Selectively Etched Halloysite Nanotubes as Performance Booster of Epoxidized Natural Rubber Composites
Abstract
:1. Introduction
2. Experimental Details
2.1. Materials
2.2. Preparation of Epoxidized Natural Rubber
2.3. Selectively Etching of HNT by Sulfuric Acid
2.4. Preparation of ENR/HNT Composites
2.5. Measurement of Curing Characteristics
2.6. Fourier Transform Infrared-Spectroscopic Analysis (FT-IR)
2.7. X-ray Diffraction Analysis (XRD)
2.8. Measurement of Mechanical Properties and Hardness
2.9. Determination of Crosslink Density
2.10. Scanning Electron Microscopy
2.11. Dynamic Properties
2.12. Wide-Angle X-ray Scattering
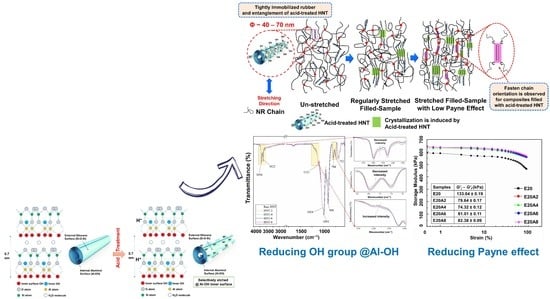
3. Results and Discussion
3.1. BET Surface Area of HNT
3.2. FT-IR Analysis
3.3. X-ray Diffraction Analysis
3.4. Curing Characteristics
3.5. Dynamic Properties
3.6. Mechanical Properties and Corresponding Morphologies
3.7. Wide-Angle X-ray Scattering
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arrighi, V.; McEwen, I.; Qian, H.; Prieto, M.S. The glass transition and interfacial layer in styrene-butadiene rubber containing silica nanofiller. Polymer 2003, 44, 6259–6266. [Google Scholar] [CrossRef]
- Ismail, H.; Pasbakhsh, P.; Fauzi, M.A.; Bakar, A.A. Morphological, thermal and tensile properties of halloysite nanotubes filled ethylene propylene diene monomer (EPDM) nanocomposites. Polym. Test. 2008, 27, 841–850. [Google Scholar] [CrossRef]
- Price, R.R.; Gaber, B.P.; Lvov, Y. In-vitro release characteristics of tetracycline HCl, khellin and nicotinamide adenine dineculeotide from halloysite; a cylindrical mineral. J. Microencapsul. 2001, 18, 713–722. [Google Scholar] [PubMed]
- Du, M.L.; Guo, B.C.; Jia, D.M. Thermal stability and flame retardant effects of halloysite nanotubes on poly(propylene). Eur. Polym. J. 2006, 42, 1362–1369. [Google Scholar] [CrossRef]
- Surya, I.; Waesateh, K.; Saiwari, S.; Ismail, H.; Othman, N.; Hayeemasae, N. Potency of Urea-Treated Halloysite Nanotubes for the Simultaneous Boosting of Mechanical Properties and Crystallization of Epoxidized Natural Rubber Composites. Polymers 2021, 13, 3068. [Google Scholar] [CrossRef] [PubMed]
- Paran, S.M.R.; Naderi, G.; Mosallanezhad, H.; Movahedifar, E.; Formela, K.; Saeb, M.R. Microstructure and mechanical properties of carboxylated nitrile butadiene rubber/epoxy/XNBR-grafted halloysite nanotubes nanocomposites. Polymers 2020, 12, 1192. [Google Scholar] [CrossRef]
- Lin, J.; Hu, D.; Luo, Y.; Zhong, B.; Chen, Y.; Jia, Z.; Jia, D. Functionalized Halloysite Nanotubes–Silica Hybrid for Enhanced Curing and Mechanical Properties of Elastomers. Polymers 2019, 11, 883. [Google Scholar] [CrossRef] [Green Version]
- Hayeemasae, N.; Waesateh, K.; Saiwari, S.; Ismail, H.; Othman, N. Detailed investigation of the reinforcing effect of halloysite nanotubes-filled epoxidized natural rubber. Polym. Bull. 2020. [Google Scholar] [CrossRef]
- Zhang, A.; Pan, L.; Zhang, Y.H.; Liu, T.S.; Ye, Y.; Xia, S.M.; Chen, X.-G. Effects of acid treatment on the physic-chemical and pore characteristics of halloysite. Colloids Surf. A Physicochem. Eng. Asp. 2012, 396, 182–188. [Google Scholar] [CrossRef]
