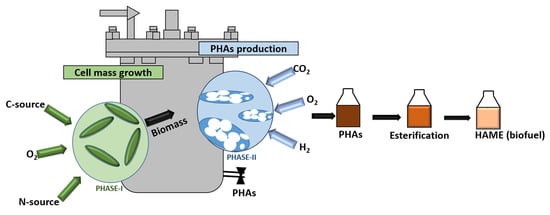
Polyhydroxyalkanoates (PHAs): Biopolymers for Biofuel and Biorefineries
Abstract
:1. Introduction
2. Sources of PHAs
2.1. Production of PHAs
2.2. Fermentation Industry
2.3. Microbial Synthesis of PHAs Homopolymers
2.4. Microbial Synthesis of PHAs Copolymers
2.5. Microbial Production of PHA Block Copolymers
3. Biofuels Based on PHAs
4. Parameters Affecting HAME-Based Biofuels Production
4.1. Reaction Time
4.2. Reaction Temperature
4.3. Type and Content of Alcohol
4.4. Catalyst Type and Concentration
5. PHAs Biorefineries
6. Conclusions and Future Directions
- Petroleum shortage for plastic materials.
- Reduced CO2 emissions.
- Environmental protection.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, G.-Q. A microbial polyhydroxyalkanoates (PHA) based bio-and materials industry. Chem. Soc. Rev. 2009, 38, 2434–2446. [Google Scholar] [CrossRef] [PubMed]
- Sudesh, K.; Abe, H.; Doi, Y. Synthesis, structure and properties of polyhydroxyalkanoates: Biological polyesters. Prog. Polym. Sci. 2000, 25, 1503–1555. [Google Scholar] [CrossRef]
- Chanprateep, S. Current trends in biodegradable polyhydroxyalkanoates. J. Biosci. Bioeng. 2010, 110, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-Q.; Patel, M.K. Plastics derived from biological sources: Present and future: A technical and environmental review. Chem. Rev. 2012, 112, 2082–2099. [Google Scholar] [CrossRef] [PubMed]
- Lemoigne, M. Produits de deshydration et de polymerisation de l’acide β= oxybutyrique. Bull. Soc. Chim. Biol. 1926, 8, 770–782. [Google Scholar]
- Raza, Z.A.; Abid, S.; Banat, I.M. Polyhydroxyalkanoates: Characteristics, production, recent developments and applications. Int. Biodeterior. Biodegrad. 2018, 126, 45–56. [Google Scholar] [CrossRef]
- Ma, Y.-M.; Wei, D.-X.; Yao, H.; Wu, L.-P.; Chen, G.-Q. Synthesis, characterization and application of thermoresponsive polyhydroxyalkanoate-graft-poly (N-isopropylacrylamide). Biomacromolecules 2016, 17, 2680–2690. [Google Scholar] [CrossRef]
- Matsumoto, K.i.; Hori, C.; Fujii, R.; Takaya, M.; Ooba, T.; Ooi, T.; Isono, T.; Satoh, T.; Taguchi, S. Dynamic changes of intracellular monomer levels regulate block sequence of polyhydroxyalkanoates in engineered Escherichia coli. Biomacromolecules 2018, 19, 662–671. [Google Scholar] [CrossRef]
- Mizuno, S.; Enda, Y.; Saika, A.; Hiroe, A.; Tsuge, T. Biosynthesis of polyhydroxyalkanoates containing 2-hydroxy-4-methylvalerate and 2-hydroxy-3-phenylpropionate units from a related or unrelated carbon source. J. Biosci. Bioeng. 2018, 125, 295–300. [Google Scholar] [CrossRef]
- Koller, M.; Mukherjee, A. Polyhydroxyalkanoates—Linking properties, applications, and end-of-life options. Chem. Biochem. Eng. Q. 2020, 34, 115–129. [Google Scholar] [CrossRef]
- David, Y.; Joo, J.C.; Yang, J.E.; Oh, Y.H.; Lee, S.Y.; Park, S.J. Biosynthesis of 2-hydroxyacid-containing polyhydroxyalkanoates by employing butyryl-CoA transferases in metabolically engineered Escherichia coli. Biotechnol. J. 2017, 12, 1700116. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Hu, D.; Che, X.; Jiang, X.; Li, T.; Chen, J.; Zhang, H.M.; Chen, G.-Q. Engineering of Halomonas bluephagenesis for low cost production of poly (3-hydroxybutyrate-co-4-hydroxybutyrate) from glucose. Metab. Eng. 2018, 47, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Chae, C.G.; Kang, K.H.; Oh, Y.H.; Joo, J.C.; Song, B.K.; Lee, S.Y.; Park, S.J. Biosynthesis of Lactate-containing polyhydroxyalkanoates in recombinant Escherichia coli by employing new CoA transferases. KSBB J. 2016, 31, 27–32. [Google Scholar] [CrossRef] [Green Version]
- Reddy, C.; Ghai, R.; Kalia, V.C. Polyhydroxyalkanoates: An overview. Bioresour. Technol. 2003, 87, 137–146. [Google Scholar] [CrossRef]
- Verlinden, R.A.; Hill, D.J.; Kenward, M.; Williams, C.D.; Radecka, I. Bacterial synthesis of biodegradable polyhydroxyalkanoates. J. Appl. Microbiol. 2007, 102, 1437–1449. [Google Scholar] [CrossRef]
- Khanna, S.; Srivastava, A.K. Recent advances in microbial polyhydroxyalkanoates. Process. Biochem. 2005, 40, 607–619. [Google Scholar] [CrossRef]
- Adeleye, A.T.; Odoh, C.K.; Enudi, O.C.; Banjoko, O.O.; Osigbeminiyi, O.O.; Toluwalope, O.E.; Louis, H. Sustainable synthesis and applications of polyhydroxyalkanoates (PHAs) from biomass. Process. Biochem. 2020, 96, 174–193. [Google Scholar] [CrossRef]
- Tamang, P.; Banerjee, R.; Köster, S.; Nogueira, R. Comparative study of polyhydroxyalkanoates production from acidified and anaerobically treated brewery wastewater using enriched mixed microbial culture. J. Environ. Sci. 2019, 78, 137–146. [Google Scholar] [CrossRef]
- Matias, F.; Rodrigues, M.F.D.A. New PHA products using unrelated carbon sources. Braz. J. Microbiol. 2011, 42, 1354–1363. [Google Scholar] [CrossRef] [Green Version]
- Pavan, F.A.; Junqueira, T.L.; Watanabe, M.D.; Bonomi, A.; Quines, L.K.; Schmidell, W.; de Aragao, G.M. Economic analysis of polyhydroxybutyrate production by Cupriavidus necator using different routes for product recovery. Biochem. Eng. J. 2019, 146, 97–104. [Google Scholar] [CrossRef]
- Harmaen, A.; Khalina, A.; Ali, H.M.; Azowa, I. Thermal, morphological, and biodegradability properties of bioplastic fertilizer composites made of oil palm biomass, fertilizer, and poly (hydroxybutyrate-co-valerate). Int. J. Polym. Sci. 2016, 2016, 3230109. [Google Scholar] [CrossRef] [Green Version]
