Efficient Synthesis and Characterization of Polyaniline@Aluminium–Succinate Metal-Organic Frameworks Nanocomposite and Its Application for Zn(II) Ion Sensing
Abstract
:1. Introduction
2. Experimental
2.1. Reagents and Instruments
2.2. Preparation of PANI@Al-SA MOFs Nanocomposite
2.3. Electrical Conductivity Measurements
2.4. Electrochemical Sensor Fabrication
2.5. Electrochemical Measurements
2.6. Real Sample Analsis
3. Results and Discussion
3.1. Physiochemical Characterization of PANI@Al-SA Nanocomposite
3.1.1. Fourier Transform Infra-Red (FT-IR) Analysis
3.1.2. Morphological and Elemental Analysis
3.1.3. X-ray Photoelectron Spectroscopy (XPS)
3.1.4. X-ray Diffraction (XRD)
3.1.5. Thermogravimetric Analysis—Differential Thermal Analysis (TGA-DTA)
3.1.6. Electrical Conductivity Study of PANI@Al-SA
3.2. Electrochemical Characterization of PANI@Al-SA Nanocomposite
3.2.1. Cyclic Voltammetry Behavior of Bare and Modified Electrodes in [Fe(CN)6] 3−/4− Solution
3.2.2. Electrochemical Impedance Spectroscopy (EIS) Analysis
3.3. Electrochemical Sensing of Zn2+ by PANI@Al-SA Modified GCE
3.3.1. Effect of pH as Supporting Electrolyte
3.3.2. Selectivity Study
3.3.3. Adjustment of Experimental Condition
3.3.4. Effect of Scan Rate
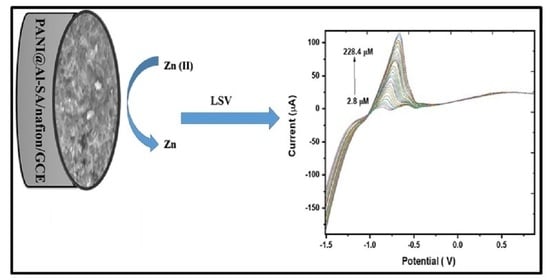
3.3.5. Quantitative Electrochemical Determination of Zinc Ion by Linear Sweep Voltammetry (LSV)
3.3.6. Interference Study
3.3.7. Stability of the Proposed Sensor
3.3.8. Application of the Proposed Sensor in Real Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, Y.; Dong, H.; He, X.; Yu, J.; Chen, R.; Liu, Q.; Liu, J.; Zhang, H.; Li, R.; Wang, J. Design of 2D mesoporous Zn/Co-based metal-organic frameworks as a flexible electrode for energy storage and conversion. J. Power Sources 2019, 438, 227057. [Google Scholar] [CrossRef]
- Hua, Y.; Li, X.; Chen, C.; Pang, H. Cobalt based metal-organic frameworks and their derivatives for electrochemical energy conversion and storage. Chem. Eng. J. 2019, 370, 37–59. [Google Scholar] [CrossRef]
- He, P.; Yu, X.-Y.; Lou, X.W. Carbon-Incorporated Nickel–Cobalt Mixed Metal Phosphide Nanoboxes with Enhanced Electrocatalytic Activity for Oxygen Evolution, Angew. Chem. Int. Ed. 2017, 56, 3897–3900. [Google Scholar] [CrossRef] [PubMed]
- Qu, C.; Zhao, B.; Jiao, Y.; Chen, D.; Dai, S.; Deglee, B.M.; Chen, Y.; Walton, K.S.; Zou, R.; Liu, M. Functionalized Bimetallic Hydroxides Derived from Metal–Organic Frameworks for High-Performance Hybrid Supercapacitor with Exceptional Cycling Stability. ACS Energy Lett. 2017, 2, 1263–1269. [Google Scholar] [CrossRef]
- Jung, K.-W.; Choi, B.H.; Lee, S.Y.; Ahn, K.-H.; Lee, Y.J. Green synthesis of aluminum-based metal organic framework for the removal of azo dye Acid Black 1 from aqueous media. J. Ind. Eng. Chem. 2018, 67, 316–325. [Google Scholar] [CrossRef]
