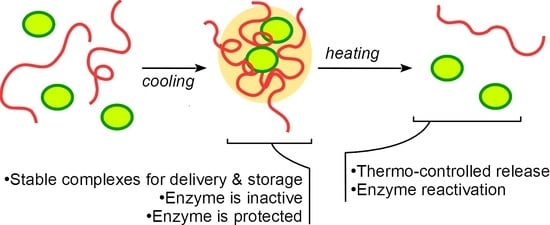
Thermocontrolled Reversible Enzyme Complexation-Inactivation-Protection by Poly(N-acryloyl glycinamide)
Abstract
:1. Introduction
2. Methods
2.1. Materials
2.2. Dynamic Light Scattering
2.3. Isothermal Titration Calorimetry
2.4. Preparation of the Complexes
2.5. Lysozyme Activity Assay
2.6. Proteinase K Proteolysis Assay
3. Results
3.1. Polymer-Enzyme Complexes Formed by the Mixture Cooling Are Stable in Cold but Dissolute When Heated
3.2. PNAGA Binds Lysozyme Only at LOW Temperature
3.3. Lysozyme in the Complexes Is Inactive
3.4. Encapsulation Protects Lysozyme from Proteolytic Degradation
4. Discussion
5. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kabanov, A.V.; Vinogradov, S.V. Nanogels as Pharmaceutical Carriers: Finite Networks of Infinite Capabilities. Angew. Chem. Int. Ed Engl. 2009, 48, 5418–5429. [Google Scholar] [CrossRef] [Green Version]
- Torchilin, V.P. Recent Advances with Liposomes as Pharmaceutical Carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef]
- Duncan, R. The Dawning Era of Polymer Therapeutics. Nat. Rev. Drug Discov. 2003, 2, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Gil, E.S.; Hudson, S.M. Stimuli-Reponsive Polymers and Their Bioconjugates. Prog. Polym. Sci. 2004, 29, 1173–1222. [Google Scholar] [CrossRef]
- Cheng, R.; Meng, F.; Deng, C.; Klok, H.-A.; Zhong, Z. Dual and Multi-Stimuli Responsive Polymeric Nanoparticles for Programmed Site-Specific Drug Delivery. Biomaterials 2013, 34, 3647–3657. [Google Scholar] [CrossRef] [PubMed]
- Mane, S.R.; Sathyan, A.; Shunmugam, R. Biomedical Applications of PH-Responsive Amphiphilic Polymer Nanoassemblies. ACS Appl. Nano Mater. 2020, 3, 2104–2117. [Google Scholar] [CrossRef]
- Rösler, A.; Vandermeulen, G.W.M.; Klok, H.-A. Advanced Drug Delivery Devices via Self-Assembly of Amphiphilic Block Copolymers. Adv. Drug Deliv. Rev. 2012, 64, 270–279. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, C.; Chang, H.; Zhang, Q.; Cheng, Y. Off-on Switching of Enzyme Activity by near-Infrared Light-Induced Photothermal Phase Transition of Nanohybrids. Sci. Adv. 2019, 5, eaaw4252. [Google Scholar] [CrossRef] [Green Version]
- Lu, D.; Liu, Z.; Zhang, M.; Wang, X.; Liu, Z. Dextran-Grafted-PNIPAAm as an Artificial Chaperone for Protein Refolding. Biochem. Eng. J. 2006, 27, 336–343. [Google Scholar] [CrossRef]
- Semenyuk, P.; Tiainen, T.; Hietala, S.; Tenhu, H.; Aseyev, V.; Muronetz, V. Artificial Chaperones Based on Thermoresponsive Polymers Recognize the Unfolded State of the Protein. Int. J. Biol. Macromol. 2019, 121, 536–545. [Google Scholar] [CrossRef] [Green Version]
- Welsch, N.; Wittemann, A.; Ballauff, M. Enhanced Activity of Enzymes Immobilized in Thermoresponsive Core−Shell Microgels. J. Phys. Chem. B 2009, 113, 16039–16045. [Google Scholar] [CrossRef]
- Schachschal, S.; Adler, H.-J.; Pich, A.; Wetzel, S.; Matura, A.; van Pee, K.-H. Encapsulation of Enzymes in Microgels by Polymerization/Cross-Linking in Aqueous Droplets. Colloid Polym. Sci. 2011, 289, 693–698. [Google Scholar] [CrossRef]
- Gawlitza, K.; Wu, C.; Georgieva, R.; Wang, D.; Ansorge-Schumacher, M.B.; von Klitzing, R. Immobilization of Lipase B within Micron-Sized Poly-N-Isopropylacrylamide Hydrogel Particles by Solvent Exchange. Phys. Chem. Chem. Phys. 2012, 14, 9594–9600. [Google Scholar] [CrossRef] [Green Version]
