Novel Poly(Methylenelactide-g-L-Lactide) Graft Copolymers Synthesized by a Combination of Vinyl Addition and Ring-Opening Polymerizations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis of Bromolactide (Br-LA)
2.2.2. Synthesis of Methylenelactide (MLA)
2.2.3. Synthesis of Poly(methylenelactide) (PMLA)
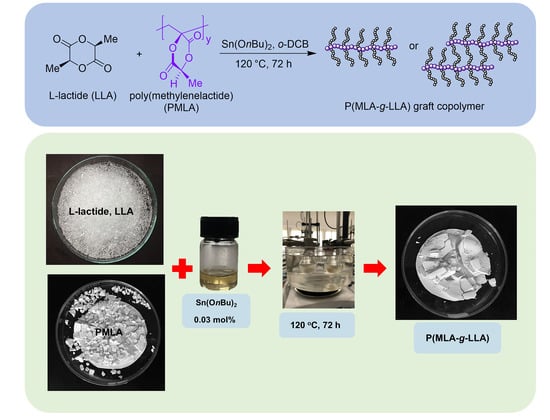
2.2.4. Synthesis of Poly(methylenelactide-g-l-lactide) (P(MLA-g-LLA)) via Solution Polymerization
2.2.5. Synthesis of Poly(l-lactide) (PLA) via Solution Polymerization
2.3. Instrumental Methods
3. Results and Discussion
3.1. Synthesis of Bromolactide (Br-LA)
3.2. Synthesis of Methylenelactide (MLA)
3.3. Synthesis of Poly(methylenelactide) (PMLA)
3.4. Synthesis of Poly(methylenelactide-g-l-lactide) (P(MLA-g-LLA))
3.5. Synthesis of Poly(L-Lactide) (PLA)
3.6. Characterization of PMLA, PLA, and P(MLA-g-LLA)
3.7. PMLA via Vinyl-Addition Polymerization
3.8. P(MLA-g-LLA) and PLA via Ring-Opening Polymerization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Castillo Martinez, F.A.; Balciunas, E.M.; Salgado, J.M.; Domínguez González, J.M.; Converti, A.; Oliveira, R.P.d.S. Lactic acid Properties, Applications and Production: A review. Trends Food Sci. Technol. 2013, 30, 70–83. [Google Scholar] [CrossRef]
- Carothers, W.H.; Dorough, G.L.; van Natta, F.J. Studies of Polymerization and Ring Formation. X. The Reversible Polymerization of Six-Membered Cyclic Esters. J. Am. Chem. Soc. 1932, 54, 761–772. [Google Scholar] [CrossRef]
- Hartmann, M.H. High Molecular Weight Polylactic Acid Polymers. In Biopolymers from Renewable Resources, 1st ed.; Springer: Berlin/Heidelberg, Germany, 1998; pp. 367–411. [Google Scholar]
- Garlotta, D. A Literature Review of Poly(Lactic Acid). J. Polym. Environ. 2001, 9, 63–84. [Google Scholar] [CrossRef]
- Kricheldorf, H.R. Syntheses and Application of Polylactides. Chemosphere 2001, 43, 49–54. [Google Scholar] [CrossRef]
- Gupta, A.P.; Kumar, V. New Emerging Trends in Synthetic Biodegradable Polymers-Polylactide: A Critique. Eur. Polym. J. 2007, 43, 4053–4074. [Google Scholar] [CrossRef]
- Groot, W.; van Krieken, J.; Sliekersl, O.; de Vos, S. Production and Purification of Lactic acid and Lactide. In Poly(lactic acid) Synthesis, Structures, Properties, Processing, and Applications, 1st ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 3–16. [Google Scholar]
- Dechy-Cabaret, O.; Martin-Vaca, B.; Bourissou, D. Polyesters from Dilactones. In Handbook of Ring-Opening Polymerization, 1st ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2009; pp. 255–280. [Google Scholar]
- Castro-Aguirre, E.; Iñiguez-Franco, F.; Samsudin, H.; Fang, X.; Auras, R. Poly(lactic acid)-Mass Production, Processing, Industrial Applications, and End of Life. Adv. Drug. Deliv. Rev. 2016, 107, 333–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.; Hong, S. An Overview of the Synthesis and Synthetic Mechanism of Poly (Lactic acid). Mod. Chem. Appl. 2014, 2, 1–5. [Google Scholar]
