Microstructure, Thermal Conductivity, and Flame Retardancy of Konjac Glucomannan Based Aerogels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
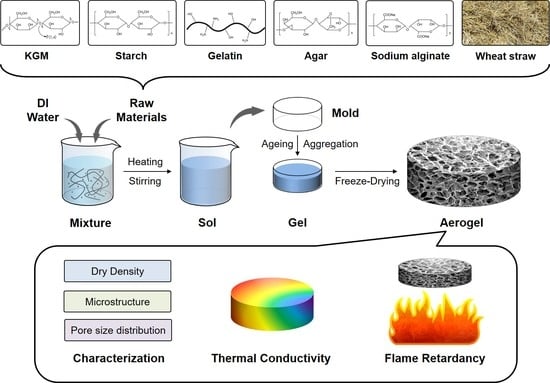
2.2. Preparation of the KGM-Based Aerogels
2.3. Characterization of KGM-Based Aerogels
2.3.1. Dry Density Determination
2.3.2. Scanning Electron Microscopy (SEM) and Pore Size Distribution
2.3.3. Thermal Conductivity Determination
2.3.4. Limiting Oxygen Index (LOI) Measurement
2.3.5. Microscale Combustion Calorimeter (MCC) Measurement
2.4. Statistical Analysis
3. Results and Discussion
3.1. Microscopic Morphology of KGM-Based Aerogels
3.2. Thermal Conductivity of KGM-Based Aerogels
3.3. Flame Retardancy of KGM-Based Aerogels
3.3.1. Limiting Oxygen Index (LOI)
3.3.2. Heat Release Behavior
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berardi, U. A cross country comparison of building energy consumption and their trends. Resour. Conserv. Recycl. 2017, 123, 230–241. [Google Scholar] [CrossRef]
- Vera, M.; Viktor, P. Saving the Architectural Appearance of the Historical Buildings due to Heat Insulation of their External Walls. Procedia Eng. 2015, 117, 891–899. [Google Scholar]
- Xu, X.F.; Chen, J.; Zhou, J.; Li, B.W. Thermal Conductivity of Polymers and Their Nanocomposites. Adv. Mater. 2018, 30, 1705544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Y.; Ruan, K.; Shi, X.; Yang, X.; Gu, J. Factors affecting thermal conductivities of the polymers and polymer composites: A review. Compos. Sci. Technol. 2020, 193, 108134. [Google Scholar] [CrossRef]
- Wi, S.; Yang, S.; Berardi, U.; Kim, S. Assessment of recycled ceramic-based inorganic insulation for improving energy efficiency and flame retardancy of buildings. Environ. Int. 2019, 130, 104900. [Google Scholar] [CrossRef]
- Schiavoni, S.; D’Alessandro, F.; Bianchi, F.; Asdrubali, F. Insulation materials for the building sector: A review and comparative analysis. Renew. Sustain. Energy Rev. 2016, 62, 988–1011. [Google Scholar] [CrossRef]
- Khoukhi, M.; Hassan, A.; Saadi, S.A.; Abdelbaqi, S. A dynamic thermal response on thermal conductivity at different temperature and moisture levels of EPS insulation. Case Stud. Therm. Eng. 2019, 14, 100481. [Google Scholar] [CrossRef]
- Septevani, A.A.; Evans, D.A.C.; Chaleat, C.; Martin, D.J.; Annamalai, P.K. A systematic study substituting polyether polyol with palm kernel oil based polyester polyol in rigid polyurethane foam. Ind. Crop. Prod. 2015, 66, 16–26. [Google Scholar] [CrossRef]
- Grace, T.C.; Salvador, C.N.; Francisco, V. Rigid foam polyurethane (PU) derived from castor oil (Ricinus communis) for thermal insulation in roof systems. Front. Archit. Res. 2012, 1, 348–356. [Google Scholar]
- Liu, L.; Li, H.; Lazzaretto, A.; Manente, G.; Tong, C.; Liu, Q.; Li, N. The development history and prospects of biomass-based insulation materials for buildings. Renew. Sustain. Energy Rev. 2017, 69, 912–932. [Google Scholar] [CrossRef]
- Berardi, U.; Naldi, M. The impact of the temperature dependent thermal conductivity of insulating materials on the effective building envelope performance. Energy Build. 2017, 144, 262–275. [Google Scholar] [CrossRef]
- Asdrubali, F.; D’Alessandro, F.; Schiavoni, S. A review of unconventional sustainable building insulation materials Sustainable Mater. Sustain. Mater. Technol. 2015, 4, 1–17. [Google Scholar]
