Characteristics and Preparation of Designed Alginate-Based Composite Scaffold Membranes with Decellularized Fibrous Micro-Scaffold Structures from Porcine Skin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of a Decellularized Extracellular Matrix Scaffold
2.3. Preparation of Decellularized Extracellular Matrix/Alginate Composite Scaffold Membranes
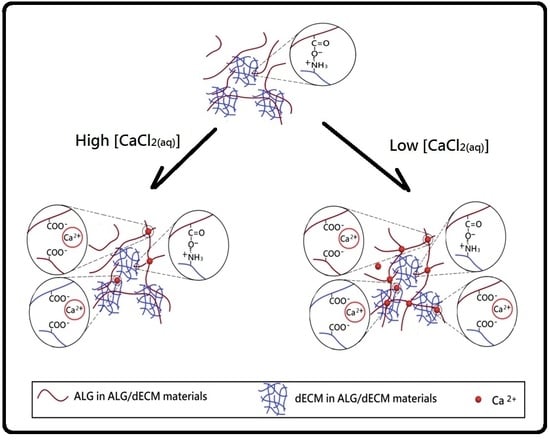
2.4. Preparation of Cross-Linked Decellularized Extracellular Matrix/Alginate Composite Scaffold Membranes
2.5. Measurements
3. Results
3.1. Fourier Transform Infrared Spectroscopy Analysis of Alginate/dECM Composite Scaffold Membranes
3.2. The Microstructure of Resulting Alginate/dECM Composite Scaffold Membranes
3.3. Thermal Stability of Resulting Alginate/dECM Composite Scaffold Memebranes
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malda, J. 25th anniversary article: Engineering hydrogels for biofabrication. Adv. Mater. 2013, 25, 5011–5028. [Google Scholar] [CrossRef]
- Jiang, T.; Munguia-Lopez, J.G.; Flores-Torres, S.; Grant, J.; Vijayakumar, S.; Leon-Rodriguez, A.D.; Kinsella, J.M. Directing the self-assembly of tumor spheroids by bioprinting cellular heterogeneous models within Alginate/Gelatin hydrogels. Sci. Rep. 2017, 7, 4575. [Google Scholar] [CrossRef] [Green Version]
- Benwood, C.; Chrenek, J.; Kirsch, R.L.; Masri, N.Z.; Richards, H.; Teetzen, K.; Willerth, S.M. Natural Biomaterials and Their Use as Bioinks for Printing Tissues. Bioengineering 2021, 8, 27. [Google Scholar] [CrossRef]
- Sherifi, I.; Bachy, M.; Laumonier, T. Use of supercritical carbon dioxide technology for fabricating a tissue engineering scaffold for anterior cruciate ligament repair. Sci. Rep. 2020, 10, 14030. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Ma, X.; Zhu, W.; Wang, P.; Miller, K.L.; Stupin, J.; Koroleva-Maharajh, A.; Hairabedian, A.; Chen, S. Scanningless and continuous 3D bioprinting of human tissues with decellularized extracellular matrix. Biomaterials 2019, 194, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.W.; Huang, C.C.; Wang, Y.Y.; Xu, J.; Wang, G.D.; Bai, X.P. Biological evaluations of decellularized extracellular matrix collagen microparticles prepared based on plant enzymes and aqueous two-phase method. Regen. Biomater. 2021, 8, rbab002. [Google Scholar] [CrossRef] [PubMed]
- Zhai, G.; Toh, S.C.; Tan, W.L.; Kang, E.T.; Neoh, K.G.; Huang, C.C.; Liaw, D.J. Poly(vinylidene fluoride) with Grafted Zwitterionic Polymer Side Chains for Electrolyte-Responsive Microfiltration Membranes. Langmuir 2003, 19, 7030–7037. [Google Scholar] [CrossRef]
- Vanaei, S.; Parizi, M.S.; Vanaei, S.; Salemizadehparizi, F.; Vanaei, H.R. An Overview on Materials and Techniques in 3D Bioprinting Toward Biomedical Application. Eng. Regen. 2021, 2, 1–18. [Google Scholar]
- Shin, Y.J.; Shafranek, R.T.; Tsui, J.H.; Walcott, J.; Nelson, A.; Kim, D.H. 3D bioprinting of mechanically tuned bioinks derived from cardiac decellularized extracellular matrix. Acta Biomater. 2021, 119, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D.H. 3D bioprinting for engineering complex tissues. Biotechnol. Adv. 2016, 34, 422–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabirian, F.; Mozafari, M. Decellularized ECM-derived bioinks: Prospects for the future. Methods 2020, 171, 108–118. [Google Scholar] [CrossRef]
- Smidsrød, O.; Skjak-Braek, G. Alginate as immobilization matrix for cells. Trends Biotechnol. 1990, 8, 71–78. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [Green Version]
- Anal, A.K.; Stevens, W.F. Chitosan-alginate multilayer beads for controlled release of ampicillin. Int. J. Pharm. 2005, 290, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, A.D.; Kiick, K.L. Polysaccharide-modified synthetic polymeric biomaterials. Biopolymers 2010, 94, 128–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Cheng, J.; Ao, Q. Preparation of Alginate-Based Biomaterials and Their Applications in Biomedicine. Mar. Drugs 2021, 19, 264. [Google Scholar] [CrossRef] [PubMed]