- Nofar, M.; Sacligil, D.; Carreau, P.J.; Kamal, M.R.; Heuzey, M.-C. Poly (lactic acid) blends: Processing, Properties and Applications. Int. J. Biol. Macromol. 2019, 125, 307–360. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Huang, Z.; Diao, X.; Weng, Y.; Wang, Y. Characterization of the Effect of REC on the Compatibility of PHBH and PLA. Polym. Test. 2015, 42, 17–25. [Google Scholar] [CrossRef]
- Hsu, Y.I.; Masutani, K.; Yamaoka, T.; Kimura, Y. Strengthening of Hydrogels made from Enantiomeric Block Copolymers of Polylactide (PLA) and Poly(ethylene glycol) (PEG) by the Chain Extending Diels-Alder Reaction at the Hydrophilic PEG Terminals. Polymer 2015, 67, 157–166. [Google Scholar] [CrossRef]
- Al-Itry, R.; Lamnawar, K.; Maazouz, A.; Billon, N.; Combeaud, C. Effect of the Simultaneous Biaxial Stretching on the Structural and Mechanical Properties of PLA, PBAT and their Blends at Rubbery State. Eur. Polym. J. 2015, 68, 288–301. [Google Scholar] [CrossRef]
- Zuo, H.; Liu, J.; Huang, D.; Bai, Y.; Cui, L.; Pan, L.; Zhang, K.; Wang, H. Sustainable and High-Performance Ternary Blends from Polylactide, CO2-Based Polyester and Microbial Polyesterswith Different Chemical Structure. J. Polym. Sci. 2021, 59, 1578–1595. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, T.-Y.; Jin, F.-L.; Park, S.-J. Fracture Toughness Improvement of Poly(lactic acid) Reinforced with Poly(ε-caprolactone) and Surface-Modified Silicon Carbide. Adv. Mater. Sci. Eng. 2018, 2018, 6537621. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Yu, L.; Dean, K.; Toikka, G.; Bateman, S.; Nguyen, T.; Yuan, Q.; Filippou, C. Improving Melt Strength of Polylactic Acid. Int. Polym. Process. 2013, 28, 64–71. [Google Scholar] [CrossRef]
- Quiles-Carrillo, L.; Duart, S.; Montanes, N.; Torres-Giner, S.; Balart, R. Enhancement of the Mechanical and Thermal Properties of Injection-Molded Polylactide Parts by the Addition of Acrylated Epoxidized Soybean Oil. Mater. Des. 2018, 140, 54–63. [Google Scholar] [CrossRef]
- Herrera, N.; Roch, H.; Salaberria, A.M.; Pino-Orellana, M.A.; Labidi, J.; Fernandes, S.C.M.; Radic, D.; Leiva, A.; Oksman, K. Functionalized Blown Films of Plasticized Polylactic Acid/Chitin Nanocomposite: Preparation and Characterization. Mater. Des. 2016, 92, 846–852. [Google Scholar] [CrossRef]
- Wojtczak, E.; Kubisa, P.; Bednarek, M. Thermal Stability of Polylactide with Different End-Groups Depending on the Catalyst used for the Polymerization. Polym. Degrad. Stab. 2018, 151, 100–104. [Google Scholar] [CrossRef]
- Nagarajan, V.; Mohanty, A.K.; Misra, M. Perspective on Polylactic Acid (PLA) Based Sustainable Materials for Durable Applications: Focus on Toughness and Heat Resistance. ACS Sustain. Chem. Eng. 2016, 4, 2899–2916. [Google Scholar] [CrossRef]
- Jin, F.-L.; Hu, R.-R.; Park, S.-J. Improvement of Thermal Behaviors of Biodegradable Poly(lactic acid) Polymer: A Review. Composites Part B: Engineering. Compos. B Eng. 2018, 164, 287–296. [Google Scholar] [CrossRef]
- Wu, F.; Misra, M.; Mohanty, A.K. Studies on Why the Heat Deflection Temperature of Polylactide Bioplastic cannot be Improved by Overcrosslinking. Polym. Cryst. 2019, 2, e10088. [Google Scholar] [CrossRef]
