Peculiarities of Oxidative Polymerization of Diarylaminodichlorobenzoquinones
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of Monomers
2.2.1. Synthesis of 2,5-Dianiline-3,6-dichloro-1,4-benzoquinone (DADCB)
2.2.2. Synthesis of 2-Methoxy-5-anilino-3,6-dichloro-1,4-benzoquinone (MADCB)
2.2.3. Synthesis of 2,5-Diphenylenediamino-3,6-dichloro-1,4-benzoquinone (DPDCB)
2.2.4. Synthesis of 2,5-Di(aniline-3-sulfo)-3,6-dichloro-1,4-benzoquinone (DASDCB)
2.2.5. Synthesis of 2-(Aniline-3-sulfo)-5-anilino-3,6-dichloro-1,4-benzoquinone (ASADCB)
2.3. Oxidative Polymerization of the Obtained Monomers
2.4. Characterization
3. Results and Discussion
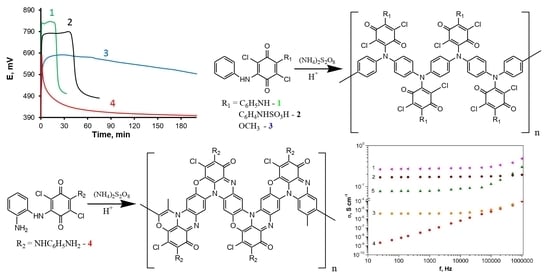
3.1. The Oxidative Polymerization of 2,5-Dianiline-3,6-dichloro-1,4-benzoquinone (DADCB)
3.2. Study of the Oxidation of 2-Methoxy-5-anilino-3,6-dichloro-1,4-benzoquinone (MADCB)
3.3. The Effect of Substituent Types in Arylamine Groups of the Quinone Ring on the Oxidation Reaction Rate and the Structure of the Resulting Polymers
3.3.1. Oxidative Polymerization of 2,5-Diphenylenediamino-3,6-dichloro-1,4-benzoquinone (DPDCB)
3.3.2. Oxidative Polymerization of 2-(Aniline-3-sulfo)-5-anilino-3,6-dichloro-1,4-benzoquinone (ASADCB)
3.4. Some Properties of the Obtained Polydiarylaminodichlorobenzoquinones
- σdc—the frequency independent part of conductivity,
- n—the exponential parameter (0 ≤ n ≤ 1),
- A—the thermally activated quantity.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tomsík, E.; Kohut, O.; Ivanko, I.; Pekarek, M.; Bieloshapka, I.; Dallas, P. Assembly and interaction of polyaniline chain: Impact on electro- and physical-chemical behavior. J. Phys. Chem. 2018, 122, 8022–8030. [Google Scholar] [CrossRef]
- Wang, Y.; Tran, H.D.; Liao, L.; Duan, X.; Kaner, R.B. Nanoscale morphology, dimensional control and electrical properties of oligoanilines. J. Am. Chem. Soc. 2010, 132, 10365–10373. [Google Scholar] [CrossRef] [Green Version]
- Zoromba, M.S.; Abdel-Aziz, M.H.; Bassiouni, M.; Attar, A.; Al-Hossainy, A.F. Synthesis and characterization of poly(orto-aminophenol-co-para-toluidine) and its application as semiconductor thin film. J. Mol. Struct. 2021, 1225, 129131. [Google Scholar] [CrossRef]
- Almurlaq, N.; Al-Hossainy, A.F.; Zoromba, M.S. Combined experimental and theoretical study, characterization and nonlinear optical properties of doped poly(p-nitro aniline-co-o-aminophenol) thin film. J. Mol. Struct. 2021, 1227, 129712. [Google Scholar] [CrossRef]
- Loza, N.V.; Falina, I.V.; Kononenko, N.A.; Kudashova, D.S. Some aspects of polyaniline template synthesis within and on the surface of perfluorinated cation exchange membrane. Synth. Met. 2020, 261, 116292. [Google Scholar] [CrossRef]
- Zoromba, M.S.; Alshehri, A.A.; Al-Hossainy, A.F.; Abdel-Aziz, M.H. Doped poly (antranilic acid-co-phenylenediamine) thin film for optoelectronic application. Opt. Mater. 2021, 111, 110621. [Google Scholar] [CrossRef]
- Matsuura, Y. Tunnel magnetoresistance in polyaniline. Synth. Met. 2018, 243, 90–96. [Google Scholar] [CrossRef]