- Gebelein, C.G. New and traditional polymers from biotechnology. In Biotechnology and Polymers; Springer: Berlin/Heidelberg, Germany, 1991; pp. 1–9. [Google Scholar]
- Francis, L.; Keshavarz, T.; Roy, I. Biosynthesis of polyhydroxyalkanoates and their applications. In Proceedings of the International Conference on Biodegradable Polymers: Their Production, Characterisation and Application, London, UK, 10 December 2007. [Google Scholar]
- Moradali, M.F.; Rehm, B.H. Bacterial biopolymers: From pathogenesis to advanced materials. Nat. Rev. Genet. 2020, 18, 195–210. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-Q.; Hajnal, I.; Wu, H.; Lv, L.; Ye, J. Engineering biosynthesis mechanisms for diversifying polyhydroxyalkanoates. Trends Biotechnol. 2015, 33, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Long, J.-Y.; Song, K.-L.; He, X.; Zhang, B.; Cui, X.-F.; Song, C.-F. Mutagenesis of phaR, a regulator gene of polyhydroxyalkanoate biosynthesis of Xanthomonas oryzae pv. oryzae caused pleiotropic phenotype changes. Front. Microbiol. 2018, 9, 3046. [Google Scholar] [CrossRef]
- Coats, E.R.; Watson, B.S.; Brinkman, C.K. Polyhydroxyalkanoate synthesis by mixed microbial consortia cultured on fermented dairy manure: Effect of aeration on process rates/yields and the associated microbial ecology. Water Res. 2016, 106, 26–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham, T.H.; Webb, J.S.; Rehm, B.H. The role of polyhydroxyalkanoate biosynthesis by Pseudomonas aeruginosa in rhamnolipid and alginate production as well as stress tolerance and biofilm formation. Microbiology 2004, 150, 3405–3413. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Miro, M.; Chen, S.; Gonzaga, Z.J.; Evert, B.; Wibowo, D.; Rehm, B.H. Polyester as antigen carrier toward particulate vaccines. Biomacromolecules 2019, 20, 3213–3232. [Google Scholar] [CrossRef]
- Gao, X.; Chen, J.-C.; Wu, Q.; Chen, G.-Q. Polyhydroxyalkanoates as a source of chemicals, polymers, and biofuels. Curr. Opin. Biotechnol. 2011, 22, 768–774. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, C.; Xian, M.; Zhang, Y.; Zhao, G. Biosynthetic pathway for poly (3-hydroxypropionate) in recombinant Escherichia coli. J. Microbiol. 2012, 50, 693–697. [Google Scholar] [CrossRef]
- Castilho, L.R.; Mitchell, D.A.; Freire, D.M. Production of polyhydroxyalkanoates (PHAs) from waste materials and by-products by submerged and solid-state fermentation. Bioresour. Technol. 2009, 100, 5996–6009. [Google Scholar] [CrossRef]
- Carvalho, G.; Oehmen, A.; Albuquerque, M.G.; Reis, M.A. The relationship between mixed microbial culture composition and PHA production performance from fermented molasses. New. Bitechnol. 2014, 31, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, S.; Hallquist, J.; Werker, A.; Welander, T. Acidogenic fermentation of industrial wastewaters: Effects of chemostat retention time and pH on volatile fatty acids production. Biochem. Eng. J. 2008, 40, 492–499. [Google Scholar] [CrossRef]
- Bhubalan, K.; Lee, W.-H.; Loo, C.-Y.; Yamamoto, T.; Tsuge, T.; Doi, Y.; Sudesh, K. Controlled biosynthesis and characterization of poly (3-hydroxybutyrate-co-3-hydroxyvalerate-co-3-hydroxyhexanoate) from mixtures of palm kernel oil and 3HV-precursors. Polym. Degrad. Stab. 2008, 93, 17–23. [Google Scholar] [CrossRef]
- Olivera, E.R.; Carnicero, D.; Jodra, R.; Miñambres, B.; García, B.; Abraham, G.A.; Gallardo, A.; Román, J.S.; García, J.L.; Naharro, G. Genetically engineered Pseudomonas: A factory of new bioplastics with broad applications. Environ. Microbiol. 2001, 3, 612–618. [Google Scholar] [CrossRef]
- Anjum, A.; Zuber, M.; Zia, K.M.; Noreen, A.; Anjum, M.N.; Tabasum, S. Microbial production of polyhydroxyalkanoates (PHAs) and its copolymers: A review of recent advancements. Int. J. Biol. Macromol. 2016, 89, 161–174. [Google Scholar] [CrossRef]
- Linares-Pastén, J.A.; Sabet-Azad, R.; Pessina, L.; Sardari, R.R.; Ibrahim, M.H.; Hatti-Kaul, R. Efficient poly (3-hydroxypropionate) production from glycerol using Lactobacillus reuteri and recombinant Escherichia coli harboring L. reuteri propionaldehyde dehydrogenase and Chromobacterium sp. PHA synthase genes. Bioresour. Technol. 2015, 180, 172–176. [Google Scholar] [CrossRef]
- Valentin, H.E.; Schönebaum, A.; Steinbüchel, A. Identification of 4-hydroxyvaleric acid as a constituent of biosynthetic polyhydroxyalkanoic acids from bacteria. Appl. Microbiol. Biotechnol. 1992, 36, 507–514. [Google Scholar] [CrossRef]
- Steinbüchel, A.; Schmack, G. Large-scale production of poly (3-hydroxyvaleric acid) by fermentation of Chromobacterium violaceum, processing, and characterization of the homopolyester. J. Environ. Polym. Degrad. 1995, 3, 243–258. [Google Scholar] [CrossRef]
- Haywood, G.W.; Anderson, A.J.; Ewing, D.F.; Dawes, E.A. Accumulation of a polyhydroxyalkanoate containing primarily 3-hydroxydecanoate from simple carbohydrate substrates by Pseudomonas sp. strain NCIMB 40135. Appl. Environ. Microbiol. 1990, 56, 3354–3359. [Google Scholar] [CrossRef] [Green Version]
- Jian, J.; Li, Z.-J.; Ye, H.-M.; Yuan, M.-Q.; Chen, G.-Q. Metabolic engineering for microbial production of polyhydroxyalkanoates consisting of high 3-hydroxyhexanoate content by recombinant Aeromonas hydrophila. Bioresour. Technol. 2010, 101, 6096–6102. [Google Scholar] [CrossRef]