- Södergård, A.; Stolt, M. Properties of Lactic Acid Based Polymers and their Correlation with Composition. Prog. Polym. Sci. 2002, 27, 1123–1163. [Google Scholar] [CrossRef]
- Zhang, Z.C.; Gao, X.R.; Hu, Z.J.; Yan, Z.; Xu, J.Z.; Xu, L.; Zhong, G.J.; Li, Z.M. Inducing Stereocomplex Crystals by Template Effect of Residual Stereocomplex Crystals during Thermal Annealing of Injection-Molded Polylactide. Ind. Eng. Chem. Res. 2016, 55, 10896–10905. [Google Scholar] [CrossRef]
- Deng, L.; Xu, C.; Wang, X.; Wang, Z. Supertoughened Polylactide Binary Blend with High Heat Deflection Temperature Achieved by Thermal Annealing above the Glass Transition Temperature. ACS Sustain. Chem. Eng. 2017, 6, 480–490. [Google Scholar] [CrossRef] [Green Version]
- Al-Itry, R.; Lamnawar, K.; Maazouz, A. Improvement of Thermal Stability, Rheological and Mechanical Properties of PLA, PBAT and their Blends by Reactive Extrusion with Functionalized Epoxy. Polym. Degrad. Stab. 2012, 97, 1898–1914. [Google Scholar] [CrossRef]
- Barczewski, M.; Mysiukiewicz, O.; Matykiewicz, D.; Kloziński, A.; Andrzejewski, J.; Piasecki, A. Synergistic Effect of Different Basalt Fillers and Annealing on the Structure and Properties of Polylactide Composites. Polym. Test. 2020, 89, 106628. [Google Scholar] [CrossRef]
- Purnama, P.; Samsuri, M.; Iswaldi, I. Properties Enhancement of High Molecular Weight Polylactide Using Stereocomplex Polylactide as a Nucleating Agent. Polymers 2021, 13, 1725. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Sun, B.; Bian, X.; Li, G.; Chen, X. High Melt Strength and High Toughness PLLA/PBS Blends by Copolymerization and In Situ Reactive Compatibilization. Ind. Eng. Chem. Res. 2016, 56, 52–62. [Google Scholar] [CrossRef]
- Safandowska, M.; Rozanski, A.; Galeski, A. Plasticization of Polylactide after Solidification:An Effectiveness and Utilization for Correct Interpretation of Thermal Properties. Polymers 2020, 12, 561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Purnama, P.; Kim, S.H. Bio-Based Composite of Stereocomplex Polylactide and Cellulose Nanowhiskers. Polym. Degrad. Stab. 2014, 109, 430–435. [Google Scholar] [CrossRef]
- Yang, S.; Wu, Z.-H.; Yang, W.; Yang, M.-B. Thermal and Mechanical Properties of Chemical Crosslinked Polylactide (PLA). Polym. Test. 2008, 27, 957–963. [Google Scholar] [CrossRef]
- Lou, X.; Detrembleur, C.; Jérôme, R. Novel Aliphatic Polyesters Based on Functional Cyclic(Di)Esters. Macromol. Rapid Commun. 2003, 24, 161–172. [Google Scholar] [CrossRef]
- Cheng, Y.; Deng, S.; Chen, P.; Ruan, R. Polylactic Acid (PLA) Synthesis and Modifications: A Review. Front. Chem. China 2009, 4, 259–264. [Google Scholar] [CrossRef]
- Agarwal, S.; Jin, Q.; Maji, A. Biobased Polymers from Plant-Derived Tulipalin A. J. Am. Chem. Soc. 2012, 1105, 197–212. [Google Scholar]
- Castillejos, S.; Cerna, J.; Meléndez, F.; Castro, M.E.; Aguilar, R.; Beltrán, C.M.; González, M. Bulk Modification of Poly(lactide) (PLA) via Copolymerization with Poly(propylene glycol) Diglycidylether (PPGDGE). Polymers 2018, 10, 1184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dechy-Cabaret, O.; Martin-Vaca, B.; Bourissou, D. Controlled Ring-Opening Polymerization of Lactide and Glycolide. Chem. Rev. 2004, 104, 6147–6176. [Google Scholar] [CrossRef]