- Lakschmi, M.S.; Wabaidur, S.M.; Althoman, Z.A.; Ragupathy, D. Novel 1D polyaniline nanorods for electrochemical supercapacitors: A facile and green approach. Synth. Met. 2020, 270, 116591. [Google Scholar] [CrossRef]
- Song, S.; Xu, G.; Wang, B.; Gu, J.; Wei, H.; Ren, Z.; Zhang, L.; Zhao, J.; Li, Y. Highly-flexible monolithic integrated infrared electrochromic device based on polyaniline conducting polymer. Synth. Met. 2021, 278, 116822. [Google Scholar] [CrossRef]
- Orlov, A.V.; Kiseleva, S.G.; Karpacheva, G.P.; Teplyakov, V.V.; Syrtsova, D.A.; Starannikova, L.E.; Lebedeva, T.L. Composite films based on polyaniline: Structure and gas separation properties. J. Appl. Pol. Sci. 2003, 89, 1379–1384. [Google Scholar] [CrossRef]
- Bhadra, S.; Khastgir, D.; Singha, N.K.; Lee, H.J. Progress in preparation, processing and applications of polyaniline. Prog. Polym. Sci. 2009, 34, 783–810. [Google Scholar] [CrossRef]
- Jaymand, M. Recent progress in chemical modification of polyaniline. Prog. Polym. Sci. 2013, 38, 1287–1306. [Google Scholar] [CrossRef]
- Sapurina, I.Y.; Stejskal, J. Effect of the pH on the oxidative polymerization of aniline and the morphology and properties of the products. Rus. Chem. Rev. 2010, 79, 1218–1239. [Google Scholar] [CrossRef]
- Ćirić-Marjanović, G. Recent advances in polyaniline research: Polymerization mechanisms, structural aspects, properties and applications. Synth. Met. 2013, 177, 1–47. [Google Scholar] [CrossRef]
- Jangid, N.K.; Jadoun, S.; Kaur, N. A review on high-throughput synthesis, deposition of thin films and properties of polyaniline. Eur. Polym. J. 2020, 125, 109485. [Google Scholar] [CrossRef]
- De Paiva, A.B.; Correrj, G.I.; Ugucioni, J.C.; Carvalho, G.R.; Jasinevicius, R.G.; de Godoy, M.P.F. On the photoconductivity behavior of emeraldine-salt polyaniline films. Synth. Met. 2021, 201, 116915. [Google Scholar] [CrossRef]
- Lin, Y.-F.; Chen, C.-H.; Xie, W.-J.; Yang, C.-S.; Hsu, C.-T.; Hsu, W.-B. Nano approach investigation of the conduction mechanism in polyaniline nanofibers. ACS Nano 2011, 5, 1541–1548. [Google Scholar] [CrossRef] [PubMed]
- Xin Li, X.; Guiyang, Y.; Wang, J.; Kong, W.; Chang, X.; Zhuang, Y.; Meng, F. Effect of a temperature threshold on the electrorheological performanceof ionic liquid crystal polyanilines. J. Mol. Liq. 2021, 326, 115299. [Google Scholar] [CrossRef]
- Zhang, Y.; Duan, Y.; Liu, J.; Ma, G.; Huang, M. Wormlike acid-doped polyaniline: Controllable electrical properties and theoretical investigation. J. Phys. Chem. C. 2018, 122, 2032–2040. [Google Scholar] [CrossRef]
- Rahy, A.; Sakrout, M.; Manohar, S.; Cho, S.J.; Ferraris, J.; Yang, D.J. Polyaniline nanofiber synthesis by co-use of ammonium peroxydisulfate and sodium hypochlorite. Chem. Mater. 2008, 20, 4808–4814. [Google Scholar] [CrossRef]
- Yu, L.; Lee, J.-I.; Shin, K.-W.; Park, C.-E.; Holze, R. Preparation of aqueous polyaniline dispersions by micellar-aided polymerization. J. Appl. Polym. Sci. 2003, 88, 1550–1555. [Google Scholar] [CrossRef]
- Sedenkova, I.; Konuschenko, E.N.; Stejskal, J.; Trchova, M.; Prokes, J. Solid-state oxidation of aniline hydrochloride with various oxidants. Synth. Met. 2011, 161, 1353–1360. [Google Scholar] [CrossRef]
