Radiation Graft-Copolymerization of Ultrafine Fully Vulcanized Powdered Natural Rubber: Effects of Styrene and Acrylonitrile Contents on Thermal Stability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental
Chemicals
2.2. Preparation of Deproteinized Natural Rubber Latex (DPNR), St, and AN Monomers
2.3. Graft Copolymer Preparation
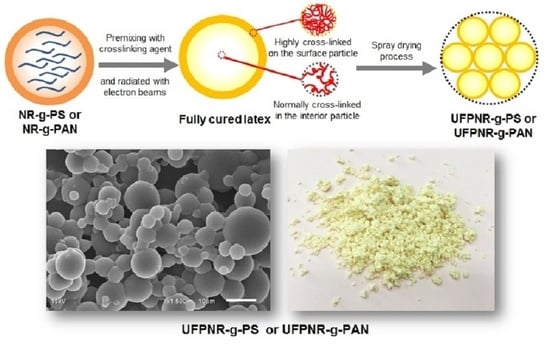
2.4. Preparation of Ultrafine Fully Vulcanized Powdered Natural Rubber (UFPNRs)
2.5. Samples Characterization
3. Results and Discussion
3.1. Chemical Structure of Graft Copolymers
3.2. Effect of St and AN Monomer Content on Monomer Conversion and Grafting Efficiency of Graft Copolymers
3.3. Effect of St and AN Monomer Content on Thermal Stability of Graft Copolymers
3.4. Effect of St and AN Monomer Content on Swelling Behavior of Graft Copolymers
3.5. Morphology of Powdered Rubber of Graft Copolymers
3.6. Chemical Structure of UFPNR-g-PS and UFPNR-g-PAN
3.7. Effect of St and AN Monomer Content and Radiation Dose on Thermal Stability of UFPNR-g-PS and UFPNR-g-PAN
3.8. Effect of Radiation Dose on Swelling Behavior of UFPNR-g-PS and UFPNR-g-PAN
3.9. Effect of Radiation Dose on Morphology and Particle Sizes of UFPNR-g-PS and UFPNR-g-PAN
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, Y.; Amornkitbamrung, L.; Mora, P.; Jubsilp, C.; Hemvichian, K.; Soottitantawat, A.; Ekgasit, S.; Rimdusit, S. Effects of Coagent Functionalities on Properties of Ultrafine Fully Vulcanized Powdered Natural Rubber Prepared as Toughening Filler in Rigid PVC. Polymers 2021, 13, 289. [Google Scholar] [CrossRef] [PubMed]
- Wongkumchai, R.; Amornkitbamrung, L.; Mora, P.; Jubsilp, C.; Rimdusit, S. Effects of coagent incorporation on properties of ultrafine fully vulcanized powdered natural rubber prepared as toughening filler in polybenzoxazine. SPE Polym. 2021, 2, 191–198. [Google Scholar] [CrossRef]
- de Paiva, L.B.; de Oliveira, A.M.; Gavioli, R.R. Preparation and properties of rubber powder from modified-SBR latex by spray drying process. Powder Technol. 2014, 264, 507–513. [Google Scholar] [CrossRef]
- Liu, W.-W.; Ma, J.-J.; Zhan, M.-S.; Wang, K. The toughening effect and mechanism of styrene-butadiene rubber nanoparticles for novolac resin. J. Appl. Polym. Sci. 2015, 132, 41533. [Google Scholar] [CrossRef]
- Jubsilp, C.; Jantaramaha, J.; Mora, P. Tribological Performance and Thermal Stability of Nanorubber-Modified Polybenzoxazine Composites for Non-Asbestos Friction Materials. Polymers 2021, 13, 2435. [Google Scholar] [CrossRef] [PubMed]
- Taewattana, R.; Jubsilp, C.; Suwanmala, P.; Rimdusit, S. Effect of gamma irradiation on properties of ultrafine rubbers as toughening filler in polybenzoxazine. Radiat. Phys. Chem. 2018, 145, 184–192. [Google Scholar] [CrossRef]
- Rezaei Abadchi, M.; Jalali-Arani, A. The use of gamma irradiation in preparation of polybutadiene rubber nanopowder; Its effect on particle size, morphology and crosslink structure of the powder. Nucl. Instrum. Methods Phys. Res. B 2014, 320, 1–5. [Google Scholar] [CrossRef]
- Sosnik, A.; Seremeta, K.P. Advantages and challenges of the spray-drying technology for the production of pure drug particles and drug-loaded polymeric carriers. Adv Colloid Interface Sci 2015, 223, 40–54. [Google Scholar] [CrossRef]
- Mini Spray Dryer B-290: The World Leading R&D Solution for Spray Drying. Available online: http://www.buchi.com/en/products/spray-drying-and-encapsulation/mini-spray-dryer-b-290 (accessed on 18 September 2019).