- Abdullayev, E.; Joshi, A.; Wei, W.; Zhao, Y.; Lvov, Y. Enlargement of halloysite clay nanotube lumen by selective etching of aluminum oxide. ACS Nano 2012, 6, 7216–7226. [Google Scholar] [CrossRef]
- Trabelsi, S.; Albouy, P.-A.; Rault, J. Stress-induced crystallization properties of natural and synthetic cis-polyisoprene. Rubber Chem. Technol. 2004, 77, 303–316. [Google Scholar]
- Toki, S.; Hsiao, B.S. Nature of strain-induced structures in natural and synthetic rubbers under stretching. Macromolecules 2003, 36, 5915–5917. [Google Scholar] [CrossRef]
- Candau, N.; Chazeau, L.; Chenal, J.-M.; Gauthier, C.; Munch, E. A comparison of the abilities of natural rubber (NR) and synthetic polyisoprene cis-1, 4 rubber (IR) to crystallize under strain at high strain rates. Phys. Chem. Chem. Phys. 2016, 18, 3472–3481. [Google Scholar] [CrossRef]
- Lake, G.J. Fatigue and Fracture of Elastomers. Rubber Chem. Technol. 1995, 68, 435–460. [Google Scholar] [CrossRef]
- Huneau, B. Strain-induced crystallization of natural rubber: A review of x-ray diffraction investigations. Rubber Chem. Technol. 2011, 84, 425–452. [Google Scholar] [CrossRef] [Green Version]
- Tosaka, M.; Murakami, S.; Poompradub, S.; Kohjiya, S.; Ikeda, Y.; Toki, S.; Sics, I.; Hsiao, B.S. Orientation and Crystallization of Natural Rubber Network As Revealed by WAXD Using Synchrotron Radiation. Macromolecules 2004, 37, 3299–3309. [Google Scholar] [CrossRef]
- Toki, S.; Sics, I.; Ran, S.; Liu, L.; Hsiao, B.S.; Murakami, S.; Tosaka, M.; Kohjiya, S.; Poompradub, S.; Ikeda, Y.; et al. Strain-Induced Molecular Orientation and Crystallization in Natural and Synthetic Rubbers under Uniaxial Deformation by In-situ Synchrotron X-ray Study. Rubber Chem. Technol. 2004, 77, 317–335. [Google Scholar] [CrossRef]
- Imbernon, L.; Pauchet, R.; Pire, M.; Albouy, P.-A.; Tencé-Girault, S.; Norvez, S. Strain-induced crystallization in sustainably crosslinked epoxidized natural rubber. Polymer 2016, 93, 189–197. [Google Scholar] [CrossRef]
- Poompradub, S.; Tosaka, M.; Kohjiya, S.; Ikeda, Y.; Toki, S.; Sics, I.; Hsiao, B.S. Mechanism of strain-induced crystallization in filled and unfilled natural rubber vulcanizates. J. Appl. Phys. 2005, 97, 103529. [Google Scholar] [CrossRef] [Green Version]
- Chenal, J.-M.; Gauthier, C.; Chazeau, L.; Guy, L.; Bomal, Y. Parameters governing strain induced crystallization in filled natural rubber. Polymer 2007, 48, 6893–6901. [Google Scholar] [CrossRef] [Green Version]
- Waesateh, K.; Saiwari, S.; Ismail, H.; Othman, N.; Soontaranon, S.; Hayeemasae, N. Features of crystallization behavior of natural rubber/halloysite nanotubes composites using synchrotron wide-angle X-ray scattering. Intern. J. Polym. Anal. Charac. 2018, 23, 260–270. [Google Scholar] [CrossRef]
- Flory, P.J.; Rehner, J., Jr. Statistical mechanics of cross-linked polymer networks I. Rubberlike elasticity. J. Chem. Phys. 1943, 11, 512–520. [Google Scholar] [CrossRef]