- Fang, X.; Zong, B.; Mao, S. Metal–Organic Framework-Based Sensors for Environmental Contaminant Sensing. Nano-Micro Lett. 2018, 10, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Wang, X.; Hou, M.; Li, X.; Wu, X.; Ge, J. Immobilization on Metal–Organic Framework Engenders High Sensitivity for Enzymatic Electrochemical Detection. ACS Appl. Mater. Interfaces 2017, 9, 13831–13836. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Deep, A.; Kim, K.-H. Metal organic frameworks for sensing applications. TRAC Trends Anal. Chem. 2015, 73, 39–53. [Google Scholar] [CrossRef]
- Al-Janabi, N.; Alfutimie, A.; Siperstein, F.R.; Fan, X. Underlying mechanism of the hydrothermal instability of Cu3(BTC)2 metal-organic framework. Front. Chem. Sci. Eng. 2016, 10, 103–107. [Google Scholar] [CrossRef]
- Cui, L.; Wu, J.; Li, J.; Ju, H. Electrochemical Sensor for Lead Cation Sensitized with a DNA Functionalized Porphyrinic Metal–Organic Framework. Anal. Chem. 2015, 87, 10635–10641. [Google Scholar] [CrossRef]
- Campbell, M.G.; Sheberla, D.; Liu, S.F.; Swager, T.M.; Dincă, M. Cu3(hexaiminotriphenylene)2: An Electrically Conductive 2D Metal–Organic Framework for Chemiresistive Sensing. Angew. Chem. Int. Ed. 2015, 54, 4349–4352. [Google Scholar] [CrossRef]
- Milakin, K.A.; Gavrilov, N.; Pašti, I.A.; Morávková, Z.; Acharya, U.; Unterweger, C.; Breitenbach, S.; Zhigunov, A.; Bober, P. Polyaniline-metal organic framework (Fe-BTC) composite for electrochemical applications. Polymer 2020, 208, 122945. [Google Scholar] [CrossRef]
- Fu, X.; Li, T.; Qi, F.; Zhang, S.; Wen, J.; Shu, W.; Luo, P.; Zhang, R.; Hu, S.; Liu, Q. Designing high electrochemical surface area between polyaniline and hydrogel polymer electrolyte for flexible supercapacitors. Appl. Surf. Sci. 2020, 507, 145135. [Google Scholar] [CrossRef]
- Neisi, Z.; Ansari-Asl, Z.; Dezfuli, A.S. Polyaniline/Cu(II) Metal-organic Frameworks Composite for High Performance Supercapacitor Electrode. J. Inorg. Organomet. Polym. Mater. 2019, 29, 1838–1847. [Google Scholar] [CrossRef]
- Zhu, C.; He, Y.; Liu, Y.; Kazantseva, N.; Saha, P.; Cheng, Q. ZnO@MOF@PANI core-shell nanoarrays on carbon cloth for high-performance supercapacitor electrodes. J. Energy Chem. 2019, 35, 124–131. [Google Scholar] [CrossRef] [Green Version]
- Du, P.; Wei, W.; Liu, D.; Kang, H.; Liu, P. Fabrication of hierarchical carbon layer encapsulated polyaniline core-shell structure nanotubes and application in supercapacitors. Chem. Eng. J. 2018, 335, 373–383. [Google Scholar] [CrossRef]
- Karim, M.R.; Shinoda, H.; Nakai, M.; Hatakeyama, K.; Kamihata, H.; Matsui, T.; Taniguchi, T.; Koinuma, M.; Kuroiwa, K.; Kurmoo, M.; et al. Electrical Conductivity and Ferromagnetism in a Reduced Graphene–Metal Oxide Hybrid. Adv. Funct. Mater. 2013, 23, 323–332. [Google Scholar] [CrossRef]
- Khan, A.A.P.; Rahman, M.M.; Asiri, A.M. High performance polyaniline/vanadyl phosphate (PANI–VOPO4) nano composite sheets prepared by exfoliation/intercalation method for sensing applications. Eur. Polym. J. 2016, 75, 388–398. [Google Scholar] [CrossRef]
- Guo, S.; Zhu, Y.; Yan, Y.; Min, Y.; Fan, J.; Xu, Q.; Yun, H. (Metal-Organic Framework)-Polyaniline sandwich structure composites as novel hybrid electrode materials for high-performance supercapacitor. J. Power Sources 2016, 316, 176–182. [Google Scholar] [CrossRef] [Green Version]