- Piras, C.C.; Smith, D.K. Multicomponent polysaccharide alginate-based bioinks. J. Mater. Chem. B 2020, 8, 8171–8188. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Yu, C.; Wang, P.; Xu, W.; Wan, X.; Sun, C.; Lai, E.; Liu, J.; Koroleva-Maharajh, A.; Chen, S. Rapid 3D bioprinting of decellularized extracellular matrix with regionally varied mechanical properties and biomimetic microarchitecture. Biomaterials 2018, 185, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Vogel, K.G.; Trotter, J.A. The Effect of Proteoglycans on the Morphology of Collagen Fibrils Formed In Vitro. Collagen Relat. Res. 1987, 7, 105–114. [Google Scholar] [CrossRef]
- Curley, C.J.; Dolan, E.B.; Otten, M. An injectable alginate/extra cellular matrix (ECM) hydrogel towards acellular treatment of heart failure. Drug Deliv. Transl. Res. 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Valencia, C.; Valencia, C.H.; Zuluaga, F.; Valencia, M.E.; Mina, J.H.; Grande-Tovar, C.D. Synthesis and Application of Scaffolds of Chitosan-Graphene Oxide by the Freeze-Drying Method for Tissue Regeneration. Molecules 2018, 23, 2651. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Sample No. | ALG/dECM a (w/w) | CaCl2 |
|---|---|---|
| AdE0N a | 100/0 | - |
| AdE1N a | 95/5 | - |
| AdE2N a | 90/10 | - |
| AdE3N a | 85/15 | - |
| AdE4N a | 80/20 | - |
| AdE0L a,b | 100/0 | 1 wt% |
| AdE1L a,b | 95/5 | 1 wt% |
| AdE2L a,b | 90/10 | 1 wt% |
| AdE3L a,b | 85/15 | 1 wt% |
| AdE4L a,b | 80/20 | 1 wt% |
| AdE0H a,c | 100/0 | 5 wt% |
| AdE1H a,c | 95/5 | 5 wt% |
| AdE2H a,c | 90/10 | 5 wt% |
| AdE3H a,c | 85/15 | 5 wt% |
| AdE4H a,c | 80/20 | 5 wt% |
| Sample No. | a | b | c |
|---|---|---|---|
| AdE0N (a) | 245 °C | ─ (d) | ─ (d) |
| AdE1N (a) | 260 °C (32 wt%) | 360 °C (11 wt%) | ─ (d) |
| AdE2N (a) | 260 °C (28 wt%) | 360 °C (15 wt%) | ─ (d) |
| AdE3N (a) | 260 °C (10 wt%) | 360 °C (26 wt%) | ─ (d) |
| AdE4N (a) | 260 °C (8 wt%) | 360 °C (27 wt%) | ─ (d) |
| dE (a) | ─ (d) | 330 | ─ (d) |
| AdE0L (a,b) | 260 °C | ─ (d) | ─ (d) |
| AdE1L (a,b) | 265 °C (20 wt%) | 350 °C (35 wt%) | ─ (d) |
| AdE2L (a,b) | 268 °C (15 wt%) | 360 °C (23 wt%) | 410 °C (17 wt%) |
| AdE3L (a,b) | 280 °C (12 wt%) | 360 °C (24 wt%) | 410 °C (19 wt%) |
| AdE4L (a,b) | 285 °C (5 wt%) | 370 °C (25 wt%) | 410 °C (25 wt%) |
| AdE0H (a,c) | 280 °C | ─ (d) | ─ (d) |
| AdE1H (a,c) | 298 °C (15 wt%) | ─ (d) | 390 °C (20 wt%) |
| AdE2H (a,c) | 298 °C (9 wt%) | 360 °C (18 wt%) | 410 °C (22 wt%) |
| AdE3H (a,c) | 298 °C (6 wt%) | 360 °C (18 wt%) | 410 °C (27 wt%) |
| AdE4H (a,c) | 300 °C (5 wt%) | 385 °C (18 wt%) | 420 °C (30 wt%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-C. Characteristics and Preparation of Designed Alginate-Based Composite Scaffold Membranes with Decellularized Fibrous Micro-Scaffold Structures from Porcine Skin. Polymers 2021, 13, 3464. https://doi.org/10.3390/polym13203464
Huang C-C. Characteristics and Preparation of Designed Alginate-Based Composite Scaffold Membranes with Decellularized Fibrous Micro-Scaffold Structures from Porcine Skin. Polymers. 2021; 13(20):3464. https://doi.org/10.3390/polym13203464
Chicago/Turabian StyleHuang, Ching-Cheng. 2021. "Characteristics and Preparation of Designed Alginate-Based Composite Scaffold Membranes with Decellularized Fibrous Micro-Scaffold Structures from Porcine Skin" Polymers 13, no. 20: 3464. https://doi.org/10.3390/polym13203464
APA StyleHuang, C. -C. (2021). Characteristics and Preparation of Designed Alginate-Based Composite Scaffold Membranes with Decellularized Fibrous Micro-Scaffold Structures from Porcine Skin. Polymers, 13(20), 3464. https://doi.org/10.3390/polym13203464