- Tian, M.; Tang, Y.-W.; Lu, Y.-L.; Qiao, J.; Li, T.; Zhang, L.-Q. Novel Rubber Blends Made from Ultra-Fine Full-Vulcanized Powdered Rubber (UFPR). Polym. J. 2006, 38, 50–56. [Google Scholar] [CrossRef] [Green Version]
- Huang, F. Interface and properties of epoxy resin modified by elastomeric nano-particles. Sci. China Ser. B 2005, 48, 148–155. [Google Scholar] [CrossRef]
- Ding, X.; Xu, R.; Yu, D.; Chen, H.; Fan, R. Effect of ultrafine, fully vulcanized acrylate powdered rubber on the mechanical properties and crystallization behavior of nylon 6. J. Appl. Polym. Sci. 2003, 90, 3503–3511. [Google Scholar] [CrossRef]
- Peng, J.; Zhang, X.; Qiao, J.; Wei, G. Radiation preparation of ultrafine carboxylated styrene-butadiene rubber powders and application for nylon 6 as an impact modifier. J. Appl. Polym. Sci. 2002, 86, 3040–3046. [Google Scholar] [CrossRef]
- Zhao, Q.; Ding, Y.; Yang, B.; Ning, N.; Fu, Q. Highly efficient toughening effect of ultrafine full-vulcanized powdered rubber on poly(lactic acid)(PLA). Polym. Test. 2013, 32, 299–305. [Google Scholar] [CrossRef]
- Petchwattana, N.; Covavisaruch, S.; Euapanthasate, N. Utilization of ultrafine acrylate rubber particles as a toughening agent for poly(lactic acid). Mater. Sci. Eng. A 2012, 532, 64–70. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, X.; Liu, S.; Gui, H.; Lai, J.; Liu, Y.; Gao, J.; Huang, F.; Song, Z.; Tan, B.; et al. Ultrafine full-vulcanized powdered rubbers/PVC compounds with higher toughness and higher heat resistance. Polymer 2005, 46, 10614–10617. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, Y.; Deng, S.; Zhang, Q.; Fu, Q. Largely enhanced mechanical properties and heat distortion temperature of β-nucleated isotactic polypropylene by adding ultrafine full-vulcanized powdered rubber. RSC Adv. 2015, 5, 62797–62804. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Gao, J.; Huang, F.; Tan, B.; Wei, G.; Qiao, J. Toughening of polypropylene by combined rubber system of ultrafine full-vulcanized powdered rubber and SBS. Polymer 2004, 45, 275–286. [Google Scholar] [CrossRef]
- Sun, D.; Yuan, Q.; Jiang, W. Thermal properties and crystallization behavior of ultrafine fully-vulcanized powdered rubber particle toughened polypropylene. J. Appl. Polym. Sci. 2008, 110, 1318–1323. [Google Scholar] [CrossRef]
- Dung, T.A.; Nhan, N.T.; Thuong, N.T.; Nghia, P.T.; Yamamoto, Y.; Kosugi, K.; Kawahara, S.; Thuy, T.T. Modification of vietnam natural rubber via graft copolymerization with styrene. J. Braz. Chem. Soc. 2017, 28, 669–675. [Google Scholar] [CrossRef]
- Hashim, A.S.; Ong, S.K. Natural rubber and its derivatives. In Elastomers; IntechOpen: Rijeka, Croatia, 2017. [Google Scholar] [CrossRef]
- Prukkaewkanjana, K.; Kawahara, S.; Sakdapipanich, J. Influence of reaction conditions on the properties of nano-matrix structure formed by graft-copolymerization of acrylonitrile onto natural rubber. Adv. Mater. Res. 2013, 844, 365–368. [Google Scholar] [CrossRef]
- Nhan, N.T.; Dung, T.A.; Khanh, P.D.; Thuong, N.T.; Tung, N.H.; Nghia, P.T.; Thuy, T.T. Investigation of cure and mechanical properties of deproteinized natural rubber-g-poly methyl methacrylate. Vietnam J. Chem. 2016, 54, 520–523. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Do, Q.V.; Tran, A.D.; Kawahara, S. Preparation of hydrogenated natural rubber with nanomatrix structure. Polym. Adv. Technol. 2019, 31, 86–93. [Google Scholar] [CrossRef]
