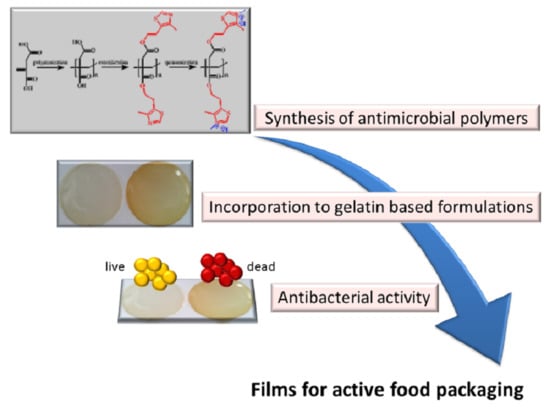
Incorporation of Poly(Itaconic Acid) with Quaternized Thiazole Groups on Gelatin-Based Films for Antimicrobial-Active Food Packaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Quaternized Polymers, MeFPIAx
2.2.1. Synthesis of Poly(Itaconic Acid) (PIA)
2.2.2. Functionalization of PIA with 1,3-Thiazole Side-Chain Groups (FPIAx)
2.2.3. Quaternization of FPIAx with Iodomethane (MeFPIAx)
2.3. Physicochemical Characterization of Synthesized Polymers
2.4. Preparation of Active Films Based on Gelatin and Starch
2.5. Mechanical Properties
2.6. Color Properties
2.7. Experimental Water Vapor Permeability (Pwexp)
2.8. Swelling Properties
Evaluation of Swelling Capacity of Films and Their Kinetics
2.9. Evaluation of the Antioxidant Properties by the ABST•+ Assay
2.9.1. Preparation of Working Solutions
2.9.2. Estimation of Film Antioxidant Capacity by Direct Contact with ABTS•+
2.10. Microbiological Assays
2.10.1. Minimal Inhibition Concentration (MIC) of MeFPIAx Quaternized Polymers
2.10.2. Antimicrobial Activity of the Films
2.11. Statistical Analysis
3. Results and Discussion
3.1. Homopolymer Synthesis and Modification
3.2. Mechanical Properties
3.3. Color Properties
3.4. Experimental Water Vapor Permeability (Pwexp)
3.5. Swelling Properties
3.6. Antioxidant Activity
3.7. Biological Assays
3.7.1. Antimicrobial Activity of MeFPIAx Quaternized Polymers
3.7.2. Antimicrobial Activity of the Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Realini, C.E.; Marcos, B. Active and intelligent packaging systems for a modern society. Meat Sci. 2014, 98, 404–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos, M.; Fortunati, E.; Peltzer, M.; Jimenez, A.; Kenny, J.M.; Garrigós, M.C. Characterization and disintegrability under composting conditions of PLA-based nanocomposite films with thymol and silver nanoparticles. Polym. Degrad. Stab. 2016, 132, 2–10. [Google Scholar] [CrossRef] [Green Version]
- Ramos, M.; Fortunati, E.; Peltzer, M.; Dominici, F.; Jiménez, A.; Garrigós, M.D.C.; Kenny, J.M. Influence of thymol and silver nanoparticles on the degradation of poly(lactic acid) based nanocomposites: Thermal and morphological properties. Polym. Degrad. Stab. 2014, 108, 158–165. [Google Scholar] [CrossRef] [Green Version]
- Sonseca, A.; Madani, S.; Rodriguez, G.; Hevilla, V.; Echeverria, C.; Fernandez-Garcia, M.; Munoz-Bonilla, A.; Charef, N.; Lopez, D. Multifunctional PLA Blends Containing Chitosan Mediated Silver Nanoparticles: Thermal, Mechanical, Antibacterial, and Degradation Properties. Nanomaterials 2019, 10, 22. [Google Scholar] [CrossRef] [Green Version]
- Campos, C.A.; Gerschenson, L.N.; Flores, S.K. Development of Edible Films and Coatings with Antimicrobial Activity. Food Bioprocess Technol. 2011, 4, 849–875. [Google Scholar] [CrossRef]
- Mi, L.; Jiang, S. Integrated Antimicrobial and Nonfouling Zwitterionic Polymers. Angew. Chem. Int. Ed. 2014, 53, 1746–1754. [Google Scholar] [CrossRef]
- Asgher, M.; Qamar, S.A.; Bilal, M.; Iqbal, H.M.N. Bio-based active food packaging materials: Sustainable alternative to conventional petrochemical-based packaging materials. Food Res. Int. 2020, 137, 109625. [Google Scholar] [CrossRef]