- Jiang, L.; Cui, Z. One-step synthesis of oriented polyaniline nanorods through electrochemical deposition. Polym. Bull. 2006, 56, 529–537. [Google Scholar] [CrossRef]
- Orlov, A.V.; Kiseleva, S.G.; Yurchenko, O.Y.; Karpacheva, G.P. Oxidative polymerization of aniline in the presence of additional substrate. Polym. Sci. A 2000, 42, 1292–1297. [Google Scholar]
- Ullah, R.; Yaseen, S.; Ali Shah, A.-U.-H.; Bilal, S.; Kamran, M.; Rahim, M. Anticorrosive polyaniline synthesized using coconut oil as the dispersion medium. Mater. Chem. Phys. 2021, 273, 125071. [Google Scholar] [CrossRef]
- Modaressi, A.R.; Amirazizi, H.A.; Movahedifar, F.M.; Farrokhzadeh, A.; Asli, G.R.; Nahavandi, H. The first report of polymerization of aniline bearing chiral alkyl group on ring via covalent bond: Poly[(±)-2-(sec-butyl)aniline]. J. Mol. Struct. 2015, 1083, 17–26. [Google Scholar] [CrossRef]
- Luo, C.; Peng, H.; Zhang, L.; Lu, G.-L.; Wang, Y.; Travas-Sejdic, J. Formation of nano-/microstructure of polyaniline and its derivatives. Macromolecules 2011, 44, 6899–6907. [Google Scholar] [CrossRef]
- Tran, H.D.; Norris, I.; D’Arcy, J.M.; Tsang, H.; Wang, Y.; Mattes, B.R.; Kaner, R.B. Substituted polyaniline nanofibers produced via rapid initiated polymerization. Macromolecules 2008, 41, 7405–7410. [Google Scholar] [CrossRef]
- Surwade, S.P.; Agnihotra, S.R.; Dua, V.; Kolla, H.S.; Zhang, X.; Manohar, S.K. Chromism and molecular weight of polyaniline derivatives. Synth. Met. 2009, 159, 2153–2156. [Google Scholar] [CrossRef]
- Sayyah, S.M.; Abd El-Khalek, A.A.; Bahgat, A.A.; Abd El-Salam, A.A. Kinetic studies of the chemical polymerization of substituted aniline in aqueous solutions and characterization of the polymer obtained. Part 1. 3-Chloraniline. Polym. Int. 2001, 50, 197–206. [Google Scholar] [CrossRef]
- Amer, I.; Mokrani, T.; Jewell, L.; Young, D.A.; Vosloo, H.C.M. Synthesis and characterization of sulfonated poly(p-phenylenediamine) prepared by different procedures. Polymer 2015, 66, 230–239. [Google Scholar] [CrossRef]
- Roman, P.; Cruz-Silva, R.; Vazquez-Duhalt, R. Peroxidase-mediated synthesis of water-soluble fully sulfonated polyaniline. Synth. Met. 2012, 162, 794–799. [Google Scholar] [CrossRef]
- Sanches, C.O.; Bustos, C.J.; Carey, M.-L.D.A. Effect of electron-withdrawing type substituents in the polyaniline ring on the electrical conductivity. Polym. Bull. 2005, 54, 263–270. [Google Scholar] [CrossRef]
- Samanta, S.; Roy, P.; Kar, P. Influence of structure of poly(o-phenylenediamine) on the doping ability and conducting property. Ionics 2017, 23, 937–947. [Google Scholar] [CrossRef]
- Yagmur, H.K.; Kaya, I. Synthesis and characterization of new polymers derived from 2-methylenediamine as an effective adsorbent for cationic dye removal. Arab. J. Chem. 2020, 13, 8183–8199. [Google Scholar] [CrossRef]
- Stejscal, J. Polymers of phenylenediamine. Prog. Polym. Sci. 2015, 41, 1–31. [Google Scholar] [CrossRef]
- Blaha, M.; Trchova, M.; Moravkova, Z.; Humpolicek, P.; Stejskal, J. Semiconducting materials from oxidative coupling of phenylenediamines under various acidic conditions. Mater. Chem. Phys. 2018, 205, 423–435. [Google Scholar] [CrossRef]