- Zhou, B.; Yoshioka, H.; Noguchi, T.; Wang, K. Experimental study of expanded polystyrene (EPS) External Thermal Insulation Composite Systems (ETICS) masonery façade reaction-to-fire performance. Therm. Sci. Eng. Prog. 2018, 8, 83–92. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, A.; Liu, X.; Zhang, F. The effectiveness of horizontal barriers in preventing fire spread on vertical insulation panels made of polystyrene foams. Fire Technol. 2015, 52, 649–662. [Google Scholar] [CrossRef]
- McKenna, S.T.; Jones, N.; Peck, G.; Dickens, K.; Pawelec, W.; Oradei, S.; Harris, S.; Stec, A.A.; Hull, T.R. Fire behaviour of modern façade materials-understanding the Grenfell Tower fire. J. Hazard. Mater. 2019, 368, 115–123. [Google Scholar] [CrossRef]
- Zhang, M.; Luo, Z.; Zhang, J.; Chen, S.; Zhou, Y. Effects of a novel phosphorus–nitrogen flame retardant on rosin-based rigid polyurethane foams. Polym. Degrad. Stab. 2015, 120, 427–434. [Google Scholar] [CrossRef]
- Chen, M.; Chen, C.; Tan, Y.; Huang, J.; Wang, X.; Chen, L.; Wang, Y. Inherently flame-retardant flexible polyurethane foam with low content of phosphorus-containing cross-linking agent. Ind. Eng. Chem. Res. 2014, 53, 1160–1171. [Google Scholar] [CrossRef]
- Thirumal, M.; Khastgir, D.; Nando, G.B.; Naik, Y.P.; Singha, N. Halogen-free flame retardant PUF: Effect of melamine compounds on mechanical, thermal and flame retardant properties. Polym. Degrad. Stab. 2010, 95, 1138–1145. [Google Scholar] [CrossRef]
- Xu, Z.B.; Kong, W.W.; Zhou, M.X.; Peng, M. Effect of surface modification of montmorillonite on the properties of rigid polyurethane foam composites. Chin. J. Polym. Sci. 2010, 28, 615–624. [Google Scholar] [CrossRef]
- Li, L.; Liu, X.; Huang, K.; Wang, Y.; Zheng, X.; Wang, J.; Du, Y.; Jiang, L.; Zhao, S. A facile strategy to fabricate intumescent fire-retardant and smoke suppression protective coatings for natural rubber. Polym. Test. 2020, 90, 106689. [Google Scholar] [CrossRef]
- Wei, G.; Li, D.; Zhuo, M.; Liao, Y.; Xie, Z.; Guo, T.; Li, J.; Zhang, S.; Liang, Z. Organophosphorus flame retardants and plasticizers: Sources, occurrence, toxicity and human exposure. Environ. Pollut. 2015, 196, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Khobragade, P.S.; Hansora, D.P.; Naik, J.B.; Chatterjee, A. Flame retarding performance of elastomeric nanocomposites: A review. Polym. Degrad. Stab. 2016, 130, 194–244. [Google Scholar] [CrossRef]
- Basak, S.; Ali, W.S. Sustainable fire retardancy of textiles using bio-macromolecules. Polym. Degrad. Stab. 2016, 133, 47–64. [Google Scholar] [CrossRef]
- Réti, C.; Casetta, M.; Duquesne, S.; Bourbigot, S.; Delobel, R. Flammability properties of intumescent PLA including starch and lignin. Polym. Adv. Technol. 2008, 19, 628–635. [Google Scholar] [CrossRef]
- Shen, D.K.; Gu, S.; Bridgwater, A.V. The thermal performance of the polysaccharides extracted from hardwood: Cellulose and hemicellulose. Carbohydr. Polym. 2010, 82, 39–45. [Google Scholar] [CrossRef]
- Han, F.; Liu, Q.; Lai, X.; Li, H.; Zeng, X. Compatibilizing effect of β-cyclodextrin in RDP/phosphorus-containing polyacrylate composite emulsion and its synergism on the flame retardancy of the latex film. Prog. Org. Coat. 2014, 77, 975–980. [Google Scholar] [CrossRef]