- Alvarez-Paino, M.; Munoz-Bonilla, A.; Fernandez-Garcia, M.; Álvarez-Paino, M.; Muñoz-Bonilla, A.; Fernández-García, M. Antimicrobial Polymers in the Nano-World. Nanomaterials 2017, 7, 48. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Bonilla, A.; Fernández-García, M. Polymeric materials with antimicrobial activity. Prog. Polym. Sci. 2012, 37. [Google Scholar] [CrossRef]
- Muñoz-Bonilla, A.; Echeverria, C.; Sonseca, Á.; Arrieta, M.P.; Fernández-García, M. Bio-Based Polymers with Antimicrobial Properties towards Sustainable Development. Materials 2019, 12, 641. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Bonilla, A.; Fernández-García, M. Poly(ionic liquid)s as antimicrobial materials. Eur. Polym. J. 2018, 105. [Google Scholar] [CrossRef]
- Muñoz-Bonilla, A.; Fernández-García, M. The roadmap of antimicrobial polymeric materials in macromolecular nanotechnology. Eur. Polym. J. 2015, 65. [Google Scholar] [CrossRef] [Green Version]
- Echeverria, C.; Torres, M.D.T.; Fernández-García, M.; De la Fuente-Nunez, C.; Muñoz-Bonilla, A. Physical methods for controlling bacterial colonization on polymer surfaces. Biotechnol. Adv. 2020, 43, 107586. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Paino, M.; Juan-Rodríguez, R.; Cuervo-Rodríguez, R.; Tejero, R.; López, D.; López-Fabal, F.; Gómez-Garcés, J.L.; Muñoz-Bonilla, A.; Fernández-García, M. Antimicrobial films obtained from latex particles functionalized with quaternized block copolymers. Colloids Surf. B Biointerfaces 2016, 140. [Google Scholar] [CrossRef] [PubMed]
- Kenawy, E.-R.; Worley, S.D.; Broughtonand, R.; Broughton, R. The Chemistry and Applications of Antimicrobial Polymers: A State-of-the-Art Review. Biomacromolecules 2007, 8, 1359–1384. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, W.; Bohrisch, J.; Laschewsky, A. Synthetic polymers with quaternary nitrogen atoms-Synthesis and structure of the most used type of cationic polyelectrolytes. Prog. Polym. Sci. 2010, 35, 511–577. [Google Scholar] [CrossRef]
- Kirimura, K.; Honda, Y.; Hattori, T. 3.14-Gluconic and Itaconic Acids. In Comprehensive Biotechnology, 2nd ed.; Moo-Young, M., Ed.; Academic Press: Burlington, MA, USA, 2011; pp. 143–147. ISBN 978-0-08-088504-9. [Google Scholar]
- Robert, T.; Friebel, S. Itaconic acid-a versatile building block for renewable polyesters with enhanced functionality. Green Chem. 2016, 18, 2922–2934. [Google Scholar] [CrossRef] [Green Version]
- Bednarz, S.; Błaszczyk, A.; Błażejewska, D.; Bogdał, D. Free-radical polymerization of itaconic acid in the presence of choline salts: Mechanism of persulfate decomposition. Catal. Today 2015, 257, 297–304. [Google Scholar] [CrossRef]
- Milosavljević, N.B.; Ristić, M.Đ.; Perić-Grujić, A.A.; Filipović, J.M.; Štrbac, S.B.; Rakočević, Z.L.; Kalagasidis Krušić, M.T. Hydrogel based on chitosan, itaconic acid and methacrylic acid as adsorbent of Cd2+ ions from aqueous solution. Chem. Eng. J. 2010, 165, 554–562. [Google Scholar] [CrossRef]
- Okuda, T.; Ishimoto, K.; Ohara, H.; Kobayashi, S. Renewable Biobased Polymeric Materials: Facile Synthesis of Itaconic Anhydride-Based Copolymers with Poly(l-lactic acid) Grafts. Macromolecules 2012, 45, 4166–4174. [Google Scholar] [CrossRef]
