surface, moldability, and high mechanical properties | Klemm et al. [112] | |
| Artificial cornea and eye bioengineering Retinal pigment epithelium (RPE) | High elastic modulus, tensile strength and elongation at break, high initial cell adhesion, porous, permeable up to 300 kDa, and dimensionally stable | Padra et al. [98] | |
| BC/polycaprolactone biocomposites | Tissue substitutes in rabbits’ cornea | Signs of the moderate inflammatory process, pro- tected ocular surface and remained stable in corneal tissue during the 45-day follow-up | Sepúlveda et al. [113] |
| BC/polycaprolactone (PCL) biocomposites | Biodegradable food packaging | Good transparency of the BC/PCL, smooth surface morphology | Barud et al. [114] |
| BC/benzoyltrifluoroacetone | Phosphors and UV to Visible energy converting devices | Improvement of the luminescence characteristics | Caiut et al. [115] |
| BC/ AgNPs/ lginate-molybdenum trioxide nanoparticles (MoO3NPs) | Hydrogen sulfide (H2S) gas sensor | Successfully detected H2S gas | Sukhavattanakul et al. [102] |
| BC/chitosan biocomposites | Wound dressing | The improved proliferation and fibroblast adhesion | Kim et al. [116] |
| BC/Lipase nanocomposites | Bioactive paper for developing a simple, handheld, and disposable devices; industrials bio- processes of detergents and food industry and biomedicine | Specific activity was higher for BC/ Lipase suspension (4.2 U/mg), improved thermal stability, reusability, and durability | Buruaga-Ramiro [78] |
| BC/ SOD (Procel-Super) and poviargol (Procel-PA) biocomposites | Skin regeneration scaffold; Membranes for skin tissue regeneration | Highly transparency, antibacterial activity | Legeza et al. [117] |
| BC/ PVOH | The food industry, food packaging | Improved mechanical properties; UV-light barrier properties; Reduce WVP and porosity value | Cazón et al. [3] |
| BC/ PHB | Food packaging applications | low dispersion of BC in the matrix; increased crystallinity of the incubated samples; low interfacial adhesion | Seoane et al. [99] |
| BC/ciprofloxacin biocomposites | Contact lens for better tissue regeneration, enhance the recovery of ocular burns, replacement for antibiotics eye drops, wound dressing after eye surgery or protection against bacteria. | No mutagenicity, genotoxicity and cytotoxicity effects | Messaddeq et al. [118] |
| BC/ polyvinyl alcohol coated biochar nanosilver biocomposites | Drinking water treatment | BC was uniformly mixed into the PVA gel; PVA/BC/C-Ag composite membranes exhibited excellent antibacterial activity; good reusability | Zhang et al. [100] |
| BC/polycaprolactone biocomposites | Tissue substitutes in rabbit cornea | High transparency and mechanical properties | Sepúlveda et al. [113] |
| BC/polyvinyl alcohol biocomposites | BC gloves | Skin cell support and fabrication of optimal moist condition | Osorio et al. [119] |
| BC/ cAgNP | Wound dressing | High cytocompatibility; high moisture content and; good level of transparency; broad-spectrum antimicrobial activity along with antioxidant properties | Gupta et al. [103] |
| Fabrication of BC and BC-based composites under agitation/shaking culture method | |||
| BC | Sewage treatment; Immobilized reaction; Adsorption of Pb2+ bio-separation and bovine serum albumin | Porous and loose structure, BC adhering to each other; diameter of composites with a size range of 0.5–6 mm | Zhu, Jia, Yang, et al. [120] |
| The production of immobilized glucoamylase was supported by BC spheres for industrial applications usage | BC spheres were produced with various range of sizes such as 0.5–1.5, 2–3, and 4–5 mm; Large functional group, as well as great surface area to connect with enzymes, resulted to the higher activity of small spheres. | Wu & Li, [121] | |
| For good viability and adhesion on the surface of the material | Sphere formation was affected by temperature; solid structure formed; diameter of composites with a size range of 2–8 mm formed | Hu et al. [122] | |