- Steinbüchel, A.; Valentin, H.E.; Schönebaum, A. Application of recombinant gene technology for production of polyhydroxyalkanoic acids: Biosynthesis of poly (4-hydroxybutyric acid) homopolyester. J. Environ. Polym. Degrad. 1994, 2, 67–74. [Google Scholar] [CrossRef]
- Anderson, A.J.; Haywood, G.W.; Dawes, E.A. Biosynthesis and composition of bacterial poly (hydroxyalkanoates). Int. J. Biol. Macromol. 1990, 12, 102–105. [Google Scholar] [CrossRef]
- Chung, C.-W.; Kim, Y.-S.; Kim, Y.-B.; Bae, K.-S.; Rhee, Y.-H. Isolation of a Pseudomonas sp. strain exhibiting unusual behavior of poly (3-hydroxyalkanoates) biosynthesis and characterization of synthesized polyesters. J. Microbiol. Biotechnol. 1999, 9, 847–853. [Google Scholar]
- Gumel, A.M.; Annuar, M.S.M.; Heidelberg, T. Biosynthesis and characterization of polyhydroxyalkanoates copolymers produced by Pseudomonas putida Bet001 isolated from palm oil mill effluent. PLoS ONE 2012, 7, e45214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tripathi, L.; Wu, L.-P.; Dechuan, M.; Chen, J.; Wu, Q.; Chen, G.-Q. Pseudomonas putida KT2442 as a platform for the biosynthesis of polyhydroxyalkanoates with adjustable monomer contents and compositions. Bioresour. Technol. 2013, 142, 225–231. [Google Scholar] [CrossRef]
- Koller, M. Advances in Polyhydroxyalkanoate (PHA) Production; MDPI: Basel, Switzerland, 2017. [Google Scholar]
- Motallebi-Veshareh, M.; Balzer, D.; Lanka, E.; Jagura-Burdzy, G.; Thomas, C.M. Conjugative transfer functions of broad-host-range plasmid RK2 are coregulated with vegetative replication. Mol. Microbiol. 1992, 6, 907–920. [Google Scholar] [CrossRef]
- Chen, G.-Q.; König, K.-H.; Lafferty, R.M. Production of poly-D (-)-3-hydroxybutyrate and poly-D (-)-3-hydroxyvalerate by strains ofAlcaligenes latus. Antonie Van Leeuwenhoek 1991, 60, 61–66. [Google Scholar] [CrossRef]
- Pohlmann, A.; Fricke, W.F.; Reinecke, F.; Kusian, B.; Liesegang, H.; Cramm, R.; Eitinger, T.; Ewering, C.; Pötter, M.; Schwartz, E. Genome sequence of the bioplastic-producing “Knallgas” bacterium Ralstonia eutropha H16. Nat. Biotechnol. 2006, 24, 1257–1262. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Luo, G.; Zhou, X.R.; Chen, G.-Q. Biosynthesis of poly (3-hydroxydecanoate) and 3-hydroxydodecanoate dominating polyhydroxyalkanoates by β-oxidation pathway inhibited Pseudomonas putida. Metab. Eng. 2011, 13, 11–17. [Google Scholar] [CrossRef]
- Wang, H.-H.; Zhou, X.-R.; Liu, Q.; Chen, G.-Q. Biosynthesis of polyhydroxyalkanoate homopolymers by Pseudomonas putida. Appl. Microbiol. Biotechnol. 2011, 89, 1497–1507. [Google Scholar] [CrossRef]
- Liu, W.; Chen, G.-Q. Production and characterization of medium-chain-length polyhydroxyalkanoate with high 3-hydroxytetradecanoate monomer content by fadB and fadA knockout mutant of Pseudomonas putida KT2442. Appl. Microbiol. Biotechnol. 2007, 76, 1153–1159. [Google Scholar] [CrossRef]
- Wang, Y.; Chung, A.; Chen, G.Q. Synthesis of medium-chain-length polyhydroxyalkanoate homopolymers, random copolymers, and block copolymers by an engineered strain of Pseudomonas entomophila. Adv. Healthc. Mater. 2017, 6, 1601017. [Google Scholar] [CrossRef]
- Sharma, P.K.; Munir, R.I.; Blunt, W.; Dartiailh, C.; Cheng, J.; Charles, T.C.; Levin, D.B. Synthesis and physical properties of polyhydroxyalkanoate polymers with different monomer compositions by recombinant Pseudomonas putida LS46 expressing a novel PHA synthase (PhaC116) enzyme. Appl. Sci. 2017, 7, 242. [Google Scholar] [CrossRef]
- Scheel, R.A.; Ji, L.; Lundgren, B.R.; Nomura, C.T. Enhancing poly (3-hydroxyalkanoate) production in Escherichia coli by the removal of the regulatory gene arcA. AMB Express 2016, 6, 120. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.C.; Liou, S.Z.; Lee, C.M. Correlation between bio-hydrogen production and polyhydroxybutyrate (PHB) synthesis by Rhodopseudomonas palustris WP3-5. Bioresour. Technol. 2012, 113, 44–50. [Google Scholar] [CrossRef]
- De Andrade Rodrigues, M.; Valentin, H.; Berger, P.; Tran, M.; Asrar, J.; Gruys, K.; Steinbüchel, A. Polyhydroxyalkanoate accumulation in Burkholderia sp.: A molecular approach to elucidate the genes involved in the formation of two homopolymers consisting of short-chain-length 3-hydroxyalkanoic acids. Appl. Microbiol. Biotechnol. 2000, 53, 453–460. [Google Scholar] [CrossRef]
- Yao, J.; Zhang, G.; Wu, Q.; Chen, G.-Q.; Zhang, R. Production of polyhydroxyalkanoates by Pseudomonas nitroreducens. Antonie Van Leeuwenhoek 1999, 75, 345–349. [Google Scholar] [CrossRef]
- Kato, M.; Bao, H.; Kang, C.-K.; Fukui, T.; Doi, Y. Production of a novel copolyester of 3-hydroxybutyric acid and medium-chain-length 3-hydroxyalkanoic acids by Pseudomonas sp. 61-3 from sugars. Appl. Microbiol. Biotechnol. 1996, 45, 363–370. [Google Scholar] [CrossRef]
- Kimura, H.; Ohura, T.; Takeishi, M.; Nakamura, S.; Doi, Y. Effective microbial production of poly (4-hydroxybutyrate) homopolymer by Ralstonia eutropha H16. Polym. Int. 1999, 48, 1073–1079. [Google Scholar] [CrossRef]
- Wang, H.-H.; Li, X.-T.; Chen, G.-Q. Production and characterization of homopolymer polyhydroxyheptanoate (P3HHp) by a fadBA knockout mutant Pseudomonas putida KTOY06 derived from P. putida KT2442. Process. Biochem. 2009, 44, 106–111. [Google Scholar] [CrossRef]