- Mielczarek, K.; Łabanowska, M.; Kurdziel, M.; Konefał, R.; Beneš, H.; Bujok, S.; Kowalski, G.; Bednarz, S. High-Molecular-Weight Polyampholytes Synthesized via Daylight-Induced, Initiator-Free Radical Polymerization of Renewable Itaconic Acid. Macromol. Rapid Commun. 2020, 41, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Niu, L.; Ma, S.; Li, J.; Tay, F.R.; Chen, J. Quaternary ammonium-based biomedical materials: State-of-the-art, toxicological aspects and antimicrobial resistance. Prog. Polym. Sci. 2017, 71, 53–90. [Google Scholar] [CrossRef] [PubMed]
- Tejero, R.; López, D.; López-Fabal, F.; Gómez-Garcés, J.L.; Fernández-García, M. Antimicrobial polymethacrylates based on quaternized 1,3-thiazole and 1,2,3-triazole side-chain groups. Polym. Chem. 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Bonilla, A.; Zagora, J.; Plachá, D.; Echeverría, C.; Chiloeches, A.; Fernández-García, M. Chemical Hydrogels Bearing Thiazolium Groups with a Broad Spectrum of Antimicrobial Behavior. Polymers 2020, 12, 2853. [Google Scholar] [CrossRef] [PubMed]
- Cuervo-Rodríguez, R.; Muñoz-Bonilla, A.; Araujo, J.; Echeverría, C.; Fernández-García, M. Influence of side chain structure on the thermal and antimicrobial properties of cationic methacrylic polymers. Eur. Polym. J. 2019, 117, 86–93. [Google Scholar] [CrossRef]
- Sapper, M.; Chiralt, A. Starch-Based Coatings for Preservation of Fruits and Vegetables. Coatings 2018, 8, 152. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, H.; Dijkstra, P.; Loos, K. The recent developments in biobased polymers toward general and engineering applications: Polymers that are upgraded from biodegradable polymers, analogous to petroleum-derived polymers, and newly developed. Polymers 2017, 9, 523. [Google Scholar] [CrossRef]
- Polysaccharides: Structural Diversity and Functional Versatility, 2nd ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2004; ISBN 978-0824754808.
- Bedell, M.L.; Navara, A.M.; Du, Y.; Du, Y.; Zhang, S.; Zhang, S.; Mikos, A.G. Polymeric Systems for Bioprinting. Chem. Rev. 2020. [Google Scholar] [CrossRef]
- Fakhouri, F.M.; Martelli, S.M.; Caon, T.; Velasco, J.I.; Mei, L.H.I. Edible films and coatings based on starch/gelatin: Film properties and effect of coatings on quality of refrigerated Red Crimson grapes. Postharvest Biol. Technol. 2015, 109, 57–64. [Google Scholar] [CrossRef]
- Mariod, A.A.; Adam, H.F. Review: Gelatin, source, extraction and industrial applications. Acta Sci. Pol. Technol. Aliment. 2013, 12, 135–147. [Google Scholar]
- Liu, Y.; Cheong, N.G.S.; Yu, J.; Tsai, W.B. Modification and crosslinking of gelatin-based biomaterials as tissue adhesives. Colloids Surf. B Biointerfaces 2019, 174, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Acosta, S.; Chiralt, A.; Santamarina, P.; Rosello, J.; González-Martínez, C.; Cháfer, M. Antifungal films based on starch-gelatin blend, containing essential oils. Food Hydrocoll. 2016, 61, 233–240. [Google Scholar] [CrossRef]
