The Quantitative Nanomechanical Mapping of Starch/Kaolin Film Surfaces by Peak Force AFM
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
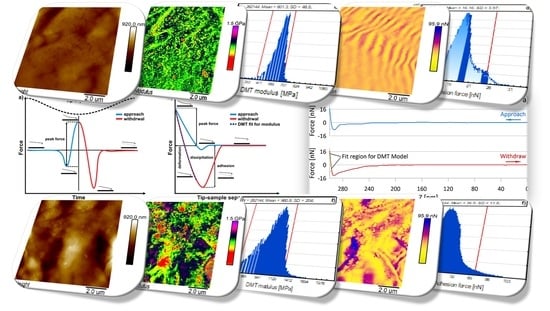
2.2. AFM PeakForce QNM
2.2.1. AFM PFQNM Calibration
2.2.2. DMT Modulus
2.2.3. Adhesion Force
2.3. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Youssef, A.M.; El-Sayed, S.M. Bionanocomposites materials for food packaging applications: Concepts and future outlook. Carbohydr. Polym. 2018, 193, 19–27. [Google Scholar] [CrossRef]
- John, M.J.; Thomas, S. Biofibres and biocomposites. Carbohydr. Polym. 2008, 71, 343–364. [Google Scholar] [CrossRef]
- Averous, L.; Boquillon, N. Biocomposites based on plasticized starch: Thermal and mechanical behaviours. Carbohydr. Polym. 2004, 56, 111–122. [Google Scholar] [CrossRef]
- Ponce, A.; Roura, S.I.; Moreira, M.R. Casein and Chitosan Polymers: Use in Antimicrobial Packaging. In Antimicrobial Food Packaging; Academic Press: Cambridge, MA, USA, 2016; pp. 455–466. [Google Scholar] [CrossRef]
- Arboleda, G.A.; Montilla, C.E.; Villada, H.S.; Varona, G.A. Obtaining a Flexible Film Elaborated from Cassava Thermoplastic Starch and Polylactic Acid. Int. J. Polym. Sci. 2015, 2015, 627268. [Google Scholar] [CrossRef]
- Panaitescu, D.M.; Frone, A.N.; Ghiurea, M.; Chiulan, I. Influence of storage conditions on starch/PVA films containing cellu-lose nanofibers. Ind. Crops Prod. 2015, 70, 170–177. [Google Scholar] [CrossRef]
- Aydemir, D.; Gardner, D.J. Biopolymer blends of polyhydroxybutyrate and polylactic acid reinforced with cellulose nano-fibrils. Carbohydr. Polym. 2020, 250, 116867. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, J.; Fortunati, E.; Vargas, M.; Chiralt, A.; Kenny, J.M. Effects of chitosan on the physicochemical and antimicrobial properties of PLA films. J. Food Eng. 2013, 119, 236–243. [Google Scholar] [CrossRef]
- Ma, X.; Yu, J.; Kennedy, J.F. Studies on the properties of natural fibers-reinforced thermoplastic starch composites. Carbohydr. Polym. 2005, 62, 19–24. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, P.; Zhang, Y.; Fan, B.; Zhu, M.; Wu, Z. Thermoplastic starch modified with hydrophobic polyurethane microparticles. Starch Stärke 2011, 63, 373–380. [Google Scholar] [CrossRef]
- Vigneshwaran, N.; Ammayappan, L.; Huang, Q. Effect of Gum arabic on distribution behavior of nanocellulose fillers in starch film. Appl. Nanosci. 2011, 1, 137–142. [Google Scholar] [CrossRef] [Green Version]
- Erba, S.Ç.; Baştürk, S.B. Fabrication and Characterization of Nanoclay-Reinforced Thermoplastic Composite Films. Mater. Tehnol. 2019, 53, 87–94. [Google Scholar] [CrossRef]
- Cyras, V.P.; Manfredi, L.B.; Ton-That, M.T.; Vázquez, A. Physical and mechanical properties of thermoplastic starch/montmorillonite nanocomposite films. Carbohydr. Polym. 2008, 73, 55–63. [Google Scholar] [CrossRef]
- Cheviron, P.; Gouanvé, F.; Espuche, E. Preparation, characterization and barrier properties of silver/montmorillonite/starch nanocomposite films. J. Membr. Sci. 2016, 497, 162–171. [Google Scholar] [CrossRef]