- Marykutty, C.; Mathew, G.; Mathew, E.; Thomas, S. Studies on novel binary accelerator system in sulfur vulcanization of natural rubber. J. Appl. Polym. Sci. 2003, 90, 3173–3182. [Google Scholar] [CrossRef]
- Osaka, N.; Kato, M.; Saito, H. Mechanical properties and network structure of phenol resin crosslinked hydrogenated acrylonitrile-butadiene rubber. J. Appl. Polym. Sci. 2013, 129, 3396–3403. [Google Scholar] [CrossRef]
- Makó, É.; Senkár, Z.; Kristóf, J.; Vágvölgyi, V. Surface modification of mechanochemically activated kaolinites by selective leaching. J. Colloid. Interface. Sci. 2006, 294, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Panda, A.K.; Mishra, B.G.; Mishra, D.K.; Singh, R.K. Effect of sulphuric acid treatment on the physico-chemical characteristics of kaolin clay. Colloids Surf. A Physicochem. Eng. Asp. 2010, 363, 98–104. [Google Scholar] [CrossRef]
- White, R.D.; Bavykin, D.V.; Walsh, F.C. The stability of halloysite nanotubes in acidic and alkaline aqueous suspensions. Nanotechnology 2012, 23, 065705. [Google Scholar] [CrossRef]
- Gelling, I.R. Modification of natural rubber latex with peracetic acid. Rubb. Chem. Technol. 1985, 58, 86–96. [Google Scholar] [CrossRef]
- Li, S.M.; Xu, T.W.; Jia, Z.X.; Zhong, B.C.; Luo, Y.F.; Jia, D.M.; Peng, Z. Preparation and stress-strain behavior of in-situ epoxidized natural rubber/SiO2 hybrid through a sol-gel method. Expre. Polym. Lett. 2018, 12, 180–185. [Google Scholar] [CrossRef]
- Saklar, S.; Yorukoglu, A. Effects of acid leaching on halloysite. Physicochem. Probl. Miner. Process. 2015, 51, 83–94. [Google Scholar]
- Jehsoh, N.; Surya, I.; Sahakaro, K.; Ismail, H.; Hayeemasae, N. Modified palm stearin compatibilized natural rubber/halloysite nanotubes composites: Reinforcement versus strain-induced crystallization. J. Elast. Plast. 2021, 53, 210–227. [Google Scholar] [CrossRef]
- Hayeemasae, N.; Sensem, Z.; Surya, I.; Sahakaro, K.; Ismail, H. Synergistic Effect of Maleated Natural Rubber and Modified Palm Stearin as Dual Compatibilizers in Composites based on Natural Rubber and Halloysite Nanotubes. Polymers 2020, 12, 766. [Google Scholar] [CrossRef] [Green Version]
- Hayeemasae, N.; Sensem, Z.; Sahakaro, K.; Ismail, H. Maleated Natural Rubber/Halloysite Nanotubes Composites. Processes 2020, 8, 286. [Google Scholar] [CrossRef] [Green Version]
- Payne, A.R.; Whittaker, R.E. Low strain dynamic properties of filled rubbers. Rubber Chem. Technol. 1971, 44, 440–478. [Google Scholar] [CrossRef]
- Nun-anan, P.; Wisunthorn, S.; Pichaiyut, S.; Nathaworn, C.D.; Nakason, C. Influence of nonrubber components on properties of unvulcanized natural rubber. Polym. Adv. Technol. 2020, 31, 44–59. [Google Scholar] [CrossRef]
- Kuang, W.; Yang, Z.; Tang, Z.; Guo, B. Wrapping of polyrhodanine onto tubular clay and its prominent effects on the reinforcement of the clay for rubber. Compos. Part A Appl. Sci. Manuf. 2016, 84, 344–353. [Google Scholar] [CrossRef]