- Zoromba, M.S.; Abdel-Aziz, M.H. Ecofriendly method to synthesize poly(o-aminophenol) based on solid state polymerization and fabrication of nanostructured semiconductor thin film. Polymer 2017, 120, 20–29. [Google Scholar] [CrossRef]
- Shenashen, M.A.; Okamoto, T.; Haraguchi, M. Study the effect of phenylenediamine compounds on the chemical polymerization of aniline. React. Funct. Polym. 2011, 71, 766–773. [Google Scholar] [CrossRef]
- Orlov, A.V.; Ozkan, S.Z.; Bondarenko, G.N.; Karpacheva, G.P. Oxidative polymerization of diphenylamine: Synthesis and structure of polymers. Polym. Sci. B 2006, 48, 5–10. [Google Scholar] [CrossRef]
- Nakahaschi, C.; Moriya, S.; Fugono, N.; Lee, H.C.; Sato, H. Preparation and characterization of poly(4-alkyltriphenylamine) by chemical oxidative polymerization. Synth. Met. 2002, 129, 123–128. [Google Scholar]
- Falcou, A.; Duchene, A.; Hourquebi, P.; Marsacq, D.; Balland-Longeau, A. A new chemical polymerization process for substituted anilines: Application to the synthesis of poly(N-alkylanilines) and poly(o-alkylanilines) and comparison of their respective properties. Synth. Met. 2005, 149, 115–122. [Google Scholar] [CrossRef]
- Batra, M.; Kriplani, P.; Batra, C.; Ojha, K.G. An efficient synthesis and biological activity of substituted p-benzoquinones. Bioorganic Med. Chem. 2006, 14, 8519–8526. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Ji, B. Investigation on the redox mechanism of polyaniline film in acidsolution by in situ rapid-scan time-resolved infraredspectroelectrochemistry. J. Electroanal. Chem. 2015, 743, 60–67. [Google Scholar] [CrossRef]
- Shimano, J.Y.; MacDiarmid, A.G. Polyaniline, a dynamic block copolymer: Key to attaining its intrinsic conductivity? Synth. Met. 2001, 123, 251–262. [Google Scholar] [CrossRef]
- Mezhuev, Y.O.; Korshak, Y.V.; Shtilman, M.I.; Pokhil, S.E.; Strakhov, I.S. Kinetic features of N-ethylaniline polymerization. Russ. J. Gen. Chem. 2015, 85, 1482–1486. [Google Scholar] [CrossRef]
- Gautam, B.P.S.; Srivatsava, M.; Prasad, R.L.; Yadav, R.A. Synthesis, characterization and quantum chemical investigation of molecular structure and vibrational spectra of 2,5-dichloro-3,6-bis-(methylamino)1,4-benzoquinone. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 129, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Bhat, N.V.; Seshadri, D.T.; Phadke, S.R. Simultaneous polymerization and crystallization of aniline. Synth. Met. 2002, 130, 185–192. [Google Scholar] [CrossRef]
- Huerta, F.; Quijada, C.; Montilla, F.; Morallón, E. Revisiting the redox transitions of Polyaniline. Semiquantitativeinterpretation of electrochemically induced IR bands. J. Electroanal. Chem. 2021, 897, 115593. [Google Scholar] [CrossRef]
- Sivakumar, R.; Saraswathi, R. Redox properties of poly(N-methylaniline). Synth. Met. 2003, 138, 381–390. [Google Scholar] [CrossRef]
- Refat, M.S.; Ibrahim, O.B.; Al-Didamony, H.; El-Noir, K.M.A.; El-Zayat, L. Spectroscopic and thermal studies on the charge transfer complexes formed between morpholine as donor with p-chloranil and 7,7′,8,8′-tetracyanodimethane. J. Saudi Chem. Soc. 2012, 16, 227–235. [Google Scholar] [CrossRef] [Green Version]