- Leon, O.; Soto, D.; Antunez, A.; Fernandez, R.; Gonzalez, J.; Pina, C.; Munoz-Bonilla, A.; Fernandez-Garcia, M. Hydrogels based on oxidized starches from different botanical sources for release of fertilizers. Int. J. Biol. Macromol. 2019, 136, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Nur Hanani, Z.A.; Roos, Y.H.; Kerry, J.P. Use and application of gelatin as potential biodegradable packaging materials for food products. Int. J. Biol. Macromol. 2014, 71, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Fakhouri, F.M.; Costa, D.; Yamashita, F.; Martelli, S.M.; Jesus, R.C.; Alganer, K.; Collares-Queiroz, F.P.; Innocentini-Mei, L.H. Comparative study of processing methods for starch/gelatin films. Carbohydr. Polym. 2013, 95, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Fakhouri, F.M.; Maria Martelli, S.; Canhadas Bertan, L.; Yamashita, F.; Innocentini Mei, L.H.; Collares Queiroz, F.P. Edible films made from blends of manioc starch and gelatin–Influence of different types of plasticizer and different levels of macromolecules on their properties. LWT 2012, 49, 149–154. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, Z. Effects of gelatin-polyphenol and gelatin–genipin cross-linking on the structure of gelatin hydrogels. Int. J. Food Prop. 2018, 20, S2822–S2832. [Google Scholar] [CrossRef]
- Yu, M.; Hwang, J.; Deming, T.J. Role of 1-3,4-dihydroxyphenylalanine in mussel adhesive proteins. J. Am. Chem. Soc. 1999, 121, 5825–5826. [Google Scholar] [CrossRef]
- Fan, C.; Fu, J.; Zhu, W.; Wang, D.A. A mussel-inspired double-crosslinked tissue adhesive intended for internal medical use. Acta Biomater. 2016, 33, 51–63. [Google Scholar] [CrossRef]
- Yen, G.C.; Hsieh, C.L. Antioxidant effects of dopamine and related compounds. Biosci. Biotechnol. Biochem. 1997, 61, 1646–1649. [Google Scholar] [CrossRef] [Green Version]
- Stawski, D.; Połowiński, S. Polymerization of itaconic acid. Polim. Polym. 2005, 50, 118–122. [Google Scholar] [CrossRef]
- ASTM E96 / E96M-16, Standard Test Methods for Water Vapor Transmission of Materials; ASTM International: West Conshohocken, PA, USA, 2016; Available online: www.astm.org (accessed on 10 December 2020).
- Delgado, J.F.; Peltzer, M.A.; Wagner, J.R.; Salvay, A.G. Hydration and water vapour transport properties in yeast biomass based films: A study of plasticizer content and thickness effects. Eur. Polym. J. 2018, 99, 9–17. [Google Scholar] [CrossRef]
- Cantor, C.R.; Schimmel, P.R. The Behavior of Biological Macromolecules, Biophysical Chemistry Part III; W.H. Freeman & Co.: New York, NY, USA, 1980; ISBN 0-7167-1192-3. [Google Scholar]
- Salvay, A.G.; Colombo, M.F.; Raúl Grigera, J. Hydration effects on the structural properties and haem–haem interaction in haemoglobin. Phys. Chem. Chem. Phys. 2003, 5, 192–197. [Google Scholar] [CrossRef]
- CLSI M07-A10. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 10th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015; ISBN 1-56238-987-4. [Google Scholar]
- ASTM E2149-01, Standard Test Method for Determining the Antimicrobial Activity of Immobilized Antimicrobial Agents under Dynamic Contact Conditions (Withdrawn 2010); ASTM International: West Conshohocken, PA, USA, 2001; Available online: www.astm.org (accessed on 10 December 2020).