- Hernández, M.; López-Manchado, M.A.; Sanz, A.; Nogales, A.; Ezquerra, T.A. Effects of strain-induced crystallization on the segmental dynamics of vulcanized natural rubber. Macromolecules 2011, 44, 6574–6580. [Google Scholar] [CrossRef] [Green Version]
- Ozbas, B.; Toki, S.; Hsiao, B.S.; Chu, B.; Register, R.A.; Aksay, I.A.; Prud’homme, R.K.; Adamson, D.H. Strain-induced crystallization and mechanical properties of functionalized graphene sheet-filled natural rubber. J. Polym. Sci. Part B Polym. Phys. 2012, 50, 718–723. [Google Scholar] [CrossRef]
- Candau, N.; Oguz, O.; Federico, C.E.; Stoclet, G.; Tahon, J.-F.; Maspoch, M.L. Strain induced crystallization in vulcanized natural rubber containing ground tire rubber particles with reinforcement and nucleation abilities. Polym. Test. 2021, 101, 107313. [Google Scholar] [CrossRef]
- White, J.L.; Spruiell, J.E. The specification of orientation and its development in polymer processing. Polym. Eng. Sci. 1983, 23, 247–256. [Google Scholar] [CrossRef]














| Raw Material | Amount (phr) |
|---|---|
| ENR 20 | 100.0 |
| Stearic acid | 1.0 |
| Zinc oxide | 5.0 |
| HNT * | 5.0 |
| CBS | 2.0 |
| Sulfur | 2.0 |
| Sample | Ts2 (min) | Tc90 (min) | ML (dN·m) | MH (dN·m) | MH–ML (dN·m) | CRI (min−1) |
|---|---|---|---|---|---|---|
| E20 | 2.29 | 4.75 | 0.76 | 8.40 | 7.64 | 40.65 |
| E20A2 | 1.11 | 3.15 | 0.81 | 8.53 | 7.72 | 49.02 |
| E20A4 | 1.10 | 3.05 | 0.77 | 8.58 | 7.81 | 51.28 |
| E20A6 | 1.07 | 3.01 | 0.75 | 8.63 | 7.88 | 51.55 |
| E20A8 | 1.05 | 2.92 | 0.74 | 8.88 | 8.14 | 53.48 |
| Sample | M100 (MPa) | M300 (MPa) | TS (MPa) | EB (%) | Ts (N/mm) | Hardness (Shore A) |
|---|---|---|---|---|---|---|
| E20 | 0.86 ± 0.03 | 2.25 ± 0.03 | 33.67 ± 1.61 | 717 ± 10 | 38.29 ± 0.94 | 39.3 ± 0.3 |
| E20A2 | 0.89 ± 0.02 | 2.50 ± 0.07 | 34.84 ± 0.90 | 676 ± 30 | 38.38 ± 0.90 | 41.4 ± 0.5 |
| E20A4 | 0.92 ± 0.02 | 2.59 ± 0.08 | 35.45 ± 0.90 | 658 ± 22 | 39.60 ± 0.60 | 42.1 ± 0.2 |
| E20A6 | 0.93 ± 0.02 | 2.62 ± 0.12 | 32.15 ± 0.40 | 655 ± 25 | 37.51 ± 1.21 | 42.8 ± 0.8 |
| E20A8 | 0.95 ± 0.02 | 2.72 ± 0.05 | 31.00 ± 0.37 | 652 ± 26 | 35.96 ± 1.08 | 43.2 ± 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Surya, I.; Waesateh, K.; Masa, A.; Hayeemasae, N. Selectively Etched Halloysite Nanotubes as Performance Booster of Epoxidized Natural Rubber Composites. Polymers 2021, 13, 3536. https://doi.org/10.3390/polym13203536
Surya I, Waesateh K, Masa A, Hayeemasae N. Selectively Etched Halloysite Nanotubes as Performance Booster of Epoxidized Natural Rubber Composites. Polymers. 2021; 13(20):3536. https://doi.org/10.3390/polym13203536
Chicago/Turabian StyleSurya, Indra, Kamaruddin Waesateh, Abdulhakim Masa, and Nabil Hayeemasae. 2021. "Selectively Etched Halloysite Nanotubes as Performance Booster of Epoxidized Natural Rubber Composites" Polymers 13, no. 20: 3536. https://doi.org/10.3390/polym13203536
APA StyleSurya, I., Waesateh, K., Masa, A., & Hayeemasae, N. (2021). Selectively Etched Halloysite Nanotubes as Performance Booster of Epoxidized Natural Rubber Composites. Polymers, 13(20), 3536. https://doi.org/10.3390/polym13203536