- Nuyken, O.; Pask, S.D. Ring-Opening Polymerization-An Introductory Review. Polymers 2013, 5, 361–403. [Google Scholar] [CrossRef] [Green Version]
- Hagiopol, C. Binary Copolymerization. In Copolymerization: Toward a Systematic Approach, 1st ed.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 1999; pp. 1–18. [Google Scholar]
- Osswald, T.A.; Menges, G. Structure of Polymers. In Materials Science of Polymers for Engineers, 3rd ed.; Hanser Publishers: Munich, Germany, 2012; pp. 49–82. [Google Scholar]
- Kaliva, M.; Georgopoulou, A.; Dragatogiannis, D.A.; Charitidis, C.A.; Chatzinikolaidou, M.; Vamvakaki, M. Biodegradable Chitosan-graft-Poly(l-lactide) Copolymers For Bone Tissue Engineering. Polymers 2020, 12, 316. [Google Scholar] [CrossRef] [Green Version]
- Xia, L.; Zhang, Z.; Hong, C.Y.; You, Y.Z. Synthesis of Copolymer via Hybrid Polymerization: From Random to Well-Defined Sequence. Eur. Polym. J. 2019, 122, 109374. [Google Scholar] [CrossRef]
- Stannett, V. Grafting. Radiat. Phys. Chem. 1981, 18, 215–222. [Google Scholar] [CrossRef]
- Athawale, V.D.; Rathi, S.C. Graft Polymerization: Starch as a Model Substrate. J. Macromol. Sci. Part C 1999, C39, 445–480. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, S.; Lu, G.; Huang, X. Constructing Well-Defined Star Graft Copolymers. Polym. Chem. 2013, 4, 1289–1299. [Google Scholar] [CrossRef]
- Gowda, R.R.; Chen, E.Y.-X. Sustainable Polymers from Biomass-Derived α-Methylene-γ-Butyrolactones. In Encyclopedia of Polymer Science and Technology; Wiley Online Library: Hoboken, NJ, USA, 2013; pp. 1–37. [Google Scholar]
- Miyake, G.M.; Zhang, Y.; Chen, E.Y.-X. Polymerizability of Exo-Methylene-Lactide Toward Vinyl Addition and Ring Opening. J. Polym. Sci. A Polym. Chem. 2015, 53, 1523–1532. [Google Scholar] [CrossRef]
- Stridberg, K.M.; Ryner, M.; Albertsson, A.C. Controlled Ring-Opening Polymerization: Polymers with Designed Macromolecular Architecture. Adv. Polym. Sci. 2002, 157, 41–65. [Google Scholar]
- Yin, M.; Baker, G.L. Preparation and Characterization of Substituted Polylactides. Macromolecules 1999, 32, 7711–7718. [Google Scholar] [CrossRef]
- Trimaille, T.; Möller, M.; Gurny, R. Synthesis and Ring-Opening Polymerization of New Monoalkyl-Substituted Lactides. J. Polym. Sci. A Polym. Chem. 2004, 42, 4379–4391. [Google Scholar] [CrossRef]
- Marcincinova Benabdillah, K.; Boustta, M.; Coudane, J.; Vert, M. Novel Degradable Polymers Combining D-Gluconic Acid, a Sugar of Vegetal Origin, with Lactic and Glycolic Acids. Biomacromolecules 2001, 2, 1279–1284. [Google Scholar] [CrossRef]
- Such, G.K.; Quinn, J.F.; Quinn, A.; Tjipto, E.; Caruso, F. Assembly of Ultrathin Polymer Multilayer Films by Click Chemistry. J. Am. Chem. Soc. 2006, 128, 9318–9319. [Google Scholar] [CrossRef]
- Jing, F.; Hillmyer, M.A. A Bifunctional Monomer Derived from Lactide for Toughening Polylactide. J. Am. Chem. Soc. 2008, 130, 13826–13827. [Google Scholar] [CrossRef] [PubMed]