- Kwasniewska, A.; Muszynski, S.; Tatarczak, J.; Gładyszewski, G.; Gładyszewska, B. Wollastonite-filled and Arabic gum-modified starch films. Part 1. Mechanical and structural properties. Przem. Chem. 2016, 95, 2239–2241. [Google Scholar] [CrossRef]
- Kwaśniewska, A.; Chocyk, D.; Gładyszewski, G.; Borc, J.; Świetlicki, M.; Gładyszewska, B. The Influence of Kaolin Clay on the Mechanical Properties and Structure of Thermoplastic Starch Films. Polymers 2020, 12, 73. [Google Scholar] [CrossRef] [Green Version]
- Mbey, J.A.; Hoppe, S.; Thomas, F. Cassava starch-kaolinite composite film. Effect of clay content and clay modification on film properties. Carbohydr. Polym. 2012, 88, 213–222. [Google Scholar] [CrossRef]
- Świetlicki, M.; Chocyk, D.; Klepka, T.; Prószyński, A.; Kwaśniewska, A.; Borc, J.; Gładyszewski, G. The structure and mechanical properties of the surface layer of polypropylene polymers with talc additions. Materials 2020, 13, 698. [Google Scholar] [CrossRef] [Green Version]
- Jose, J.; De, S.K.; Ma’adeed, M.A.A.; Dakua, J.B.; Sreekumar, P.A.; Sougrat, R.; Al-Harthi, M.A. Compatibilizing role of carbon nanotubes in poly(vinyl alcohol)/starch blend. Starch Stärke 2015, 67, 147–153. [Google Scholar] [CrossRef]
- Yurdakul, H.; Durukan, O.; Seyhan, A.T.; Celebi, H.; Oksuzoglu, M.; Turan, S. Microstructural characterization of corn starch-based porous thermoplastic composites filled with multiwalled carbon nanotubes. J. Appl. Polym. Sci. 2013, 127, 812–820. [Google Scholar] [CrossRef]
- Bootklad, M.; Kaewtatip, K. Biodegradability, mechanical, and thermal properties of thermoplastic starch/cuttlebone composites. Polym. Polym. Compos. 2015, 36, 101–113. [Google Scholar] [CrossRef]
- Campos-Requena, V.H.; Rivas, B.L.; Pérez, M.A.; Figueroa, C.R.; Figueroa, N.E.; Sanfuentes, E.A. Thermoplastic starch/clay nanocomposites loaded with essential oil constituents as packaging for strawberries—In vivo antimicrobial synergy over Bo-trytis cinerea. Postharvest Biol. Technol. 2017, 129, 29–36. [Google Scholar] [CrossRef]
- Souza, A.C.; Goto, G.E.O.; Mainardi, J.A.; Coelho, A.C.V.; Tadini, C.C. Cassava starch composite films incorporated with cinnamon essential oil: Antimicrobial activity, microstructure, mechanical and barrier properties. LWT Food Sci. Technol. 2013, 54, 346–352. [Google Scholar] [CrossRef]
- Muszyński, S.; Kwaśniewska, A.; Sołowiej, B.; Tomczyk, A.; Leus, A.; Szymanek, M.; Siedliska, K.; Gladyszewska, B. Physical properties of kaolin clay-containing pectin gels. Właściwości fizyczne żeli pektynowych zawierających glinkę kaolinową. Przem. Chem. 2017, 96, 422–426. [Google Scholar] [CrossRef]
- Zhang, B.; Chang, Z.; Li, J.; Li, X.; Kan, Y.; Gao, Z. Effect of kaolin content on the performances of kaolin-hybridized soybean meal-based adhesives for wood composites. Compos. Part B Eng. 2019, 173, 1–8. [Google Scholar] [CrossRef]
- Adamcik, J.; Berquand, A.; Mezzenga, R. Single-step direct measurement of amyloid fibrils stiffness by peak force quantitative nanomechanical atomic force microscopy. Appl. Phys. Lett. 2011, 98, 193701. [Google Scholar] [CrossRef]