- Shang, K.; Liao, W.; Wang, J.; Wang, Y.; Wang, Y.; Schiraldi, D.A. Nonflammable Alginate Nanocomposite Aerogels Prepared by a Simple Freeze-Drying and Post-Cross-Linking Method. ACS Appl. Mater. Interfaces 2016, 8, 643–650. [Google Scholar] [CrossRef]
- Xiao, Y.; Zheng, Y.; Wang, X.; Chen, Z.; Xu, Z. Preparation of a chitosan-based flame-retardant synergist and its application in flame-retardant polypropylene. J. Appl. Polym. Sci. 2014, 131, 40845. [Google Scholar] [CrossRef]
- Yang, J.; Wu, H.; He, S.; Wang, M. Prediction of thermal conductivity of fiber/aerogel composites for optimal thermal insulation. J. Porous Media 2015, 18, 971–984. [Google Scholar] [CrossRef]
- Kistler, S.S. Coherent expanded aerogels and jellies. Nature 1931, 127, 741. [Google Scholar] [CrossRef]
- Schwan, M.; Tannert, R.; Ratke, L. New soft and spongy resorcinol-formaldehyde aerogels. J. Supercrit. Fluids 2016, 107, 201–208. [Google Scholar] [CrossRef]
- Mikkonen, K.S.; Parikka, K.; Ghafar, A.; Tenkanen, M. Prospects of poly- saccharide aerogels as modern advanced food materials. Trends Food Sci. Technol. 2013, 34, 124–136. [Google Scholar] [CrossRef]
- Mehrez, E.E.; Sarah, I.O.; Osama, M.M. Synthesis, drying process and medical application of polysaccharide-based aerogels. Int. J. Biol. Macromol. 2020, 145, 1115–1128. [Google Scholar]
- Zuo, L.; Zhang, Y.; Zhang, L.; Miao, Y.; Fan, W.; Liu, T. Polymer/Carbon-Based Hybrid Aerogels: Preparation. Prop. Appl. Mater. 2015, 8, 6806–6848. [Google Scholar]
- Pragya, G.; Balwant, S.; Pradip, K.M. Low density and high strength nanofibrillated cellulose aerogel for thermal insulation application. Mater. Des. 2018, 158, 224–236. [Google Scholar]
- Xu, C.; Luo, X.; Lin, X.; Zhuo, X.; Liang, L. Preparation and characterization of polylactide/thermoplastic konjac glucomannan blends. Polymer 2009, 50, 3698–3705. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Kuang, Y.; Xiao, M.; Su, Y.; Jiang, F. Microstructure and filtration performance of konjac glucomannan-based aerogels strengthened by wheat straw. Int. J. Low Carbon Technol. 2018, 13, 67–75. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wu, K.; Xiao, M.; Riffat, S.B.; Su, Y.; Jiang, F. Thermal conductivity, structure and mechanical properties of konjac glucomannan/starch based aerogel strengthened by wheat straw. Carbohydr. Polym. 2018, 197, 284–291. [Google Scholar] [CrossRef]
- Ganesan, K.; Budtova, T.; Ratke, L.; Gurikov, P.; Baudron, V.; Preibisch, I.; Niemeyer, P.; Smirnova, I.; Milow, B. Review on the Production of Polysaccharide Aerogel Particles. Materials 2018, 11, 2144. [Google Scholar] [CrossRef] [Green Version]
- Price, C.J. Take some solid steps to improve crystallization. Chem. Eng. Prog. 1997, 93, 34–43. [Google Scholar]
- Kiani, H.; Sun, D.W. Water crystallization and its importance to freezing of foods: A review. Trends Food Sci. Technol. 2011, 22, 407–426. [Google Scholar] [CrossRef]
- Wu, K.; Fang, Y.; Wu, H.; Wan, Y.; Qian, H.; Jiang, F.; Chen, S. Improving konjac glucomannan-based aerogels filtration properties by combining aerogel pieces in series with different pore size distributions. Int. J. Biol. Macromol. 2020, in press. [Google Scholar]
- Jeong, Y.; Choi, H.; Kim, K.; Choi, G.; Kang, J.; Yang, K. A study on the thermal conductivity of resilient materials. Thermochim. Acta 2009, 490, 47–50. [Google Scholar] [CrossRef]