- Li, M.; Zhuang, B.; Yu, J. Functional Zwitterionic Polymers on Surface: Structures and Applications. Chem. Asian J. 2020, 15, 2060–2075. [Google Scholar] [CrossRef]
- Blackman, L.D.; Gunatillake, P.A.; Cass, P.; Locock, K.E.S. An introduction to zwitterionic polymer behavior and applications in solution and at surfaces. Chem. Soc. Rev. 2019, 48, 757–770. [Google Scholar] [CrossRef]
- Zheng, L.; Sundaram, H.S.; Wei, Z.; Li, C.; Yuan, Z. Applications of zwitterionic polymers. React. Funct. Polym. 2017, 118, 51–61. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Ren, B.; Zhang, D.; Xie, S.; Chang, Y.; Yang, J.; Wu, J.; Xu, L.; Zheng, J. Fundamentals and applications of zwitterionic antifouling polymers. J. Phys. D Appl. Phys. 2019, 52, 403001. [Google Scholar] [CrossRef]
- Keenan, T.R. 10.13-Gelatin; Matyjaszewski, K., Möller, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 237–247. ISBN 978-0-08-087862-1. [Google Scholar]
- Wu, X.; Liu, Y.; Wang, W.; Han, Y.; Liu, A. Improved mechanical and thermal properties of gelatin films using a nano inorganic filler. J. Food Process Eng. 2017, 40, 1–10. [Google Scholar] [CrossRef]
- Kang, S.M.; Hwang, N.S.; Yeom, J.; Park, S.Y.; Messersmith, P.B.; Choi, I.S.; Langer, R.; Anderson, D.G.; Lee, H. One-Step Multipurpose Surface Functionalization by Adhesive Catecholamine. Adv. Funct. Mater. 2012, 22, 2949–2955. [Google Scholar] [CrossRef] [Green Version]
- Strauss, G.; Gibson, S.M. Plant phenolics as cross-linkers of gelatin gels and gelatin-based coacervates for use as food ingredients. Food Hydrocoll. 2004, 18, 81–89. [Google Scholar] [CrossRef]
- Peltzer, M.A.; Salvay, A.G.; Delgado, J.F.; De la Osa, O.; Wagner, J.R. Use of Residual Yeast Cell Wall for New Biobased Materials Production: Effect of Plasticization on Film Properties. Food Bioprocess Technol. 2018, 11, 1995–2007. [Google Scholar] [CrossRef]
- Han, J.H. Chapter 9-Edible Films and Coatings: A Review. In Food Science and Technology, 2nd ed.; Han, J.H., Ed.; Academic Press: San Diego, CA, USA, 2014; pp. 213–255. ISBN 978-0-12-394601-0. [Google Scholar]
- Molyneux, P. “Transition-site” model for the permeation of gases and vapors through compact films of polymers. J. Appl. Polym. Sci. 2001, 79, 981–1024. [Google Scholar] [CrossRef]
- Sothornvit, R.; Krochta, J.M. Plasticizers in edible films and coatings. Innov. Food Packag. 2005, 403–433. [Google Scholar] [CrossRef]
- Kuijpers, A.J.; Engbers, G.H.; Krijgsveld, J.; Zaat, S.A.; Dankert, J.; Feijen, J. Cross-linking and characterisation of gelatin matrices for biomedical applications. J. Biomater. Sci. Polym. Ed. 2000, 11, 225–243. [Google Scholar] [CrossRef] [PubMed]
- Jopling, D.W. The Swelling of Gelatin Films the Effects of Drying. J. Appl. Chem. 1956, 6, 79–84. [Google Scholar] [CrossRef]
- Crank, J.; Park, G.S.; Crank, J.; Park, G.S. Diffusion of Polymers; Academic Press Incorporated: New York, NY, USA, 1968; ISBN 9780121970505. [Google Scholar]