- Gawlitza, K.; Georgieva, R.; Tavraz, N.; Keller, J.; von Klitzing, R. Immobilization of Water-Soluble HRP within Poly-N-Isopropylacrylamide Microgel Particles for Use in Organic Media. Langmuir 2013, 29, 16002–16009. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wang, C.; Guo, W. Multifunctional Poly-N-Isopropylacrylamide/DNAzyme Microgels as Highly Efficient and Recyclable Catalysts for Biosensing. Adv. Funct. Mater. 2018, 28, 1705876. [Google Scholar] [CrossRef]
- Reinicke, S.; Fischer, T.; Bramski, J.; Pietruszka, J.; Böker, A. Biocatalytically Active Microgels by Precipitation Polymerization of N-Isopropyl Acrylamide in the Presence of an Enzyme. RSC Adv. 2019, 9, 28377–28386. [Google Scholar] [CrossRef] [Green Version]
- Cummings, C.; Murata, H.; Koepsel, R.; Russell, A.J. Dramatically Increased PH and Temperature Stability of Chymotrypsin Using Dual Block Polymer-Based Protein Engineering. Biomacromolecules 2014, 15, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Tenhu, H.; Hietala, S. Bicatalytic Poly(N-Acryloyl Glycinamide) Microgels. Eur. Polym. J. 2020, 133, 109760. [Google Scholar] [CrossRef]
- Kotsuchibashi, Y. Recent Advances in Multi-Temperature-Responsive Polymeric Materials. Polym. J. 2020, 52, 681–689. [Google Scholar] [CrossRef]
- Shimada, N.; Nakayama, M.; Kano, A.; Maruyama, A. Design of UCST Polymers for Chilling Capture of Proteins. Biomacromolecules 2013, 14, 1452–1457. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, W. Poly(N-Acryloyl Glycinamide): A Fascinating Polymer That Exhibits a Range of Properties from UCST to High-Strength Hydrogels. Chem. Commun. 2018, 54, 10540–10553. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, H.; Kasmi, S.; Van Herck, S.; Deswarte, K.; Lambrecht, B.N.; Hoogenboom, R.; Nuhn, L.; De Geest, B.G. A Synthetic, Transiently Thermoresponsive Homopolymer with UCST Behaviour within a Physiologically Relevant Window. Angew. Chem. Int. Ed. 2019, 58, 7866–7872. [Google Scholar] [CrossRef] [PubMed]
- Seuring, J.; Bayer, F.M.; Huber, K.; Agarwal, S. Upper Critical Solution Temperature of Poly(N-Acryloyl Glycinamide) in Water: A Concealed Property. Macromolecules 2012, 45, 374–384. [Google Scholar] [CrossRef]
- Seuring, J.; Agarwal, S. Polymers with Upper Critical Solution Temperature in Aqueous Solution. Macromol. Rapid Commun. 2012, 33, 1898–1920. [Google Scholar] [CrossRef]
- Mäkinen, L.; Varadharajan, D.; Tenhu, H.; Hietala, S. Triple Hydrophilic UCST–LCST Block Copolymers. Macromolecules 2016, 49, 986–993. [Google Scholar] [CrossRef]
- Deng, Y.; Käfer, F.; Chen, T.; Jin, Q.; Ji, J.; Agarwal, S. Let There Be Light: Polymeric Micelles with Upper Critical Solution Temperature as Light-Triggered Heat Nanogenerators for Combating Drug-Resistant Cancer. Small 2018, 14, 1802420. [Google Scholar] [CrossRef]
- Tomita, S.; Ito, L.; Yamaguchi, H.; Konishi, G.; Nagasaki, Y.; Shiraki, K. Enzyme Switch by Complementary Polymer Pair System (CPPS). Soft Matter 2010, 6, 5320–5326. [Google Scholar] [CrossRef] [Green Version]
- Evstafyeva, D.B.; Izumrudov, V.A.; Muronetz, V.I.; Semenyuk, P.I. Tightly Bound Polyelectrolytes Enhance Enzyme Proteolysis and Destroy Amyloid Aggregates. Soft Matter 2018, 14, 3768–3773. [Google Scholar] [CrossRef]
- Vikulina, A.S.; Feoktistova, N.A.; Balabushevich, N.G.; von Klitzing, R.; Volodkin, D. Cooling-Triggered Release from Mesoporous Poly(N-Isopropylacrylamide) Microgels at Physiological Conditions. ACS Appl. Mater. Interfaces 2020, 12, 57401–57409. [Google Scholar] [CrossRef]