- Moosburger-Will, J.; Jäger, J.; Horn, S.; Wellhausen, C. Investigation of phase morphology of polyetherimide-toughened epoxy resin by scanning probe microscopy. Polym. Test. 2012, 31, 1008–1018. [Google Scholar] [CrossRef]
- Pittenger, B.; Erina, N.; Su, C. Application Note—Quantitative Mechanical Property Mapping at the Nanoscale with PeakForce QNM.; Bruker: Billerica, MA, USA, 2010. [Google Scholar] [CrossRef]
- Young, T.J.; Monclus, M.A.; Burnett, T.L.; Broughton, W.R.; Ogin, S.L.; Smith, P.A. The use of the PeakForceTM quantitative nanomechanical mapping AFM-based method for high-resolution Young’s modulus measurement of polymers. Meas. Sci. Technol. 2011, 22, 125703. [Google Scholar] [CrossRef] [Green Version]
- Nahar, S.N.; Schmets, A.J.M.; Schitter, G.; Scarpas, A. Quantitative nanomechanical property mapping of bitumen micro-phases by peak-force Atomic Force Microscopy. In Proceedings of the International Conference Asphalt Pavements, ISAP 2014, Raleigh, NC, USA, 1–5 June 2014; Volume 2, pp. 1397–1406. [Google Scholar] [CrossRef]
- Banerjee, S.S.; Kumar, K.D.; Sikder, A.K.; Bhowmick, A.K. Nanomechanics and Origin of Rubber Elasticity of Novel Nanostructured Thermoplastic Elastomeric Blends Using Atomic Force Microscopy. Macromol. Chem. Phys. 2015, 216, 1666–1674. [Google Scholar] [CrossRef]
- Ren, M.; Shi, T.; Corr, D.J.; Shah, S.P. Mechanical Properties of Micro-regions in Cement-based Material based on the Peak-Force QNM Mode of AFM. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2019, 34, 893–899. [Google Scholar] [CrossRef]
- Zhao, B.; Song, Y.; Wang, S.; Dai, B.; Zhang, L.; Dong, Y.; Lü, J.; Hu, J. Mechanical mapping of nanobubbles by PeakForce atomic force microscopy. Soft Matter 2013, 9, 8837–8843. [Google Scholar] [CrossRef]
- Smolyakov, G.; Formosa-Dague, C.; Severac, C.; Duval, R.E.; Dague, E. High speed indentation measures by FV, QI and QNM introduce a new understanding of bionanomechanical experiments. Micron 2016, 85, 8–14. [Google Scholar] [CrossRef]
- Dokukin, M.E.; Sokolov, I. Quantitative Mapping of the Elastic Modulus of Soft Materials with HarmoniX and PeakForce QNM AFM Modes. Langmuir 2012, 28, 16060–16071. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, H.; Gou, J.; Ying, J.; Wang, Y.; Liu, C.; Shen, C. Quantitative nanomechanical mapping on poly(lactic ac-id)/poly(ε-caprolactone)/carbon nanotubes bionanocomposites using atomic force microscopy. Polym. Test. 2019, 77, 105904. [Google Scholar] [CrossRef]
- Qu, Z.; Bu, J.; Pan, X.; Hu, X. Probing the nanomechanical properties of PLA/PC blends compatibilized with compatibilizer and nucleation agent by AFM. J. Polym. Res. 2018, 25, 138. [Google Scholar] [CrossRef]
- Smolyakov, G.; Pruvost, S.; Cardoso, L.; Alonso, B.; Belamie, E.; Duchet-Rumeau, J. AFM PeakForce QNM mode: Evidencing nanometre-scale mechanical properties of chitin-silica hybrid nanocomposites. Carbohydr. Polym. 2016, 151, 373–380. [Google Scholar] [CrossRef]
- Hopf, J.; Pierce, E.M. Topography and Mechanical Property Mapping of International Simple Glass Surfaces with Atomic Force Microscopy. Procedia Mater. Sci. 2014, 7, 216–222. [Google Scholar] [CrossRef] [Green Version]