- Shen, X.-W.; Yang, Y.; Jian, J.; Wu, Q.; Chen, G.-Q. Production and characterization of homopolymer poly (3-hydroxyvalerate)(PHV) accumulated by wild type and recombinant Aeromonas hydrophila strain 4AK4. Bioresour. Technol. 2009, 100, 4296–4299. [Google Scholar] [CrossRef]
- Ramachander, T.; Rohini, D.; Belhekar, A.; Rawal, S. Synthesis of PHB by recombinant E. coli harboring an approximately 5 kb genomic DNA fragment from Streptomyces aureofaciens NRRL 2209. Int. J. Biol. Macromol. 2002, 31, 63–69. [Google Scholar] [CrossRef]
- Valappil, S.P.; Peiris, D.; Langley, G.; Herniman, J.; Boccaccini, A.R.; Bucke, C.; Roy, I. Polyhydroxyalkanoate (PHA) biosynthesis from structurally unrelated carbon sources by a newly characterized Bacillus spp. J. Biotechnol. 2007, 127, 475–487. [Google Scholar] [CrossRef]
- Rodríguez-Contreras, A.; Koller, M.; Miranda-de Sousa Dias, M.; Calafell-Monfort, M.; Braunegg, G.; Marqués-Calvo, M.S. Influence of glycerol on poly (3-hydroxybutyrate) production by Cupriavidus necator and Burkholderia sacchari. Biochem. Eng. J. 2015, 94, 50–57. [Google Scholar] [CrossRef]
- Pais, J.; Farinha, I.; Freitas, F.; Serafim, L.S.; Martínez, V.; Martínez, J.C.; Arévalo-Rodríguez, M.; Prieto, M.A.; Reis, M.A. Improvement on the yield of polyhydroxyalkanotes production from cheese whey by a recombinant Escherichia coli strain using the proton suicide methodology. Enzyme Microb. Technol. 2014, 55, 151–158. [Google Scholar] [CrossRef]
- Kahar, P.; Tsuge, T.; Taguchi, K.; Doi, Y. High yield production of polyhydroxyalkanoates from soybean oil by Ralstonia eutropha and its recombinant strain. Polym. Degrad. Stab. 2004, 83, 79–86. [Google Scholar] [CrossRef]
- Chanprateep, S.; Buasri, K.; Muangwong, A.; Utiswannakul, P. Biosynthesis and biocompatibility of biodegradable poly (3-hydroxybutyrate-co-4-hydroxybutyrate). Polym. Degrad. Stab. 2010, 95, 2003–2012. [Google Scholar] [CrossRef]
- López-Cuellar, M.; Alba-Flores, J.; Rodríguez, J.G.; Pérez-Guevara, F. Production of polyhydroxyalkanoates (PHAs) with canola oil as carbon source. Int. J. Biol. Macromol. 2011, 48, 74–80. [Google Scholar] [CrossRef]
- Koller, M.; Bona, R.; Chiellini, E.; Fernandes, E.G.; Horvat, P.; Kutschera, C.; Hesse, P.; Braunegg, G. Polyhydroxyalkanoate production from whey by Pseudomonas hydrogenovora. Bioresour. Technol. 2008, 99, 4854–4863. [Google Scholar] [CrossRef]
- Althuri, A.; Mathew, J.; Sindhu, R.; Banerjee, R.; Pandey, A.; Binod, P. Microbial synthesis of poly-3-hydroxybutyrate and its application as targeted drug delivery vehicle. Bioresour. Technol. 2013, 145, 290–296. [Google Scholar] [CrossRef]
- Suwannasing, W.; Imai, T.; Kaewkannetra, P. Cost-effective defined medium for the production of polyhydroxyalkanoates using agricultural raw materials. Bioresour. Technol. 2015, 194, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.C.; Dias, M.L.; Castilho, L.R.; Freire, D.M. Characterization of poly (3-hydroxybutyrate) produced by Cupriavidus necator in solid-state fermentation. Bioresour. Technol. 2007, 98, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Cavalheiro, J.M.; de Almeida, M.C.M.; Grandfils, C.; Da Fonseca, M. Poly (3-hydroxybutyrate) production by Cupriavidus necator using waste glycerol. Process. Biochem. 2009, 44, 509–515. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, R.-H.; Koutinas, A.; Webb, C. Microbial biodegradable plastic production from a wheat-based biorefining strategy. Process. Biochem. 2010, 45, 153–163. [Google Scholar] [CrossRef]
- Jung, H.-R.; Choi, T.-R.; Han, Y.H.; Park, Y.-L.; Park, J.Y.; Song, H.-S.; Yang, S.-Y.; Bhatia, S.K.; Gurav, R.; Park, H. Production of blue-colored polyhydroxybutyrate (PHB) by one-pot production and coextraction of indigo and PHB from recombinant Escherichia coli. Dyes Pigment. 2020, 173, 107889. [Google Scholar] [CrossRef]
- Gu, J.J.; Zhou, Y.; Tong, Y.B.; Lu, J.J.; Ye, B.C. Efficient production and characterization of homopolymeric poly (3-hydroxyvalerate) produced by Bacillus strain PJC48. Biotechnol. Appl. Biochem. 2018, 65, 622–629. [Google Scholar] [CrossRef]
- Hiroe, A.; Watanabe, S.; Kobayashi, M.; Nomura, C.T.; Tsuge, T. Increased synthesis of poly (3-hydroxydodecanoate) by random mutagenesis of polyhydroxyalkanoate synthase. Appl. Microbiol. Biotechnol. 2018, 102, 7927–7934. [Google Scholar] [CrossRef]
- Chobchuenchom, W.; Tanadchangsaeng, N. Production of medium chain length polyhydroxyalkanoates by Pseudomonas putida ATCC47054 using glycerol and sodium octanoate as substrates. In Proceedings of the RSU International Research Conference, Riga, Latvia, 1–3 April 2019. [Google Scholar]
- Yamada, M.; Yukita, A.; Hanazumi, Y.; Yamahata, Y.; Moriya, H.; Miyazaki, M.; Yamashita, T.; Shimoi, H. Poly (3-hydroxybutyrate) production using mannitol as a sole carbon source by Burkholderia sp. AIU M5M02 isolated from a marine environment. Fish. Sci. 2018, 84, 405–412. [Google Scholar] [CrossRef]
- Kourmentza, C.; Costa, J.; Azevedo, Z.; Servin, C.; Grandfils, C.; De Freitas, V.; Reis, M. Burkholderia thailandensis as a microbial cell factory for the bioconversion of used cooking oil to polyhydroxyalkanoates and rhamnolipids. Bioresour. Technol. 2018, 247, 829–837. [Google Scholar] [CrossRef]
- Możejko-Ciesielska, J.; Kiewisz, R. Bacterial polyhydroxyalkanoates: Still fabulous? Microbiol. Res. 2016, 192, 271–282. [Google Scholar] [CrossRef]