- Silva, C.H.B.; Ferreira, D.C.; Ando, R.A.; Temperini, M.L.A. Aniline-1,4-benzoquinone as a model system for the characterization of products from aniline olygomerization in low acidic media. Chem. Phys. Lett. 2012, 551, 130–133. [Google Scholar] [CrossRef]
- Mezhuev, Y.O.; Korshak, Y.V. Theory of chain growth in chemical oxidative polymerization of aniline derivatives. Synthetic Met. 2020, 267, 116445. [Google Scholar] [CrossRef]
- Surwade, S.P.; Dua, V.; Manohar, N.; Manohar, S.K.; Beck, E.; Ferraris, J.P. Oligoaniline intermediates in the aniline-peroxydisulfate system. Synth. Met. 2009, 159, 445–455. [Google Scholar] [CrossRef]
- Stejskal, J.; Bober, P.; Trchova, M.; Horsky, J.; Pilar, J. The oxidation of aniline with p-benzoquinone and its impact on the preparation of the conducting polymer, polyaniline. Synth. Met. 2014, 192, 66–73. [Google Scholar] [CrossRef]
- Kiseleva, S.G.; Orlov, A.V.; Bondarenko, G.N.; Karpacheva, G.P. Oxidative polymerization of 3,6-dianiline-2,5-dichlorbenzoquinone and its copolymerization with aniline. Polym. Sci. B 2018, 60, 717–726. [Google Scholar] [CrossRef]
- Orlov, A.V.; Ozkan, S.Z.; Karpacheva, G.P. Oxidative polymerization of diphenylamine: A mechanism study. Polym. Sci. B 2006, 48, 11–17. [Google Scholar] [CrossRef]
- Nateghi, M.R.; Zahedi, M.; Mosslemin, M.H.; Hashemian, S.; Behzad, S.; Minnai, A. Autoacceleration/degradation of electrochemical polymerization of substituted anilines. Polymer 2005, 46, 11476–11483. [Google Scholar] [CrossRef]
- Mazaikiene, R.; Niaura, G.; Malinauskas, A. Voltammetric study of the redox processes of self-doped sulfonated polyaniline. Synth. Met. 2003, 139, 89–94. [Google Scholar] [CrossRef]
- Rakič, A.A.; Vukomanovic, M.; Trifunovic, S.; Traves-Sejdic, J.; Chaudhaty, O.J.; Horsky, J.; Ćirić-Marjanović, G. Solvent effects on dopant-free pH-falling polymerization of aniline. Synth. Met. 2015, 209, 279–296. [Google Scholar] [CrossRef]
- Ansari, M.H.; Parsa, J.B.; Arjomandi, J. Application of conducting polyaniline, o-anisidine, o-phenetidine and o-chloraniline in removal of nitrate from water via electrically switching ion exchange: Modeling and optimization using a response surface methodology. Sep. Purif. Technol. 2017, 179, 104–117. [Google Scholar] [CrossRef]
- Yalcinkaya, S. Electrochemical synthesis of poly(o-anisidine)/chitosan composite on platinum and mild steel electrodes. Prog. Org. Coat. 2013, 76, 181–187. [Google Scholar] [CrossRef]
- Zang, L.; Yuan, W.; Yan, Y. In situ UV–vis spectroeletrochemical studies on the copolymerization of o-phenylenediamine and o-methoxy aniline. Electrochim. Acta 2013, 113, 218–228. [Google Scholar] [CrossRef]
- Refat, M.S.; El-Zayat, L.; Yesilel, O.Z. Synthesis and spectroscopic characterization of piperidine/chloranil and piperidine/7,7′,8,8′-tetracyanoquinodimethane charge transfer complexes: X-ray cristal structure of a 7,7′-dicyano-8,8′-piperidinoquinodimethane adduct. Polyhedron 2008, 27, 475–484. [Google Scholar] [CrossRef]