- Fiore, G.L.; Jing, F.; Young, V.G., Jr.; Cramer, C.J.; Hillmyer, M.A. High Tg Aliphatic Polyesters by the Polymerization of Spirolactide Derivatives. Polym. Chem. 2010, 1, 870–877. [Google Scholar] [CrossRef]
- Castillo, J.A.; Borchmann, D.E.; Cheng, A.Y.; Wang, Y.; Hu, C.; García, A.J.; Weck, M. Well-Defined Poly(lactic acid)s Containing Poly(ethylene glycol) Side Chains. Macromolecules 2012, 45, 62–69. [Google Scholar] [CrossRef] [Green Version]
- Beuille, E.; Darcos, V.; Coudane, J.; Lacroix-Desmazes, P.; Nottelet, B. Regioselective Halogenation of Poly(lactide) by Free-Radical Process. Macromol. React. Eng. 2013, 8, 141–148. [Google Scholar] [CrossRef]
- Britner, J.; Ritter, H. Self-Activation of Poly(methylenelactide) through Neighboring-Group Effects: A Sophisticated Type of Reactive Polymer. Macromolecules 2015, 48, 3516–3522. [Google Scholar] [CrossRef]
- Britner, J.; Ritter, H. Methylenelactide: Vinyl polymerization and spatial reactivity effects. Beilstein J. Org. Chem. 2016, 12, 2378–2389. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.B.; Tang, X.; Falivene, L.; Caporaso, L.; Cavallo, L.; Chen, E.Y.-X. Organocatalytic Coupling of Bromo-Lactide with Cyclic Ethers and Carbonates to Chiral Bromo-Diesters: NHC or Anion Catalysis? ACS Catal. 2017, 7, 3929–3933. [Google Scholar] [CrossRef]
- Mauldin, T.C.; Wertz, J.T.; Boday, D.J. Acrylic Platform from Renewable Resources via a Paradigm Shift in Lactide Polymerization. ACS Macro Lett. 2016, 5, 544–546. [Google Scholar] [CrossRef]
- Sinclair, F.; Shaver, M.P. Cross-Metathesis Functionalized Exo-Olefin Derivatives of Lactide. J. Polym. Sci. A Polym. Chem. 2018, 56, 741–748. [Google Scholar] [CrossRef]
- Boday, D.J.; Garcia, J.M.; Hedrick, J.L.; Kobilka, B.M.; Wertz, J.T.; Wojtecki, R.J. Lactide-Derived Polymers with Improved Materials Properties via Polyhexahydrotriazines (PHT) Reaction. U.S. Patent 10,329,380 B2, 25 June 2019. [Google Scholar]
- Meepowpan, P.; Punyodom, W.; Molloy, R. Process for the Preparation of Liquid Tin(II) Alkoxides. U.S. Patent 9,637,507 B2, 2 May 2017. [Google Scholar]
- Chaiwon, T.; Sriyai, M.; Meepowpan, P.; Molloy, R.; Nalampang, K.; Kaabbuathong, N.; Punyodom, W. Kinetic and Mechanistic Studies of Bulk Copolymerization of L-lactide and Glycolide Initiated by Liquid Tin(II) n-Butoxide. Chiang Mai J. Sci. 2021, 48, 489–505. [Google Scholar]
- Sriyai, M.; Chaiwon, T.; Molloy, R.; Meepowpan, P.; Punyodom, W. Efficiency of Liquid Tin(II) n-Alkoxide Initiators in the Ring-Opening Polymerization of L-lactide: Kinetic Studies by Non-Isothermal Differential Scanning Calorimetry. RSC Adv. 2020, 10, 43566–43578. [Google Scholar] [CrossRef]
- Sattayanon, C.; Sontising, W.; Jitonnom, J.; Meepowpan, P.; Punyodom, W.; Kungwan, N. Theoretical Study on the Mechanism and Kinetics of Ring-Opening Polymerization of Cyclic Esters Initiated by Tin(II) n-Butoxide. Comput. Theor. Chem. 2014, 1044, 29–35. [Google Scholar] [CrossRef]