- Li, Z.-J.; Shi, Z.-Y.; Jian, J.; Guo, Y.-Y.; Wu, Q.; Chen, G.-Q. Production of poly (3-hydroxybutyrate-co-4-hydroxybutyrate) from unrelated carbon sources by metabolically engineered Escherichia coli. Metab. Eng. 2010, 12, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Koller, M. Polyhydroxyalkanoate biosynthesis at the edge of water activitiy-haloarchaea as biopolyester factories. Bioengineering 2019, 6, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aldor, I.S.; Kim, S.-W.; Prather, K.L.J.; Keasling, J.D. Metabolic engineering of a novel propionate-independent pathway for the production of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) in recombinant Salmonella enterica serovar typhimurium. Appl. Environ. Microbiol. 2002, 68, 3848–3854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, Y.-Z.; Han, J.; Guo, J.-J.; Chen, G.-Q. Production of poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) from gluconate and glucose by recombinant Aeromonas hydrophila and Pseudomonas putida. Biotechnol. Lett. 2005, 27, 1381–1386. [Google Scholar] [CrossRef] [PubMed]
- Matsusaki, H.; Abe, H.; Taguchi, K.; Fukui, T.; Doi, Y. Biosynthesis of poly (3-hydroxybutyrate-co-3-hydroxyalkanoates) by recombinant bacteria expressing the PHA synthase gene phaC1 from Pseudomonas sp. 61-3. Appl. Microbiol. Biotechnol. 2000, 53, 401–409. [Google Scholar] [CrossRef]
- Yim, K.S.; Lee, S.Y.; Chang, H.N. Synthesis of poly-(3-hydroxybutyrate-co-3-hydroxyvalerate) by recombinant Escherichia coli. Biotechnol. Bioeng. 1996, 49, 495–503. [Google Scholar] [CrossRef]
- Kichise, T.; Fukui, T.; Yoshida, Y.; Doi, Y. Biosynthesis of polyhydroxyalkanoates (PHA) by recombinant Ralstonia eutropha and effects of PHA synthase activity on in vivo PHA biosynthesis. Int. J. Biol. Macromol. 1999, 25, 69–77. [Google Scholar] [CrossRef]
- Tsuge, T.; Yano, K.; Imazu, S.i.; Numata, K.; Kikkawa, Y.; Abe, H.; Taguchi, S.; Doi, Y. Biosynthesis of polyhydroxyalkanoate (PHA) copolymer from fructose using wild-type and laboratory-evolved PHA synthases. Macromol. Biosci. 2005, 5, 112–117. [Google Scholar] [CrossRef]
- Lee, S.H.; Oh, D.H.; Ahn, W.S.; Lee, Y.; Choi, J.i.; Lee, S.Y. Production of poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) by high-cell-density cultivation of Aeromonas hydrophila. Biotechnol. Bioeng. 2000, 67, 240–244. [Google Scholar] [CrossRef]
- Han, J.; Qiu, Y.-Z.; Liu, D.-C.; Chen, G.-Q. Engineered Aeromonas hydrophila for enhanced production of poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) with alterable monomers composition. FEMS Microbiol. Lett. 2004, 239, 195–201. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.S.; Lee, B.H.; Kim, B.S. Production of poly (3-hydroxybutyrate-co-4-hydroxybutyrate) by Ralstonia eutropha. Biochem. Eng. J. 2005, 23, 169–174. [Google Scholar] [CrossRef]
- Rao, U.; Sridhar, R.; Sehgal, P. Biosynthesis and biocompatibility of poly (3-hydroxybutyrate-co-4-hydroxybutyrate) produced by Cupriavidus necator from spent palm oil. Biochem. Eng. J. 2010, 49, 13–20. [Google Scholar] [CrossRef]
- Zhao, W.; Chen, G.-Q. Production and characterization of terpolyester poly (3-hydroxybutyrate-co-3-hydroxyvalerate-co-3-hydroxyhexanoate) by recombinant Aeromonas hydrophila 4AK4 harboring genes phaAB. Process. Biochem. 2007, 42, 1342–1347. [Google Scholar] [CrossRef]
- Lee, W.-H.; Azizan, M.N.; Sudesh, K. Effects of culture conditions on the composition of poly (3-hydroxybutyrate-co-4-hydroxybutyrate) synthesized by Comamonas acidovorans. Polym. Degrad. Stab. 2004, 84, 129–134. [Google Scholar] [CrossRef]
- Lee, W.-H.; Loo, C.-Y.; Nomura, C.T.; Sudesh, K. Biosynthesis of polyhydroxyalkanoate copolymers from mixtures of plant oils and 3-hydroxyvalerate precursors. Bioresour. Technol. 2008, 99, 6844–6851. [Google Scholar] [CrossRef]
- Meng, D.-C.; Shi, Z.-Y.; Wu, L.-P.; Zhou, Q.; Wu, Q.; Chen, J.-C.; Chen, G.-Q. Production and characterization of poly (3-hydroxypropionate-co-4-hydroxybutyrate) with fully controllable structures by recombinant Escherichia coli containing an engineered pathway. Metab. Eng. 2012, 14, 317–324. [Google Scholar] [CrossRef]
- Reddy, S.V.; Thirumala, M.; Mahmood, S. A novel Bacillus sp. accumulating poly (3-hydroxybutyrate-co-3-hydroxyvalerate) from a single carbon substrate. J. Ind. Microbiol. Biotechnol. 2009, 36, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Masood, F.; Hasan, F.; Ahmed, S.; Hameed, A. Biosynthesis and characterization of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) from Bacillus cereus FA11 isolated from TNT-contaminated soil. Ann. Microbiol. 2012, 62, 1377–1384. [Google Scholar] [CrossRef]
- Wong, Y.-M.; Brigham, C.J.; Rha, C.; Sinskey, A.J.; Sudesh, K. Biosynthesis and characterization of polyhydroxyalkanoate containing high 3-hydroxyhexanoate monomer fraction from crude palm kernel oil by recombinant Cupriavidus necator. Bioresour. Technol. 2012, 121, 320–327. [Google Scholar] [CrossRef] [Green Version]
- Tsuge, T.; Yamamoto, T.; Yano, K.; Abe, H.; Doi, Y.; Taguchi, S. Evaluating the ability of polyhydroxyalkanoate synthase mutants to produce P (3HB-co-3HA) from soybean oil. Macromol. Biosci. 2009, 9, 71–78. [Google Scholar] [CrossRef]