- Mahipal, V.; Venkatesh, N.; Neveen, B.; Suresh, G.; Manaiah, V.; Parthasarathy, T. Catalytic activity and DNA binding applications of Benzhydrylpiperazine and p-Chloranil charge transfer complex: Synthesis, spectroscopic, and DFT studies. Chem. Data Collect. 2020, 28, 100474. [Google Scholar] [CrossRef]
- Sharma, K.; Sharma, P.S.; Lahiri, S.C. Spectrophotometric, Fourier transform infrared spectroscopic and theoretical studies of the charge-transfer complexes between methyldopa[(S)-2-amino-3-(3,4-dihydroxyphenyl)-2-methyl propanoic acid] and the acceptors (chloranilic acid, o-chloranil and dichlorodicyanobenzoquinone) in acetonitrile and their thermodynamic properties. Spectrochim. Acta. Part A Mol. Biomol. Spectrosc. 2012, 92, 212–224. [Google Scholar] [CrossRef]
- Cruz-Estrada, R.H. On the characterization of an electrically conductive polyaniline complex. J. Mater. Sci. 2004, 39, 511–518. [Google Scholar] [CrossRef]
- Bazzi, H.S.; Mostafa, A.; AlQaradawi, S.Y.; Nour, E.-M. Synthesis and spectroscopic structural investigations of the charge-transfer complexes formed in the reaction of 2,6-diaminopyridine with π-acceptors TCNE, chloranil and DDQ. J. Mol. Struct. 2007, 842, 1–5. [Google Scholar] [CrossRef]
- Prasad, R.L.; Kushwaha, A.; Kumar, S.M.; Yadav, R.A. Infrared and ab initio studies of conducting molecules: 2,5-Diamino-3,6-dichloro-1,4-benzoquinone. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2008, 69, 304–311. [Google Scholar] [CrossRef]
- Benyoucef, A.; Huerta, F.; Vazquez, J.L.; Morallon, E. Synthesis and in situ FTIRS characterization of conducting polymers obtained from aminobenzoic acid isomers at platinum electrodes. Eur. Polym. J. 2005, 41, 843–852. [Google Scholar] [CrossRef] [Green Version]
- Ren, S.; Yang, X.; Zhao, X.; Zhang, Y.; Huang, W. An m-phenylenediamine-based benzoxazine with favorable processability and its high-performance thermoset. J. Appl. Polym. Sci. 2016, 133, 43368–43378. [Google Scholar] [CrossRef]
- Fatuch, J.C.; Soto-Oviedo, M.A.; Avellaneda, C.O.; Franco, M.F.; Romao, W.; De Paoli, M.-A.; Nogueira, A.F. Synthesis and characterization of aniline copolymers containing carboxylic groups and their application as sensitizer and hole conductor in solar cells. Synth. Met. 2009, 159, 2348–2354. [Google Scholar] [CrossRef]
- Tucceri, R.; Arnal, P.M.; Scian, A.N. Spectroscopic Characterization of Poly(ortho-Aminophenol) Film Electrodes: A Review Article. J. Spectrosc. 2013, 951604. [Google Scholar] [CrossRef]
- Marjanović, B.; Yuranić, I.; Ćirić-Marjanović, G.; Pašti, I.; Trchova, M. Chemical oxidative polymerization of benzocaine. React. Funct. Polym. 2011, 71, 704–712. [Google Scholar] [CrossRef]
- Ozkan, S.Z.; Karpacheva, G.P.; Bondarenko, G.N. Polymers of phenoxazine: Synthesis, structure. Russ. Chem. Bull. Int. Ed. 2011, 60, 1651–1656. [Google Scholar] [CrossRef]
- Resada, N.; Park, J.; Ryu, K. Laccase-catalyzed polymerization of m-phenylenediamine in aqueous buffers. Korean J. Chem. Eng. 2016, 33, 3011–3015. [Google Scholar] [CrossRef]
- Zhang, L.; Chai, L.; Wang, H.; Yang, Z. Facile synthesis of one-dimensional self-assembly oligo(o-phenylenediamine) materials by ammonium persulfate in acidic solution. Mater. Lett. 2010, 64, 1193–1196. [Google Scholar] [CrossRef]