- Angnanon, S.; Prasassarakich, P.; Hinchiranan, N. Styrene/Acrylonitrile Graft Natural Rubber as Compatibilizer in Rubber Blends. Polym.-Plast. Technol. Eng. 2011, 50, 1170–1178. [Google Scholar] [CrossRef]
- Prasassarakich, P.; Sintoorahat, P.; Wongwisetsirikul, N. Enhanced graft copolymerization of styrene and acrylonitrile onto natural rubber. J. Chem. Eng. Jpn. 2001, 34, 249–253. [Google Scholar] [CrossRef]
- Tuti, I.S.; Asep, H.S.; Setijo, B.; Dadi, R.M.; Adi, C. The effect of styrene monomer in the graft copolymerization of arcylonitrile onto deproteinized natural rubber. Int. J. Technol. 2015, 6, 1164–1173. [Google Scholar] [CrossRef]
- Lv, F.; Fan, J.; Huang, J.; Cao, L.; Yan, X.; Ge, L.; Abubakar, S.; Chen, Y. Preparation of polypropylene/ethylene-propylene-diene terpolymer/nitrile rubber ternary thermoplastics vulcanizates with good mechanical properties and oil resistance by core-shell dynamic vulcanization. Polym. Adv. Technol. 2020, 31, 2161–2171. [Google Scholar] [CrossRef]
- BGS—Beta Gama Service. Radiation Crosslinking and Radiation Sterilization. Available online: http://bgs.eu (accessed on 7 October 2021).
- Chirinos, H.; Salvador; Yoshii, F.; Takasaki, K.M. Natural rubber latex using gamma rays and electron beam radiation. Elastomers Plast. 2007, 60, 535–541. [Google Scholar]
- Manaila, E.; Craciun, G.; Stelescu, M.-D.; Ighigeanu, D.; Ficai, M. Radiation vulcanization of natural rubber with polyfunctional monomers. Polym. Bull. 2013, 71, 57–82. [Google Scholar] [CrossRef]
- Sritragool, K.; Michael, H.; Gehde, M.; Chemnitz; Gohs, U.; Heinrich, G.; Dresden. PP/rubber particle blends by high energy electron treatment under stationary condition. Test. Meas. 2010, 63, 377–382. [Google Scholar]
- Pisuttisap, A. Modified Natural Rubber Latex by Graft Copolymerization with Styrene and Diimide Hydrogenation. Master’s Thesis, Chulalongkorn University, Bangkok, Thailand, 2011. [Google Scholar]
- Bandzierz, K.S.; Reuvekamp, L.A.E.M.; Przybytniak, G.; Dierkes, W.K.; Blume, A.; Bielinski, D. Effect of electron beam irradiation on structure and properties of styrene-butadiene rubber. Radiat. Phys. Chem. 2017, 149, 14–25. [Google Scholar] [CrossRef]
- Chueangchayaphan, W.; Tanrattanakul, V.; Chueangchayaphan, N.; Muangsap, S.; Borapak, W. Synthesis and thermal properties of natural rubber grafted with poly(2-hydroxyethyl acrylate). J. Polym. Res. 2017, 24, 107. [Google Scholar] [CrossRef]
- Dinsmore, H.L.; Smith, D.C. Analysis of Natural and Synthetic Rubber by Infrared Spectroscopy. Anal. Chem. 1948, 20, 11–24. [Google Scholar] [CrossRef]
- Kishore, K.; Pandey, H.K. Spectral studies on plant rubbers. Prog. Polym. Sci. 1986, 12, 155–178. [Google Scholar] [CrossRef]
- Nallasamy, P.; Mohan, S. Vibrational spectra of cis-1,4-polyisoprene. Arab. J. Sci. Eng. 2004, 28, 17–26. [Google Scholar]
- Tancharernrat, T.; Rempel, G.L.; Prasassarakich, P. Preparation of styrene butadiene copolymer–silica nanocomposites via differential microemulsion polymerization and NR/SBR–SiO2 membranes for pervaporation of water–ethanol mixtures. Chem. Eng. J. 2014, 258, 290–300. [Google Scholar] [CrossRef]