- Zeng, G.; Dirscherl, K.; Garnæs, J. Toward Accurate Quantitative Elasticity Mapping of Rigid Nanomaterials by Atomic Force Microscopy: Effect of Acquisition Frequency, Loading Force, and Tip Geometry. Nanomaterials 2018, 8, 616. [Google Scholar] [CrossRef] [Green Version]
- Xu, K.; Sun, W.; Shao, Y.; Wei, F.; Zhang, X.; Wang, W.; Li, P. Recent development of PeakForce Tapping mode atomic force microscopy and its applications on nanoscience. Nanotechnol. Rev. 2018, 7, 605–621. [Google Scholar] [CrossRef]
- Derjaguin, B.V.; Muller, V.M.; Toporov, Y.P. Effect of contact deformations on the adhesion of particles. J. Colloid Interface Sci. 1975, 53, 314–326. [Google Scholar] [CrossRef]
- Świetlicka, I.; Muszyński, S.; Kwaśniewska, A.; Świetlicki, M.; Gołacki, K.; Gładyszewska, B. Wollastonite-filled and arabic gum-modified starch films. Part 4. Surface nanostructure. Folie skrobiowe napełniane wollastonitem i modyfikowane gumaą arabskaą. Cz. IV. Nanostruktura powierzchni. Przem. Chem. 2017, 96, 1135–1138. [Google Scholar] [CrossRef]
- Chivrac, F.; Pollet, E.; Schmutz, M.; Avérous, L. Starch nano-biocomposites based on needle-like sepiolite clays. Carbohydr. Polym. 2010, 80, 145–153. [Google Scholar] [CrossRef]
- Krotil, H.U.; Stifter, T.; Waschipky, H.; Weishaupt, K.; Hild, S.; Marti, O. Pulsed force mode: A new method for the investiga-tion of surface properties. Surf. Interface Anal. 1999, 27, 336–340. [Google Scholar] [CrossRef]
- Vlassov, S.; Oras, S.; Antsov, M.; Sosnin, I.; Polyakov, B.; Shutka, A.; Dorogin, L.M. Adhesion and Mechanical Properties of PDMS-Based Materials Probed with AFM: A Review. Rev. Adv. Mater. Sci. 2018, 56, 62–78. [Google Scholar] [CrossRef]
- Laitinen, O.; Bauer, K.; Niinimäki, J.; Peuker, U.A. Validity of the Rumpf and the Rabinovich adhesion force models for alu-mina substrates with nanoscale roughness. Powder Technol. 2013, 246, 545–552. [Google Scholar] [CrossRef]






| Sample | DMT Modulus, (MPa) | Adhesion Force, (nN) | Contact Angle, (°) |
|---|---|---|---|
| k0 | 598.54 ± 65.12 a | 14.18 ± 3.0 a | 92.69 ± 8.82 a |
| k5 | 757.60 ± 115.25 b | 34.5 ± 8.53 ab | 48.03 ± 12.1 b |
| k10 | 1018.71 ± 297.21 c | 42.57 ± 11.92 b | 46.66 ± 11.58 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwaśniewska, A.; Świetlicki, M.; Prószyński, A.; Gładyszewski, G. The Quantitative Nanomechanical Mapping of Starch/Kaolin Film Surfaces by Peak Force AFM. Polymers 2021, 13, 244. https://doi.org/10.3390/polym13020244
Kwaśniewska A, Świetlicki M, Prószyński A, Gładyszewski G. The Quantitative Nanomechanical Mapping of Starch/Kaolin Film Surfaces by Peak Force AFM. Polymers. 2021; 13(2):244. https://doi.org/10.3390/polym13020244
Chicago/Turabian StyleKwaśniewska, Anita, Michał Świetlicki, Adam Prószyński, and Grzegorz Gładyszewski. 2021. "The Quantitative Nanomechanical Mapping of Starch/Kaolin Film Surfaces by Peak Force AFM" Polymers 13, no. 2: 244. https://doi.org/10.3390/polym13020244
APA StyleKwaśniewska, A., Świetlicki, M., Prószyński, A., & Gładyszewski, G. (2021). The Quantitative Nanomechanical Mapping of Starch/Kaolin Film Surfaces by Peak Force AFM. Polymers, 13(2), 244. https://doi.org/10.3390/polym13020244