- Al-Kaddo, K.B.; Mohamad, F.; Murugan, P.; Tan, J.S.; Sudesh, K.; Samian, M.R. Production of P (3HB-co-4HB) copolymer with high 4HB molar fraction by Burkholderia contaminans Kad1 PHA synthase. Biochem. Eng. J. 2020, 153, 107394. [Google Scholar] [CrossRef]
- Norhafini, H.; Huong, K.-H.; Amirul, A. High PHA density fed-batch cultivation strategies for 4HB-rich P (3HB-co-4HB) copolymer production by transformant Cupriavidus malaysiensis USMAA1020. Int. J. Biol. Macromol. 2019, 125, 1024–1032. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Gurav, R.; Choi, T.-R.; Jung, H.-R.; Yang, S.-Y.; Song, H.-S.; Jeon, J.-M.; Kim, J.-S.; Lee, Y.-K.; Yang, Y.-H. Poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) production from engineered Ralstonia eutropha using synthetic and anaerobically digested food waste derived volatile fatty acids. Int. J. Biol. Macromol. 2019, 133, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.-M.; Brigham, C.J.; Kim, Y.-H.; Kim, H.-J.; Yi, D.-H.; Kim, H.; Rha, C.; Sinskey, A.J.; Yang, Y.-H. Biosynthesis of poly (3-hydroxybutyrate-co-3-hydroxyhexanoate)(P (HB-co-HHx)) from butyrate using engineered Ralstonia eutropha. Appl. Microbiol. Biotechnol. 2014, 98, 5461–5469. [Google Scholar] [CrossRef]
- Jeon, J.-M.; Kim, H.-J.; Bhatia, S.K.; Sung, C.; Seo, H.-M.; Kim, J.-H.; Park, H.-Y.; Lee, D.; Brigham, C.J.; Yang, Y.-H. Application of acetyl-CoA acetyltransferase (AtoAD) in Escherichia coli to increase 3-hydroxyvalerate fraction in poly (3-hydroxybutyrate-co-3-hydroxyvalerate). Bioprocess. Biosyst. Eng. 2017, 40, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.K.; Kim, J.-H.; Kim, M.-S.; Kim, J.; Hong, J.W.; Hong, Y.G.; Kim, H.-J.; Jeon, J.-M.; Kim, S.-H.; Ahn, J. Production of (3-hydroxybutyrate-co-3-hydroxyhexanoate) copolymer from coffee waste oil using engineered Ralstonia eutropha. Bioprocess. Biosyst. Eng. 2018, 41, 229–235. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Gurav, R.; Choi, T.-R.; Jung, H.-R.; Yang, S.-Y.; Moon, Y.-M.; Song, H.-S.; Jeon, J.-M.; Choi, K.-Y.; Yang, Y.-H. Bioconversion of plant biomass hydrolysate into bioplastic (polyhydroxyalkanoates) using Ralstonia eutropha 5119. Bioresour. Technol. 2019, 271, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Kurita, S.; Orita, I.; Nakamura, S.; Fukui, T. Modification of acetoacetyl-CoA reduction step in Ralstonia eutropha for biosynthesis of poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) from structurally unrelated compounds. Microb. Cell Fact. 2019, 18, 147. [Google Scholar] [CrossRef] [Green Version]
- Sudo, M.; Hori, C.; Ooi, T.; Mizuno, S.; Tsuge, T.; Matsumoto, K.i. Synergy of valine and threonine supplementation on poly (2-hydroxybutyrate-block-3-hydroxybutyrate) synthesis in engineered Escherichia coli expressing chimeric polyhydroxyalkanoate synthase. J. Biosci. Bioeng. 2020, 129, 302–306. [Google Scholar] [CrossRef]
- Steinbüchel, A.; Hofrichter, M.; Koyama, T.; Vandamme, E.; De Baets, S. Biopolymers: Biology, Chemistry, Biotechnology, Applications: Polysaccharides I: Polysaccharides from Prokaryotes; Wiley-VCH: Weinheim, Germany, 2001. [Google Scholar]
- McChalicher, C.W.; Srienc, F. Investigating the structure–property relationship of bacterial PHA block copolymers. J. Biotechnol. 2007, 132, 296–302. [Google Scholar] [CrossRef]
- Pederson, E.N.; McChalicher, C.W.; Srienc, F. Bacterial synthesis of PHA block copolymers. Biomacromolecules 2006, 7, 1904–1911. [Google Scholar] [CrossRef]
- Li, S.Y.; Dong, C.L.; Wang, S.Y.; Ye, H.M.; Chen, G.-Q. Microbial production of polyhydroxyalkanoate block copolymer by recombinant Pseudomonas putida. Appl. Microbiol. Biotechnol. 2011, 90, 659–669. [Google Scholar] [CrossRef]
- Tripathi, L.; Wu, L.-P.; Chen, J.; Chen, G.-Q. Synthesis of Diblock copolymer poly-3-hydroxybutyrate-block-poly-3-hydroxyhexanoate [PHB-b-PHHx] by a β-oxidation weakened Pseudomonas putida KT2442. Microb. Cell Fact. 2012, 11, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brännström, H.; Kumar, H.; Alén, R. Current and potential biofuel production from plant oils. BioEnergy Res. 2018, 11, 592–613. [Google Scholar] [CrossRef]
- Chhetri, A.B.; Tango, M.S.; Budge, S.M.; Watts, K.C.; Islam, M.R. Non-edible plant oils as new sources for biodiesel production. Int. J. Mol. Sci. 2008, 9, 169–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demirbas, A.; Bafail, A.; Ahmad, W.; Sheikh, M. Biodiesel production from non-edible plant oils. Energy Explor. Exploit. 2016, 34, 290–318. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Luo, R.; Wang, Z.; Deng, Y.; Chen, G.-Q. Application of (R)-3-hydroxyalkanoate methyl esters derived from microbial polyhydroxyalkanoates as novel biofuels. Biomacromolecules 2009, 10, 707–711. [Google Scholar] [CrossRef]
- Choonut, A.; Yunu, T.; Pichid, N.; Sangkharak, K. The optimization conditions of polyhydroxybutyrate methyl ester from polyhydroxybutyrate via acid-catalyst. Energy Proc. 2017, 138, 435–440. [Google Scholar] [CrossRef]
- Sangkharak, K.; Pichid, N.; Yunu, T.; Srinak, K.; Sornnum, S.; Prasertsan, P. Biofuel production, characterization and degradation of 3-hydroxybutyate methyl ester from polyhydroxybutyrate. Chiang Mai J. Sci. 2016, 43, 808–817. [Google Scholar]
- Keunun, P.; Rakkarn, T.; Yunu, T.; Paichid, N.; Prasertsan, P.; Sangkharak, K. The production of polyhydroxybutyrate by two-step fermentation and the application of polyhydroxybutyrate as a novel substrate for a biolubricant. J. Polym. Environ. 2018, 26, 2459–2466. [Google Scholar] [CrossRef]