- Wang, Q.; Wang, T.; Lv, Z.; Cui, M.; Zhao, Z.; Cao, X.; Wei, Q. A Comprehensive Review on Water Diffusion in Polymers Focusing on the Polymer. Polymers 2020, 12, 138. [Google Scholar]
- Shahidi, F.; Janitha, P.K.; Wanasundara, P.D. Phenolic antioxidants. Crit. Rev. Food Sci. Nutr. 1992, 32, 67–103. [Google Scholar] [CrossRef]
- Takahashi, H.; Palermo, E.F.; Yasuhara, K.; Caputo, G.A.; Kuroda, K. Molecular design, structures, and activity of antimicrobial peptide-mimetic polymers. Macromol. Biosci. 2013, 13, 1285–1299. [Google Scholar] [CrossRef] [Green Version]
- Palermo, E.F.; Kuroda, K. Structural determinants of antimicrobial activity in polymers which mimic host defense peptides. Appl. Microbiol. Biotechnol. 2010, 87, 1605–1615. [Google Scholar] [CrossRef]
- Timofeeva, L.; Kleshcheva, N. Antimicrobial polymers: Mechanism of action, factors of activity, and applications. Appl. Microbiol. Biotechnol. 2010, 89, 475–492. [Google Scholar] [CrossRef]
- Tejero, R.; Gutiérrez, B.; López, D.; López-Fabal, F.; Gómez-Garcés, J.L.J.L.; Muñoz-Bonilla, A.; Fernández-García, M. Tailoring macromolecular structure of cationic polymers towards efficient contact active antimicrobial surfaces. Polymers 2018, 10, 241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palermo, E.F.; Kuroda, K. Chemical Structure of Cationic Groups in Amphiphilic Polymethacrylates Modulates the Antimicrobial and Hemolytic Activities. Biomacromolecules 2009, 10, 1416–1428. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Xu, Q.; Shi, R.; Zheng, Z.; Mao, H.; Yan, F. Polyanionic Antimicrobial Membranes: An Experimental and Theoretical Study. Langmuir 2017, 33, 4346–4355. [Google Scholar] [CrossRef] [PubMed]
- Koser, S.A.; Chinn, B.D.; Saunders, F. Gelatin as a Source of Growth-Promoting Substances for Bacteria1. J. Bacteriol. 1938, 36, 57–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| Sample Names | Description |
|---|---|
| GS | Films based on gelatin, starch, and glycerol |
| GS-MeFPIA1 | Films based on gelatin, starch, glycerol, and MeFPIA1 |
| GS-MeFPIA2 | Films based on gelatin, starch, glycerol, and MeFPIA2 |
| GS-D | Films based on gelatin, starch, glycerol, and dopamine |
| GS-MeFPIA1D | Films based on gelatin, starch, glycerol, MeFPIA1, and dopamine |
| GS-MeFPIA2D | Films based on gelatin, starch, glycerol, MeFPIA2, and dopamine |
| Formulations | Tensile Strength (MPa) | Young’s Modulus (Mpa) | Elongation at Break (%) |
|---|---|---|---|
| GS | 2.86 ± 0.32 a | 15.7 ± 3.0 a | 140 ± 6 a |
| GS-MeFPIA1 | 5.24 ± 0.26 c | 12.2 ± 2.4 abc | 199 ± 7 c |
| GS-MeFPIA2 | 4.18 ± 0.51 b | 12.6 ± 4.8 abc | 170 ± 7 bd |
| GS-D | 3.07 ± 0.15 ad | 16.4 ± 2.0 a | 146 ± 10 a |
| GS-MeFPIA1D | 3.59 ± 0.29 d | 10.9 ± 4.0 bc | 183 ± 5 d |
| GS-MeFPIA2D | 3.32 ± 0.29 ad | 9.9 ± 1.9 bc | 166 ± 12 b |
| Formulations | L* | a* | b* | ΔL* | Δa* | Δb* | ΔE* |
|---|---|---|---|---|---|---|---|