- Liu, D.; Kang, J.; Chen, P.; Liu, X.; Cao, Y. 1H NMR and 13C NMR Investigation of Microstructures of Carboxyl-Terminated Butadiene Acrylonitrile Rubbers. J. Macromol. Sci. 2013, 52, 127–137. [Google Scholar] [CrossRef]
- Arayapranee, W.; Prasassarakich, P.; Rempel, G.L. Synthesis of graft copolymers from natural rubber using cumene hydroperoxide redox initiator. J. Appl. Polym. Sci. 2002, 83, 2993–3001. [Google Scholar] [CrossRef]
- Seleem, S.; Hopkins, M.; Olivio, J.; Schiraldi, D.A. Comparison of Thermal Decomposition of Polystyrene Products vs. Bio-Based Polymer Aerogels. Ohio J. Sci. 2017, 117, 50–60. [Google Scholar] [CrossRef] [Green Version]
- Samthong, C.; Kunanusont, N.; Deetuam, C.; Wongkhan, T.; Supannasud, T.; Somwangthanaroj, A. Effect of acrylonitrile content of acrylonitrile butadiene rubber on mechanical and thermal properties of dynamically vulcanized poly(lactic acid) blends. Polym. Int. 2019, 68, 2004–2016. [Google Scholar] [CrossRef]
- Manshaie, R.; Nouri Khorasani, S.; Jahanbani Veshare, S.; Rezaei Abadchi, M. Effect of electron beam irradiation on the properties of natural rubber (NR)/styrene–butadiene rubber (SBR) blend. Radiat. Phys. Chem. 2011, 80, 100–106. [Google Scholar] [CrossRef]
- Dave, P.N.; Gor, A. Natural Polysaccharide-Based Hydrogels and Nanomaterials. In Handbook of Nanomaterials for Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 36–66. [Google Scholar] [CrossRef]
- Gibson, J.; McKee, J.; Freihofer, G.; Raghavan, S.; Gou, J. Enhancement in ballistic performance of composite hard armor through carbon nanotubes. Int. J. Smart Nano Mater. 2013, 4, 212–228. [Google Scholar] [CrossRef]
- Dawes, K.; Glovery, L.C.; Vroomy, D.A. The effects of electron beam and gamma-irradiation on polymeric materials. In Physical Properties of Polymers Handbook; Springer: New York, NY, USA, 2017; pp. 867–887. [Google Scholar]
- Anderson, H.R. Compounding rubber for radiation resistance. J. Appl. Polym. Sci. 1960, 3, 316–330. [Google Scholar] [CrossRef]


















Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rimdusit, N.; Jubsilp, C.; Mora, P.; Hemvichian, K.; Thuy, T.T.; Karagiannidis, P.; Rimdusit, S. Radiation Graft-Copolymerization of Ultrafine Fully Vulcanized Powdered Natural Rubber: Effects of Styrene and Acrylonitrile Contents on Thermal Stability. Polymers 2021, 13, 3447. https://doi.org/10.3390/polym13193447
Rimdusit N, Jubsilp C, Mora P, Hemvichian K, Thuy TT, Karagiannidis P, Rimdusit S. Radiation Graft-Copolymerization of Ultrafine Fully Vulcanized Powdered Natural Rubber: Effects of Styrene and Acrylonitrile Contents on Thermal Stability. Polymers. 2021; 13(19):3447. https://doi.org/10.3390/polym13193447
Chicago/Turabian StyleRimdusit, Niratchaporn, Chanchira Jubsilp, Phattarin Mora, Kasinee Hemvichian, Tran Thi Thuy, Panagiotis Karagiannidis, and Sarawut Rimdusit. 2021. "Radiation Graft-Copolymerization of Ultrafine Fully Vulcanized Powdered Natural Rubber: Effects of Styrene and Acrylonitrile Contents on Thermal Stability" Polymers 13, no. 19: 3447. https://doi.org/10.3390/polym13193447
APA StyleRimdusit, N., Jubsilp, C., Mora, P., Hemvichian, K., Thuy, T. T., Karagiannidis, P., & Rimdusit, S. (2021). Radiation Graft-Copolymerization of Ultrafine Fully Vulcanized Powdered Natural Rubber: Effects of Styrene and Acrylonitrile Contents on Thermal Stability. Polymers, 13(19), 3447. https://doi.org/10.3390/polym13193447