- Sadegh, F.; Modarresi-Alam, A.R.; Noroozifar, M.; Kerman, K. A facile and green synthesis of superparamagnetic Fe3O4@PANI nanocomposite with a core–shell structure to increase of triplet state population and efficiency of the solar cells. J. Environ. Chem. Eng. 2021, 9, 104942. [Google Scholar] [CrossRef]
- Su, N. Polyaniline-Doped Spherical Polyelectrolyte Brush Nanocomposites with Enhanced Electrical Conductivity, Thermal Stability, and Solubility Property. Polymers 2015, 7, 1599–1616. [Google Scholar] [CrossRef] [Green Version]
- Eltayeb, N.E.; Khan, A. Preparation and properties of newly synthesized Polyaniline@Graphene oxide/Ag nanocomposite for highly selective sensor application. J. Mater. Res. Technol. 2020, 9, 10459–10467. [Google Scholar] [CrossRef]
- Khan, A.A.P.; Hussein, M.A.; Neppolian, B.; Asiri, A.M. Preparation of new and novel wave like poly(2-anisidine) zirconium tungstate nanocomposite: Thermal, electrical and ion-selective studies. Chin. J. Chem. Eng. 2019, 27, 459–466. [Google Scholar] [CrossRef]
- Khan, A.A.; Akhtar, T. Preparation, physico-chemical characterization and electrical conductivity measurement studies of an organic–inorganic nanocomposite cation-exchanger: Poly-o-toluidine Zr(IV) phosphate. Electrochim. Acta. 2008, 53, 5540–5548. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications. Electrochem. Methods 2001, 2, 580–632. [Google Scholar]
- Xu, H.; Zeng, L.; Huang, D.; Xian, Y.; Jin, L. A Nafion-coated bismuth film electrode for the determination of heavy metals in vegetable using differential pulse anodic stripping voltammetry: An alternative to mercury-based electrodes. Food Chem. 2008, 109, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Gosser, D.K. Cyclic Voltammetry: Simulation and Analysis of Reaction Mechanisms; VCH: New York, NY, USA, 1993. [Google Scholar]
- Adeosun, W.A.; Asiri, A.M.; Marwani, H.M. Sensitive determination of 2-nitrophenol using electrochemically deposited polymethyl red film for healthcare and environmental safety. Synth. Met. 2020, 261, 116321. [Google Scholar] [CrossRef]
- Obisesan, O.R.; Adekunle, A.S.; Oyekunle, J.A.; Sabu, T.; Nkambule, T.T.; Mamba, B.B. Development of Electrochemical Nanosensor for the Detection of Malaria Parasite in Clinical Samples. Front Chem. 2019, 7, 89. [Google Scholar] [CrossRef] [Green Version]
- Sui, L.; Wu, T.; Liu, L.; Wang, H.; Wang, Q.; Hou, H.; Guo, Q. A Sensitive Pyrimethanil Sensor Based on Electrospun TiC/C Film. Sensors 2019, 19, 1531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramswaroop, A.S.; Jhankal, K.K.; Sharma, D.K. Electrochemical Behavior of 1-Methyl-2-oxo-4,5-dichloro- quinolone in Aprotic Media at Glassy Carbon Electrode. Chem. Sci. 2015, 4, 604–610. [Google Scholar]
- Lide, D.R.; Kehiaian, H.V. CRC Handbook of Thermophysical and Thermochemical Data; CRC Press: Boca Raton, FL, USA, 1994. [Google Scholar]