| Fermentation | IR: 6.52–3.85; Crystallinity: 81.43–84.35 %; Flocky asterisk-like; diameter of composites with a size range of 5–10 mm, | Bi et al. [24] | |
| Food, healthcare, and materials applications | Diameter is less than 1–8 mm at 150 rpm; Form solid structure however the central region is not layered; Layer spacing 10 μm (150 rpm) and 20 μm (125 rpm) | Hu & Catchmark [123] | |
| Good production yield | Thinner microfibrils structure; IR: 4.48; crystallinity: 84%; large and unique spheres; diameter of composites with a size range of 5–10 mm | Czaja et al. [124] | |
| High-efficiency lipase-immobilization system for large-scale industrial hydrolysis of fats and oils Suitable for enzymatic immobilization. | High hydrolytic activity; High operational activity; Lipase immobilized BC sphere; Size of diameter between 3–9 mm. | Cai et al. [125] | |
| Pectin and xyloglucan can be used to enhance cellulose growth and cellulose assembly. | Xyloglucan: Layered structure, densely packed cellulose bundles with the layered structure were formed; Central core is not obviously seen; diameter of composites with a size range of 4–5 mm; aster-likePectin: Densely packed cellulose bundles with layered structure; diameter of composites with a size range of 5–6 mm; aster-like Xylan: Pore structure of cellulose bundles with a few tails formed on the surface of sphere; diameter of composites with a size range of 7–8 mm; layered structure Arabinogalactan: Cellulose linkage between layered structure; diameter of composites with a size range of 4–6 mm; Sphere | Gu & Catchmark [126] | |
| BC/ schizophyllan (SPG) biopolymers | Anti-wrinkle dressing masks, wound healing and absorption materials | Mechanical, swelling and antibacterial properties were improved; moderate antibacterial activity | Hamedi et al. [101] |
| BC/CNT biocomposites | -- | BC: IR index 2.23, crystallinity 67.2%; snow like structuredBC/CNT composites: IR index 2.56; crystallinity 76.2%, the diameter of composites with a size range of 2–5 mm, rice-like structured | Yan et al. [127] |
| BC/Fe3O4 biocomposites | Elution: Mn2+ > Pb2+ > Cr3+ Superparamagnetic Adsorption: Pb2+ > Mn2+ > Cr3+ | Iron(II,III) oxide (Fe3O4) particles with a size of 15 nm were distributed uniformly in spheres The diameter of composites with a size range of 3–5 mm | Zhu, Jia, Wan, et al. [128] |
| BC/GO biocomposites | Superabsorbent for water environmental protection | Superior absorption capacity; Interconnected structure with a honeycomb-like surface pattern; diameter of composites with a size range of 3–7 mm | Hu [129] |
| Modification | Production Specification and Advantages | BC Production | References |
|---|---|---|---|
| Enriched Oxygen Bioreactors | |||
| Bubble column (controlled pH) Aeration rate:1.0 vvm (30 L/min) | Attributes: Low concentrated solution state culture; Low shear stress; Low mechanical properties: 17.15 to 11.66 MPa; Low crystallinity: 86 to 79.6%, Low degree of polymerization and molecular weight Advantages: High oxygen transfer rate | 0.07–0.09 g/L/h | Choi et al. [192] |
| High oxygen concentration | Attribute: After 30 h the production decreased Advantages: Higher productivity; High oxygen transfer rate; Low power requirement. | 0.20 g/L/h | Chao et al. [193] |
| Internal loop airlift with controlled pH/ fresh and glucose medium | Attribute: The highest concentration: 10.4 g/L at 60–70 g/L fructose Advantages: Formed a unique ellipse; Low mechanical strength; High hydrodynamic characteristic; High volumetric oxygen transfer | 0.22 g/L/h | Chao et al. [194] |
| Internal loop airlift with enriched oxygen | Advantages: Unique ellipse was formed; High hydrodynamic characteristic; High volumetric oxygen transfer | 0.116 g/L/h | Chao et al. [195] |
| Shaking flask with controlled pH/ Hestrin & Schramm medium | Attribute: A membrane-type BC was produced Advantages: Varying the net plates number would result in high Young’s modulus and water holding capacity | - | Wu and Li [121] |
| Rotating disc bioreactors | |||