- Matsumoto, K.; Kimura, S.; Itai, S.; Kondo, H.; Iwao, Y. In Vivo Temperature-Sensitive Drug Release System Trigged by Cooling Using Low-Melting-Point Microcrystalline Wax. J. Controlled Release 2019, 303, 281–288. [Google Scholar] [CrossRef]
- Käfer, F.; Lerch, A.; Agarwal, S. Tunable, Concentration-Independent, Sharp, Hysteresis-Free UCST Phase Transition from Poly(N-Acryloyl Glycinamide-Acrylonitrile) System. J. Polym. Sci. Part Polym. Chem. 2017, 55, 274–279. [Google Scholar] [CrossRef]
- Liu, F.; Seuring, J.; Agarwal, S. Controlled Radical Polymerization of N-Acryloylglycinamide and UCST-Type Phase Transition of the Polymers. J. Polym. Sci. Part Polym. Chem. 2012, 50, 4920–4928. [Google Scholar] [CrossRef]
- Sponchioni, M.; Bassam, P.R.; Moscatelli, D.; Arosio, P.; Palmiero, U.C. Biodegradable Zwitterionic Nanoparticles with Tunable UCST-Type Phase Separation under Physiological Conditions. Nanoscale 2019, 11, 16582–16591. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Hao, B.; Tang, Y.; Li, H.; Lee, T.-C.; Feng, A.; Zhang, L.; Thang, S.H. Effect of End-Groups on Sulfobetaine Homopolymers with the Tunable Upper Critical Solution Temperature (UCST). Eur. Polym. J. 2020, 132, 109704. [Google Scholar] [CrossRef]
- Xue, X.; Thiagarajan, L.; Braim, S.; Saunders, B.R.; Shakesheff, K.M.; Alexander, C. Upper Critical Solution Temperature Thermo-Responsive Polymer Brushes and a Mechanism for Controlled Cell Attachment. J. Mater. Chem. B 2017, 5, 4926–4933. [Google Scholar] [CrossRef] [PubMed]
- Tamarov, K.P.; Osminkina, L.A.; Zinovyev, S.V.; Maximova, K.A.; Kargina, J.V.; Gongalsky, M.B.; Ryabchikov, Y.; Al-Kattan, A.; Sviridov, A.P.; Sentis, M.; et al. Radio Frequency Radiation-Induced Hyperthermia Using Si Nanoparticle-Based Sensitizers for Mild Cancer Therapy. Sci. Rep. 2014, 4, 7034. [Google Scholar] [CrossRef]
- Fox, M.E.; Szoka, F.C.; Fréchet, J.M.J. Soluble Polymer Carriers for the Treatment of Cancer: The Importance of Molecular Architecture. Acc. Chem. Res. 2009, 42, 1141–1151. [Google Scholar] [CrossRef] [Green Version]
- Boustta, M.; Colombo, P.-E.; Lenglet, S.; Poujol, S.; Vert, M. Versatile UCST-Based Thermoresponsive Hydrogels for Loco-Regional Sustained Drug Delivery. J. Controlled Release 2014, 174, 1–6. [Google Scholar] [CrossRef]
- Wu, Q.; Wei, J.; Xu, B.; Liu, X.; Wang, H.; Wang, W.; Wang, Q.; Liu, W. A Robust, Highly Stretchable Supramolecular Polymer Conductive Hydrogel with Self-Healability and Thermo-Processability. Sci. Rep. 2017, 7, 41566. [Google Scholar] [CrossRef] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semenyuk, P.I.; Kurochkina, L.P.; Mäkinen, L.; Muronetz, V.I.; Hietala, S. Thermocontrolled Reversible Enzyme Complexation-Inactivation-Protection by Poly(N-acryloyl glycinamide). Polymers 2021, 13, 3601. https://doi.org/10.3390/polym13203601
Semenyuk PI, Kurochkina LP, Mäkinen L, Muronetz VI, Hietala S. Thermocontrolled Reversible Enzyme Complexation-Inactivation-Protection by Poly(N-acryloyl glycinamide). Polymers. 2021; 13(20):3601. https://doi.org/10.3390/polym13203601
Chicago/Turabian StyleSemenyuk, Pavel I., Lidia P. Kurochkina, Lauri Mäkinen, Vladimir I. Muronetz, and Sami Hietala. 2021. "Thermocontrolled Reversible Enzyme Complexation-Inactivation-Protection by Poly(N-acryloyl glycinamide)" Polymers 13, no. 20: 3601. https://doi.org/10.3390/polym13203601
APA StyleSemenyuk, P. I., Kurochkina, L. P., Mäkinen, L., Muronetz, V. I., & Hietala, S. (2021). Thermocontrolled Reversible Enzyme Complexation-Inactivation-Protection by Poly(N-acryloyl glycinamide). Polymers, 13(20), 3601. https://doi.org/10.3390/polym13203601