- Dumklang, M.; Pattawong, N.; Punyodom, W.; Meepowpan, P.; Molloy, R.; Hoffman, M. Novel Tin(II) Butoxides for Use as Initiators in the Ring-Opening Polymerisation of ε-Caprolactone. Chiang Mai J. Sci. 2009, 36, 136–148. [Google Scholar]













| Sample | Initiator | Composition Ratio PMLA:LLA (w/w) | Tg (°C) a | Tc (°C) a | Tm (°C) a | Td (°C) b | c | c | Dispersity (Đ) c |
|---|---|---|---|---|---|---|---|---|---|
| PMLA-5 | AIBN | - | 244.0 | - | - | 368.0 | - | - | - |
| PMLA-10 | AIBN | - | 225.7 | - | - | 360.1 | - | - | - |
| PMLA-15 | AIBN | - | 223.7 | - | - | 361.1 | - | - | - |
| PMLA-20 | AIBN | - | 221.4 | - | - | 356.9 | - | - | - |
| PMLA-25 | AIBN | - | 220.3 | - | - | 356.4 | - | - | - |
| PLA | Sn(OnBu)2 | - | 71.5 | 110.8 | 168.3 | 301.1 | 1.68 × 104 | 1.11 × 104 | 1.5 |
| P(MLA5-g-LLA) | Sn(OnBu)2 | 1:30 | 70.1 | - | 159.8 | 318.5 | 9.04 × 104 | 6.78 × 103 | 1.3 |
| P(MLA5-g-LLA) | Sn(OnBu)2 | 1:50 | 73.3 | - | 164.2 | 328.8 | 1.14 × 104 | 8.24 × 103 | 1.4 |
| P(MLA10-g-LLA) | Sn(OnBu)2 | 1:30 | 70.6 | - | 155.5 | 318.5 | 7.08 × 103 | 5.66 × 103 | 1.2 |
| P(MLA10-g-LLA) | Sn(OnBu)2 | 1:50 | 74.8 | - | 159.7 | 325.3 | 8.74 × 103 | 6.63 × 103 | 1.3 |
| P(MLA15-g-LLA) | Sn(OnBu)2 | 1:30 | 73.4 | - | 155.8 | 321.4 | 6.11 × 103 | 5.15 × 103 | 1.2 |
| P(MLA15-g-LLA) | Sn(OnBu)2 | 1:50 | 66.8 | - | 160.3 | 326.8 | 8.21 × 103 | 6.34 × 103 | 1.3 |
| P(MLA20-g-LLA) | Sn(OnBu)2 | 1:30 | 72.3 | - | 144.0 | 314.4 | 4.30 × 103 | 3.76 × 103 | 1.1 |
| P(MLA20-g-LLA) | Sn(OnBu)2 | 1:50 | 73.0 | - | 161.0 | 324.1 | 8.45 × 103 | 6.48 × 103 | 1.3 |
| P(MLA25-g-LLA) | Sn(OnBu)2 | 1:30 | 73.6 | - | 163.3 | 328.1 | 4.87 × 103 | 4.08 × 103 | 1.2 |
| P(MLA25-g-LLA) | Sn(OnBu)2 | 1:50 | 73.5 | - | 163.8 | 386.7 | 4.59 × 103 | 3.81 × 103 | 1.2 |
| P(MLA20-g-LLA) | Sn(OnBu)2 | 1:300 | 68.8 | - | 164.3 | 335.4 | 1.01 × 104 | 7.86 × 103 | 1.4 |
| P(MLA20-g-LLA) | Sn(OnBu)2 | 1:600 | 68.4 | - | 162.8 | 328.9 | 1.10 × 104 | 7.88 × 103 | 1.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mekpothi, T.; Meepowpan, P.; Sriyai, M.; Molloy, R.; Punyodom, W. Novel Poly(Methylenelactide-g-L-Lactide) Graft Copolymers Synthesized by a Combination of Vinyl Addition and Ring-Opening Polymerizations. Polymers 2021, 13, 3374. https://doi.org/10.3390/polym13193374
Mekpothi T, Meepowpan P, Sriyai M, Molloy R, Punyodom W. Novel Poly(Methylenelactide-g-L-Lactide) Graft Copolymers Synthesized by a Combination of Vinyl Addition and Ring-Opening Polymerizations. Polymers. 2021; 13(19):3374. https://doi.org/10.3390/polym13193374
Chicago/Turabian StyleMekpothi, Tanyaluck, Puttinan Meepowpan, Montira Sriyai, Robert Molloy, and Winita Punyodom. 2021. "Novel Poly(Methylenelactide-g-L-Lactide) Graft Copolymers Synthesized by a Combination of Vinyl Addition and Ring-Opening Polymerizations" Polymers 13, no. 19: 3374. https://doi.org/10.3390/polym13193374
APA StyleMekpothi, T., Meepowpan, P., Sriyai, M., Molloy, R., & Punyodom, W. (2021). Novel Poly(Methylenelactide-g-L-Lactide) Graft Copolymers Synthesized by a Combination of Vinyl Addition and Ring-Opening Polymerizations. Polymers, 13(19), 3374. https://doi.org/10.3390/polym13193374