| GS | 89 ± 1 a | 1 ± 0.02 a | −1 ± 0.1 a | ||||
| GS-MeFPIA1 | 87 ± 1 a | −3 ± 0.2 c | 18 ± 1 c | −2 | −4 | 18 | 19 |
| GS-MeFPIA2 | 83 ± 1 c | −3 ± 0.2 c | 34 ± 4 b | −6 | −4 | 35 | 36 |
| GS-D | 56 ± 3 b | 9 ± 1 b | 34 ± 1 b | −33 | 7 | 35 | 49 |
| GS-MeFPIA1D | 62 ± 2 e | 8 ± 1 b | 37 ± 1 b | −27 | 6 | 38 | 47 |
| GS-MeFPIA2D | 47 ± 1 d | 16 ± 0.4 d | 36 ± 2 b | −42 | 14 | 37 | 58 |
| Formulations | Pwexp (g·s−1·m−1·Pa−1) |
|---|---|
| GS | 8.7 ± 0.1 b |
| GS-MeFPIA1 | 11.1 ± 0.1 e |
| GS-MeFPIA2 | 9.3 ± 0.1 c |
| GS-D | 8.4 ± 0.1 a |
| GS-MeFPIA1D | 9.9 ± 0.1 d |
| GS-MeFPIA2D | 8.5 ± 0.1 a |
| Formulations | Temp. (°C) | h∞ (g/g) | Δh1 (g/g) | τ1 (min) | φ1 | Δh2 (g/g) | τ2 (min) | φ2 |
|---|---|---|---|---|---|---|---|---|
| GS | 25 | 336 ± 7 | 118 ± 4 | 7.6 ± 0.4 | 0.35 | 218 ± 3 | 85 ± 4 | 0.65 |
| GS-MeFPIA1 | 215 ± 5 | 109 ± 3 | 4.0 ± 0.2 | 0.51 | 106 ± 2 | 50 ± 2 | 0.49 | |
| GS-MeFPIA2 | 277 ± 7 | 114 ± 4 | 4.6 ± 0.3 | 0.41 | 163 ± 3 | 65 ± 3 | 0.59 | |
| GS-D | 436 ±10 | 132 ± 6 | 5.7 ± 0.4 | 0.30 | 304 ± 4 | 93 ± 5 | 0.70 | |
| GS-MeFPIA1D | 260 ± 11 | 123 ± 6 | 3.0 ± 0.4 | 0.47 | 137 ± 5 | 38 ± 3 | 0.53 | |
| GS-MeFPIA2D | 273 ± 5 | 117 ± 3 | 5.1 ± 0.3 | 0.43 | 156 ± 2 | 73 ± 3 | 0.57 | |
| GS | 37 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| GS-MeFPIA1 | 436 ± 7 | 118 ± 4 | 3.4 ± 0.2 | 0.27 | 318 ± 3 | 36 ± 1 | 0.73 | |
| GS-MeFPIA2 | 345 ± 7 | 137 ± 4 | 4.5 ± 0.3 | 0.40 | 208 ± 3 | 76 ± 4 | 0.60 | |
| GS-D | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| GS-MeFPIA1D | 324 ± 10 | 125 ± 6 | 3.1 ± 0.4 | 0.39 | 199 ± 4 | 46 ± 2 | 0.61 | |
| GS-MeFPIA2D | 388 ± 8 | 129 ± 5 | 4.6 ± 0.4 | 0.33 | 259 ± 3 | 78 ± 4 | 0.67 |
| Polymers | MIC (mg/mL) | ||
|---|---|---|---|
| E. coli | P. aeruginosa | S. aureus | |
| MeFPIA1 | 8 | 2 | 0.5 |
| MeFPIA2 | 8 | 4 | 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cottet, C.; Salvay, A.G.; Peltzer, M.A.; Fernández-García, M. Incorporation of Poly(Itaconic Acid) with Quaternized Thiazole Groups on Gelatin-Based Films for Antimicrobial-Active Food Packaging. Polymers 2021, 13, 200. https://doi.org/10.3390/polym13020200
Cottet C, Salvay AG, Peltzer MA, Fernández-García M. Incorporation of Poly(Itaconic Acid) with Quaternized Thiazole Groups on Gelatin-Based Films for Antimicrobial-Active Food Packaging. Polymers. 2021; 13(2):200. https://doi.org/10.3390/polym13020200
Chicago/Turabian StyleCottet, Celeste, Andrés G. Salvay, Mercedes A. Peltzer, and Marta Fernández-García. 2021. "Incorporation of Poly(Itaconic Acid) with Quaternized Thiazole Groups on Gelatin-Based Films for Antimicrobial-Active Food Packaging" Polymers 13, no. 2: 200. https://doi.org/10.3390/polym13020200
APA StyleCottet, C., Salvay, A. G., Peltzer, M. A., & Fernández-García, M. (2021). Incorporation of Poly(Itaconic Acid) with Quaternized Thiazole Groups on Gelatin-Based Films for Antimicrobial-Active Food Packaging. Polymers, 13(2), 200. https://doi.org/10.3390/polym13020200