- Carbone, M.E.; Ciriello, R.; Granafai, S.; Guerriari, A.; Salvi, A.M. Electrosynthesis of conducting poly(o-aminophenol) films on Pt substrates: A combined electrochemical and XPS investigation. Electrochim. Acta 2014, 144, 174–185. [Google Scholar] [CrossRef]
- Al-Mashat, L.; Shin, K.; Kalantar-Zadeh, K.; Plessis, J.D.; Kojima, H.R.W.; Kaner, R.B.; Li, D.; Gou, X.; Ippolito, S.J.; Wodarski, W.W. Graphene/Polyaniline Nanocomposite for Hydrogen Sensing. J. Phys. Chem. C. 2010, 114, 16168–16173. [Google Scholar] [CrossRef]
- Bian, L.-J.; Luan, F.; Liu, S.-S.; Liu, X.-X. Self-doped polyaniline on functionalized carbon cloth as electroactive materials for supercapacitor. Electrochim. Acta 2012, 64, 17–22. [Google Scholar] [CrossRef]
- Xie, P.; Li, Y.; Hou, Q.; Sui, K.; Liu, C.; Fu, X.; Zhang, J.; Murugadoss, V.; Fan, J.; Wang, Y.; et al. Tunneling-induced negative permittivity in Ni/MnO nanocomposites by a bio-gel derived strategy. J. Mater. Chem. C. 2020, 8, 3029–3039. [Google Scholar] [CrossRef]
- Ozkan, S.Z.H.; Kostev, A.I.; Karpacheva, G.P.; Chernavsky, P.A.; Vasilev, A.A.; Muratov, D.G. Hybrid Electromagnetic nanomaterials based on polydiphenylamine-2-carboxylic acid. Polymers 2020, 12, 1568. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, S.Z.; Karpacheva, G.P.; Efimov, M.N.; Vasilev, A.A.; Muratov, D.G.; Petrov, V.A.; Chernavskii, P.A.; Pankina, G.V. One-step synthesis, characterization and properties of novel hybrid electromagnetic nanomaterials based on polydiphenylamine and Co-Fe particles in the absence and presence of single-walled carbon nanotubes. RSC Advances 2021, 11, 24772. [Google Scholar] [CrossRef]



















| Sample | C | N | O | Cl | O/C | N/C | Cl/C |
|---|---|---|---|---|---|---|---|
| DPDCB | 71.2 | 15.3 | 7.1 | 6.4 | 0.1 | 0.21 | 0.09 |
| poly-DPDCB | 72.0 | 16.7 | 8.2 | 3.1 | 0.11 | 0.23 | 0.04 |
| Sample | σdc, S/cm | σac (25 Hz) | σac (1 MHz) | n |
|---|---|---|---|---|
| PANI | 1.7 × 10−1 | 1.8 × 10−1 | 3 × 10−1 | 0.39 |
| poly-DADCB | 2.6 × 10−1 | 2.6 × 10−1 | 4.8 × 10−1 | 0.79 |
| poly-MADCB | 1.4 × 10−9 | 1.8 × 10−9 | 9.2 × 10−5 | 0.99 |
| poly-DPDCB | 3.6 × 10−6 | 5.4 × 10−7 | 1.1 × 10−4 | 0.99 |
| poly-ASADCB | 7.7 × 10−2 | 7.7 × 10−2 | 2.9 × 10−1 | 0.76 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orlov, A.V.; Kiseleva, S.G.; Karpacheva, G.P.; Muratov, D.G. Peculiarities of Oxidative Polymerization of Diarylaminodichlorobenzoquinones. Polymers 2021, 13, 3657. https://doi.org/10.3390/polym13213657
Orlov AV, Kiseleva SG, Karpacheva GP, Muratov DG. Peculiarities of Oxidative Polymerization of Diarylaminodichlorobenzoquinones. Polymers. 2021; 13(21):3657. https://doi.org/10.3390/polym13213657
Chicago/Turabian StyleOrlov, Andrey V., Svetlana G. Kiseleva, Galina P. Karpacheva, and Dmitriy G. Muratov. 2021. "Peculiarities of Oxidative Polymerization of Diarylaminodichlorobenzoquinones" Polymers 13, no. 21: 3657. https://doi.org/10.3390/polym13213657
APA StyleOrlov, A. V., Kiseleva, S. G., Karpacheva, G. P., & Muratov, D. G. (2021). Peculiarities of Oxidative Polymerization of Diarylaminodichlorobenzoquinones. Polymers, 13(21), 3657. https://doi.org/10.3390/polym13213657