- Okwundu, O.S.; El-Shazly, A.H.; Elkady, M. Comparative effect of reaction time on biodiesel production from low free fatty acid beef tallow: A definition of product yield. SN Appl. Sci. 2019, 1, 140. [Google Scholar] [CrossRef] [Green Version]
- Weerachanchai, P.; Tangsathitkulchai, C.; Tangsathitkulchai, M. Effect of reaction conditions on the catalytic esterification of bio-oil. Korean J. Chem. Eng. 2012, 29, 182–189. [Google Scholar] [CrossRef]
- Farag, H.; El-Maghraby, A.; Taha, N.A. Optimization of factors affecting esterification of mixed oil with high percentage of free fatty acid. Fuel Process. Technol. 2011, 92, 507–510. [Google Scholar] [CrossRef]
- Villegas Calvo, M.; Colombo, B.; Corno, L.; Eisele, G.; Cosentino, C.; Papa, G.; Scaglia, B.; Pilu, R.; Simmons, B.; Adani, F. Bioconversion of giant cane for integrated production of biohydrogen, carboxylic acids, and polyhydroxyalkanoates (PHAs) in a multistage biorefinery approach. ACS Sustain. Chem. Eng. 2018, 6, 15361–15373. [Google Scholar] [CrossRef]
- De Jong, E.; Higson, A.; Walsh, P.; Wellisch, M. Bio-Based Chemicals Value Added Products from Biorefineries; IEA Bioenergy: Utrecht, The Netherlands, 2012; Volume 34. [Google Scholar]
- Snell, K.D.; Peoples, O.P. PHA bioplastic: A value-added coproduct for biomass biorefineries. Biofuels Bioprod. Biorefin. Innovat. Sustain. Econ. 2009, 3, 456–467. [Google Scholar] [CrossRef]
- Budzianowski, W.M. High-value low-volume bioproducts coupled to bioenergies with potential to enhance business development of sustainable biorefineries. Renew. Sustain. Energy Rev. 2017, 70, 793–804. [Google Scholar] [CrossRef]
- Jiang, G.; Hill, D.J.; Kowalczuk, M.; Johnston, B.; Adamus, G.; Irorere, V.; Radecka, I. Carbon sources for polyhydroxyalkanoates and an integrated biorefinery. Int. J. Mol. Sci. 2016, 17, 1157. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.-H.; Shinde, S.; Xie, S.; Hao, N.; Lin, F.; Li, M.; Yoo, C.G.; Ragauskas, A.J.; Yuan, J.S. Cooperative valorization of lignin and residual sugar to polyhydroxyalkanoate (PHA) for enhanced yield and carbon utilization in biorefineries. Sustain. Energy Fuels 2019, 3, 2024–2037. [Google Scholar] [CrossRef]
- Raza, Z.A.; Riaz, S.; Banat, I.M. Polyhydroxyalkanoates: Properties and chemical modification approaches for their functionalization. Biotechnol. Progr. 2018, 34, 29–41. [Google Scholar] [CrossRef]









| Properties | PHA | PLA |
|---|---|---|
| Structure | Over 150 monomer units | D- and L-lactic acids (LA) monomer. |
| Synthesis method | Biosynthesized as intracellular polyester | Biological synthesis of LA and Chemical synthesis of PLA |
| Cost | Twice than PLA | Comparable with conventional plastics. |
| Material properties | Brittle (could be controlled by structural modifications) | Could be varied by adjusting D- and L- ratios. |
| Technology advancement | Over 10 companies Producing PHA up to 2000 ton/year by fermentation of microbes. | NatureWorks, WeforYou, Evonik, and Total-Corbion are largest PLA producers. |
| Applications | Almost all areas of conventional plastic industry. | Packaging, medical implants, printing and coatings. |
| Strain | Carbon Source | DNA Manipulation | Final PHA (%) | Manufacturer |
|---|---|---|---|---|
| Ralstonia eutropha | Glucose | No | >80% | Tianjin North. Food, China |
| Alcaligenes latus | Glucose or sucrose | No | >75% | Chemie Linz, btF, Austria, Biomer, Germany |
| E. coli | Glucose | P3HBCAB + vgb | >80% | Jiang Su Nan Tian, China |
| R. eutropha | Glucose + propionate | No | >75% | ICI, UK, Zhejiang Tian An, China |
| R. eutropha E. coli | Glucose + 1,4-BD | No P3HBCAB | >75% | Metabolix, USA Escherichia coli P3HBCAB Tianjin Green Biosci. China |
| R. eutropha | Fatty acids | PhaCAc | >80% | P&G, Kaneka, Japan |
| Aeromonas hydrophila | Lauric acid | No | <50% | P&G, Jiangmen Biotech Ctr, China |
| A. hydrophila | Lauric acid | P3HBCAB + vgb | >50% | Shandong Lukang, |
| Pseudomonas putida, P. oleovorans | Fatty acids | No | >60% | ETH, Switzerland |
| Bacillus spp. | Sucrose | No | >50% | Biocycles, Brazil |
| R. eutropha + recombinant E. coli | Glucose | No | >80% | Tianjin North. Food, China, and Lantian Group China |
| Burkholderia sp. | Sucrose | No | >70% | Industrial Usina da Pedra-Acucare Alcool Brazil |
| A. latus | Sucrose | No | >90% | Chemie Linz, Austria |
| R. eutropha | Glucose | No | >80% | NingBo TianAn, China |
| A. hydrophila | Glucose + Lauric acid | No | 50% | Guangdong Jiangmen Center for Biotech Development, China, Procter & Gamble, USA |
| Sr No. | Homopolymer | Strain or Plasmid | Involved Genes/Genes Sequence | Carbon Source | Ref. |
|---|---|---|---|---|---|
| 1 | P3HB 3H4PE a | Burkholderia sp. | phaC | Gluconate and sucrose | [59] |
| 2 | P3HB PHO b PHD c | Pseudomonas nitroreducens AS 1.2343 | Hexanoate and octanoate, butyrate, decanoate, lauric acid and tetradecanoic acid | [60] | |
| 3 | P3HB | Pseudomonas sp. 61-3 | P3HB | Glucose | [61] |
| 4 | P3HB | R. eutropha H16 | Propionic acid | [62] | |
| 5 | PHHp d | P. putida KTOY06 | fadBA | Heptanoate | [63] |
| 6 | PHV | hydrophila 4AK4 | vgb and fadD | undecanoic acid | [64] |
| 7 | P3HB | Recombinant E. coli | Sau3A I | Glucose | [65] |
| 8 | P3HB P4HB PHV | Bacillus cereus | 16S rRNA | Fructose, sucrose, and gluconate | [66] |