- Desmond, D.; Lane, B.; Alderman, J.; Hill, M.; Arrigan, D.W.M.; Glennon, J.D. An environmental monitoring system for trace metals using stripping voltammetry. Sens. Actuators B Chem. 1998, 48, 409–414. [Google Scholar] [CrossRef]
- Kadara, R.O.; Jenkinson, N.; Banks, C.E. Disposable Bismuth Oxide Screen Printed Electrodes for the High Throughput Screening of Heavy Metals. Electroanalysis 2009, 21, 2410–2414. [Google Scholar] [CrossRef]
- Jothimuthu, P.; Wilson, R.A.; Herren, J.; Pei, X.; Kang, W.; Daniels, R.; Wong, H.; Beyette, F.; Heineman, W.R.; Papautsky, I. Zinc Detection in Serum by Anodic Stripping Voltammetry on Microfabricated Bismuth Electrodes. Electroanalysis 2013, 25, 401–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feier, B.; Floner, D.; Cristea, C.; Bodoki, E.; Sandulescu, R.; Geneste, F. Flow electrochemical analyses of zinc by stripping voltammetry on graphite felt electrode. Talanta 2012, 98, 152–156. [Google Scholar] [CrossRef] [PubMed]










| Modified Electrode | Technique | LR(µM) | LOD (µM) | Ref |
|---|---|---|---|---|
| Hg thin film SPE | DPASV | Up to 15.29 | 0.85 | [33] |
| Bismuth oxide modified ink SPE | SWASV | 0.6–2.29 | 0.45 | [34] |
| Microfabricated bismuth electrodes | ASV | 5–50 | 6 | [35] |
| Graphite felt electrode | LSSV | 1–100 | 0.5 | [36] |
| PANI@Al-SA /nafion modified GCE | LSV | 2.8–228.6 | 0.5 | This study |
| Sample | Spiked Concentration (µM) | Found Concentration (µM) | RSD (%) (n = 3) | Bias | Recovery (%) |
|---|---|---|---|---|---|
| Tap water | 0 | 0 | - | - | - |
| 3 | 2.94 | 1.4 | −0.06 | 98.06 | |
| 6 | 5.8 | 1 | −0.23 | 96.11 | |
| Bottled water | 0 | 0 | - | - | - |
| 3 | 2.91 | 1.45 | −0.08 | 97.22 | |
| 6 | 5.86 | 0.8 | −0.13 | 97.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsafrani, A.E.; Adeosun, W.A.; Marwani, H.M.; Khan, I.; Jawaid, M.; Asiri, A.M.; Khan, A. Efficient Synthesis and Characterization of Polyaniline@Aluminium–Succinate Metal-Organic Frameworks Nanocomposite and Its Application for Zn(II) Ion Sensing. Polymers 2021, 13, 3383. https://doi.org/10.3390/polym13193383
Alsafrani AE, Adeosun WA, Marwani HM, Khan I, Jawaid M, Asiri AM, Khan A. Efficient Synthesis and Characterization of Polyaniline@Aluminium–Succinate Metal-Organic Frameworks Nanocomposite and Its Application for Zn(II) Ion Sensing. Polymers. 2021; 13(19):3383. https://doi.org/10.3390/polym13193383
Chicago/Turabian StyleAlsafrani, Amjad E., Waheed A. Adeosun, Hadi M. Marwani, Imran Khan, Mohammad Jawaid, Abdullah M. Asiri, and Anish Khan. 2021. "Efficient Synthesis and Characterization of Polyaniline@Aluminium–Succinate Metal-Organic Frameworks Nanocomposite and Its Application for Zn(II) Ion Sensing" Polymers 13, no. 19: 3383. https://doi.org/10.3390/polym13193383
APA StyleAlsafrani, A. E., Adeosun, W. A., Marwani, H. M., Khan, I., Jawaid, M., Asiri, A. M., & Khan, A. (2021). Efficient Synthesis and Characterization of Polyaniline@Aluminium–Succinate Metal-Organic Frameworks Nanocomposite and Its Application for Zn(II) Ion Sensing. Polymers, 13(19), 3383. https://doi.org/10.3390/polym13193383