- Li, X.; He, Y.; Qin, Z.; Chen, M.; Chen, H. Facile fabrication, mechanical property and flame retardancy of aerogel composites based on alginate and melamine-formaldehyde. Polymer 2019, 181, 121783. [Google Scholar] [CrossRef]
- Hou, Y.; Hu, Y. Preparation of layered organic-inorganic aluminum phosphonate for enhancing fire safety of polystyrene. Mater. Chem. Phys. 2017, 196, 109–117. [Google Scholar] [CrossRef]
- Lu, H.; Wilkie, C. Synergistic effect of carbon nanotubes and decabromodiphenyl oxide/Sb2O3 in improving the flame retardancy of polystyrene. Polym. Degrad. Stab. 2010, 95, 564–571. [Google Scholar] [CrossRef] [Green Version]
- Zhao, B.; Liu, D.; Liang, W.; Li, F.; Wang, J.; Liu, Y. Bi-phase flame-retardant actions of water-blown rigid polyurethane foam containing diethyl-N, N-bis(2-hydroxyethyl) phosphoramide and expandable graphite. J. Anal. Appl. Pyrolysis 2017, 124, 247–255. [Google Scholar] [CrossRef]



| Sample | Density (g/cm−3) | Thermal Conductivity (W/mK) |
|---|---|---|
| K1A1 | 0.0320 ± 0.0020 c | 0.05127 ± 0.00124 c |
| K1G1 | 0.0247 ± 0.0008 b | 0.04817 ± 0.00133 abc |
| K1AL1 | 0.0234 ± 0.0012 b | 0.04705 ± 0.00120 ab |
| K1A1S1 | 0.0497 ± 0.0033 e | 0.04980 ± 0.00156 bc |
| K1G1S1 | 0.0373 ± 0.0003 d | 0.04795 ± 0.00163 abc |
| K1AL1S1 | 0.0362 ± 0.0008 d | 0.04700 ± 0.00078 ab |
| K1A1S1WS1 | 0.0559 ± 0.0005 f | 0.04748 ± 0.00156 ab |
| K1G1S1WS1 | 0.0489 ± 0.0024 e | 0.04633 ± 0.00096 ab |
| K1AL1S1WS1 | 0.0464 ± 0.0005 e | 0.04573 ± 0.00183 a |
| PU | 0.0539 ± 0.0021 f | 0.04795 ± 0.00120 abc |
| EPS | 0.00787 ± 0.00005 a | 0.05170 ± 0.00053 c |
| Sample | LOI (%) | PHRR (W/g) | TPHRR (°C) | THR (kJ/g) |
|---|---|---|---|---|
| K1 | <20.00 | 165.5 ± 1.7 | 320.1 ± 3.6 | 16.2 ± 0.6 |
| K1A1 | 22.33 | 129.2 ± 2.1 | 301.5 ± 4.2 | 15.5 ± 0.2 |
| K1G1 | 25.09 | 122.9 ± 1.3 | 313.5 ± 2.6 | 10.3 ± 0.2 |
| K1AL1 | 24.53 | 46.7 ± 2.4 | 252.3 ± 3.2 | 6.2 ± 0.3 |
| K1A1S1 | 22.33 | 124.1 ± 2.8 | 313.3 ± 1.7 | 10.9 ± 0.2 |
| K1G1S1 | 24.53 | 117.5 ± 1.4 | 309.4 ± 4.1 | 10.0 ± 0.1 |
| K1AL1S1 | 23.81 | 47.8 ± 2.5 | 280.7 ± 4.8 | 5.7 ± 0.08 |
| K1A1S1WS1 | 22.33 | 96.7 ± 1.5 | 318.5 ± 2.4 | 9.2 ± 0.3 |
| K1G1S1WS1 | 23.81 | 91.7 ± 2.3 | 303.5 ± 1.1 | 9.1 ± 0.2 |
| K1AL1S1WS1 | 23.81 | 60.2 ± 0.9 | 293.9 ± 1.5 | 7.4 ± 0.1 |
| PU | 20.5 | 226.4 ± 2.0 | 378.2 ± 1.3 | 24.3 ± 0.1 |
| EPS | 17 | 813.0 ± 5.2 | 431.0 ± 3.2 | 53.0 ± 1.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuang, Y.; Chen, L.; Zhai, J.; Zhao, S.; Xiao, Q.; Wu, K.; Qiao, D.; Jiang, F. Microstructure, Thermal Conductivity, and Flame Retardancy of Konjac Glucomannan Based Aerogels. Polymers 2021, 13, 258. https://doi.org/10.3390/polym13020258
Kuang Y, Chen L, Zhai J, Zhao S, Xiao Q, Wu K, Qiao D, Jiang F. Microstructure, Thermal Conductivity, and Flame Retardancy of Konjac Glucomannan Based Aerogels. Polymers. 2021; 13(2):258. https://doi.org/10.3390/polym13020258
Chicago/Turabian StyleKuang, Ying, Lijun Chen, Junjun Zhai, Si Zhao, Qinjian Xiao, Kao Wu, Dongling Qiao, and Fatang Jiang. 2021. "Microstructure, Thermal Conductivity, and Flame Retardancy of Konjac Glucomannan Based Aerogels" Polymers 13, no. 2: 258. https://doi.org/10.3390/polym13020258
APA StyleKuang, Y., Chen, L., Zhai, J., Zhao, S., Xiao, Q., Wu, K., Qiao, D., & Jiang, F. (2021). Microstructure, Thermal Conductivity, and Flame Retardancy of Konjac Glucomannan Based Aerogels. Polymers, 13(2), 258. https://doi.org/10.3390/polym13020258