| 9 | P3HB | C. necator and Burkholderia sacchari | Glycerol and Glucose | [67] | |
| 10 | P3HB | Recombinant E. coli | phaCAB | Cheese whey | [68] |
| 11 | P3HB | R. eutropha H16 and its recombinant strain | phaCAc | Soybean oil | [69] |
| 12 | P3HB | C. necator strain A-04 | Refined sugarcane, Brown sugarcane, Coconut palm sugar, rock sugar, toddy palm sugar and | [70] | |
| 13 | P3HB PHV PHO PHDD e | Wautersia eutropha | Canola oil | [71] | |
| 14 | P3HB | Pseudomonas hydrogenovora | Lactose, glucose, and galactose | [72] | |
| 15 | P3HB | Bacillus firmus NII 0830 | Pineapple Crude glycerol | [73] | |
| 16 | P3HB | Bacillus sp. SV13 | and sugarcane | [74] | |
| 17 | P3HB | C. necator DSM 545 | PhaA | Soy cake and molasses. | [75] |
| 18 | P3HB | C. necator DSM 545 | PhaA | Waste glycerol | [76] |
| 19 | P3HB | W. eutropha | Wheat based bio refinery | [77] | |
| 20 | P3HB | E. coli DH5 α and KSYH(DE3) | bktB-phaB-phaC under trc promotor | Tryptone | [78] |
| 21 | PHV | Bacillus strain PJC48 | 16S rDNA | Glucose | [79] |
| 22 | PHDD | P. putida KT2440 | phaC1Pp | Sodium dodecanoate | [80] |
| 23 | PHO | P. putida ATCC47054 | Glycerol and sodium octanoate | [81] | |
| 24 | P3HB | Burkholderia sp. AIU M5M02 | 16S rRNA | Mannitol | [82] |
| 25 | P3HB | Burkholderia thailandensis | rhlA, rhlB and rlh | Cooking oil | [83] |
| Sr No. | Copolymer | Strain or Plasmid | Involved Genes/Genes Sequence | Carbon Source | Ref. |
|---|---|---|---|---|---|
| 1 | P3HB-co-HA | Pseudomonas sp. 61-3 | phaC1 | Gluconate alkanoates | [89] |
| 2 | P3HB-co-HA | P. sp. 61-3 | Glucose | [61] | |
| 3 | P3HB-co-HV | Recombinant E. coli | phaA | Glucose propionate | [90] |
| 4 | P3HB-co-HHx, P3HB-co-HV-co-HHp | Recombinant R. eutropha P3HB–4 | P3HB phaCAc | Hexanoate and octanoate, pentanoate and nonanoate | [91] |
| 5 | P3HB-co-P4HB | R. eutropha H16 | n-alkanoic acids | [62] | |
| 6 | P3HB-co-HA | R. eutropha P3HB-4 | phaC1Ps | Fructose | [92] |
| 7 | P3HB-co-HHx | A. hydrophila | lauric acid, and oleic acid | [93] | |
| 8 | P3HB-co-HHx | A. hydrophila | phaC coexpressed with phaP phaJ | Dodecanoate | [94] |
| 9 | P3HB-co-P4HB | R. eutropha ATCC 17699 | Fructose and γ-butyrolactone | [95] | |
| 10 | P3HB-co-P4HB | necator | Spent palm oil | [96] | |
| 11 | P3HB-co-HHx | R. eutropha H16 and its recombinant strain | phaCAc | Soybean oil | [69] |
| 12 | P3HB-co-4HB | C. necator strain A-04 | sugarcane, rock sugar, toddy palm and coconut palm sugar | [70] | |
| 13 | P3HB-co-P3HV | P. hydrogenovora | Lactose, glucose and galactose | [72] | |
| 14 | P3HB-co-P3HV-co-P3HHx | Recombinant A. hydrophila 4AK4 | phaA and phaB | Dodecanoic acid and propionic acid | [97] |
| 15 | P3HB-co-P4HB | Comamonas acidovorans | phaA | Glucose and 1,4-butanediol | [98] |
| 16 | P3HB-co-P3HV | C. necator H16 | phaA | palm oil and palm olein | [99] |
| 17 | P3HB-co-P3HV-co-P3HHx | Recombinant C. necator | PhaCAc | Palm kernel oil | [35] |
| 18 | P(3HP-co-4HB) a | Recombinant E. coli | OrfZ, pcs, dhaT, aldD, and phaC1 | Glycerol | [100] |
| 19 | P3HB-co-P3HV | Bacillus sp. | 16S rDNA | Glucose, glycerol, sod. acetate | [101] |
| 20 | P3HB-co-P3HV | Bacillus cereus FA11 | 16S rRNA | Glucose | [102] |
| 21 | P3HB-co-P3HHx | Recombinant C. necator strain Re2160/pCB113 | phaJ | palm kernel oil, soybean oil, corn oil, and coconut oil | [103] |
| 22 | P3HB-co-P3HA | Recombinant R. eutropha | phaC1Ps | Soybean oil, fructose | [104] |
| 23 | P3HB-co-P4HB | Burkholderia contaminans | phaCBcon | sodium-4-hydroxybutyrate | [105] |
| 24 | P3HB-co-P4HB | Cupriavidus malaysiensis USMAA1020 | phaC | 1,4-butanediol and 1,6-hexanediol | [106] |
| 25 | P3HB-co-P3HHx | R. eutropha Re2133/pCB81 | phaC2 | sodium acetate, sodium butyrate, sodium lactate and sodium propionate | [107] |
| 26 | P3HB-co-P3HHx | R. eutropha H16 | phaC2 | Butyrate | [108] |
| 27 | P3HB-co-P3HV | E. coli YH090 | atoAD overexpressed with bktB, phaB, and phaC | propionate | [109] |
| 28 | P3HB-co-P3HHx | R. eutropha Re2133 | phaJ and phaC2 | coffee waste oil | [110] |
| 29 | P3HB-co-P3HV | R. eutropha 5119 | hmfH | Glucose | [111] |
| 30 | P3HB-co-P3HHx | R. eutropha H16 | phaB1 and phaB2 | Glucose, fructose and glycerol | [112] |
| Liquid | Solid | Gaseous |
|---|---|---|
| Biodiesel, Methanol, Ethanol, biobutanol gasohol, biogasoline, Hydrotreated Vegetable Oil (HVO), hydroxyalkanoates methyl ester (HAME) or hydroxybutyrate methyl ester (HBME) | Wood fuel, sawdust, charcoal and bagasse, dried animal dung | Methane (CH4), Butane (C4H10) and Hydrogen (H2) gas |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riaz, S.; Rhee, K.Y.; Park, S.J. Polyhydroxyalkanoates (PHAs): Biopolymers for Biofuel and Biorefineries. Polymers 2021, 13, 253. https://doi.org/10.3390/polym13020253
Riaz S, Rhee KY, Park SJ. Polyhydroxyalkanoates (PHAs): Biopolymers for Biofuel and Biorefineries. Polymers. 2021; 13(2):253. https://doi.org/10.3390/polym13020253
Chicago/Turabian StyleRiaz, Shahina, Kyong Yop Rhee, and Soo Jin Park. 2021. "Polyhydroxyalkanoates (PHAs): Biopolymers for Biofuel and Biorefineries" Polymers 13, no. 2: 253. https://doi.org/10.3390/polym13020253
APA StyleRiaz, S., Rhee, K. Y., & Park, S. J. (2021). Polyhydroxyalkanoates (PHAs): Biopolymers for Biofuel and Biorefineries. Polymers, 13(2), 253. https://doi.org/10.3390/polym13020253