| A rotating disk bioreactor | Attribute: A consistent product Advantages: Produced strong and intact cellulosic matrix, BC pore size of 10–15 μm; High tensile strength | - | Mormino & Bungay [196] Zahan et al. [197] |
| Rotating disk bioreactor supported by plastic composites | Attribute: A semi-continuous process Advantages: Low mechanical property (Young’s modulus of 372.5 MPa); Low crystallinity: 66.9%; similar thermostability and water content with BC produced by static culture | 0.01 g/L/day | Lin et al. [198] |
| Rotating disk bioreactor with different additions supported by plastic composites | Attribute: A semi-continuous process Advantages: Similar strain but lower stress for carboxymethylcellulose and avicel, respectively; High water retention properties of 98.6–99%; Disc rotation speed and oxygen concentration improved the fermentation process; Fructose concentration was decreased from 50 to 10 g/L; No re-inoculation | 0.64 g/slice with 0.8% carboxymethylcellulose and avicel | Lin et al. [199] |
| Rotating magnetic field | Advantages: Yield BC with an altered degree of porosity and microstructure; Increased biochemical properties; Positive impact on the growth of bacteria; Increased water retention by 26% as compared to the control sample; high density with tangled and long fibres | - | Fijałkowski et al. [200,201,202,203] |
| Other bioreactors for BC production | |||
| Spin filter supporting bioreactor | Advantages: BC concentration was increased from 5.65 to 11.52 g/L/140 h; An abundance of Cel + cells were converted into Cel- mutants | 0.02 to 0.06 g/L/h | Jung et al. [204] |
| Fed-batch principle | Advantages: The gradient of a graph in the load-displacement diagram: (aerosol bioreactor = 34.7 N/10 mm, usual surface culture = 8.9 N/10 mm); High tensile strength: 114 N; High-quality cellulose; the degree of polymerization of BC is 5200; Best time interval: 6 h; BC layer or slices (3–4 cm); Culture box: low cost | - | Hornung et al. [205] |
| Biofilm reactor | Advantages: High crystallinity: 93% with a crystal size of 5.2 nm; high biomass density; Water retention ability up to 95 %; better thermal performance | 7.05 g/L | Cheng et al. [206] |
| Biofilm reactor with additives | Advantages: Continuous BC production; High biomass density; High Young’s modulus and tensile strength; High crystallinity: 80% with a crystal size of 4.2 nm; potential application of BC paper sheets | 13 g/L | Cheng et al. [207] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadier, A.; Ilyas, R.A.; Huzaifah, M.R.M.; Harihastuti, N.; Sapuan, S.M.; Harussani, M.M.; Azlin, M.N.M.; Yuliasni, R.; Ibrahim, R.; Atikah, M.S.N.; et al. Use of Industrial Wastes as Sustainable Nutrient Sources for Bacterial Cellulose (BC) Production: Mechanism, Advances, and Future Perspectives. Polymers 2021, 13, 3365. https://doi.org/10.3390/polym13193365
Kadier A, Ilyas RA, Huzaifah MRM, Harihastuti N, Sapuan SM, Harussani MM, Azlin MNM, Yuliasni R, Ibrahim R, Atikah MSN, et al. Use of Industrial Wastes as Sustainable Nutrient Sources for Bacterial Cellulose (BC) Production: Mechanism, Advances, and Future Perspectives. Polymers. 2021; 13(19):3365. https://doi.org/10.3390/polym13193365
Chicago/Turabian StyleKadier, Abudukeremu, R. A. Ilyas, M. R. M. Huzaifah, Nani Harihastuti, S. M. Sapuan, M. M. Harussani, M. N. M. Azlin, Rustiana Yuliasni, R. Ibrahim, M. S. N. Atikah, and et al. 2021. "Use of Industrial Wastes as Sustainable Nutrient Sources for Bacterial Cellulose (BC) Production: Mechanism, Advances, and Future Perspectives" Polymers 13, no. 19: 3365. https://doi.org/10.3390/polym13193365
APA StyleKadier, A., Ilyas, R. A., Huzaifah, M. R. M., Harihastuti, N., Sapuan, S. M., Harussani, M. M., Azlin, M. N. M., Yuliasni, R., Ibrahim, R., Atikah, M. S. N., Wang, J., Chandrasekhar, K., Islam, M. A., Sharma, S., Punia, S., Rajasekar, A., Asyraf, M. R. M., & Ishak, M. R. (2021). Use of Industrial Wastes as Sustainable Nutrient Sources for Bacterial Cellulose (BC) Production: Mechanism, Advances, and Future Perspectives. Polymers, 13(19), 3365. https://doi.org/10.3390/polym13193365