Polyamidoamines Derived from Natural α-Amino Acids as Effective Flame Retardants for Cotton
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Synthesis of PAAs
2.4. Treatment of Cotton Fabrics with PAAs
2.5. Combustion Tests of PAAs and PAA-Treated Cotton Fabrics
3. Results and Discussion
3.1. Synthesis of PAAs
3.2. Thermal Stability of PAAs
3.3. Ignitability of PAAs
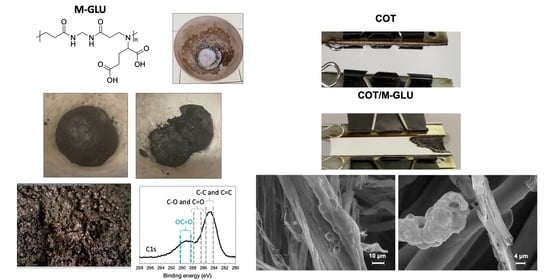
3.4. Morphological Characterization of Cotton Fabrics Treated with 7% Add-On PAAs
3.5. Thermal Characterization of PAA-Treated Cotton Fabrics
3.6. Combustion Studies of PAA-Treated Cotton Fabrics
3.6.1. Horizontal Flame Spread Tests (HFSTs)
3.6.2. Cone Calorimetry Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Danusso, F.; Ferruti, P. Synthesis of tertiary amine polymers. Polymer 1970, 11, 88–113. [Google Scholar] [CrossRef]
- Ferruti, P. Polyamidoamines: Past, Present and Perspectives. J. Polym. Sci. Polym. Chem. 2013, 51, 2319–2353. [Google Scholar] [CrossRef]
- Ranucci, E.; Manfredi, A. Polyamidoamines: Versatile bioactive polymers with potential for biotechnological applications. Chem. Afr. 2019, 2, 167–193. [Google Scholar] [CrossRef] [Green Version]
- Mather, B.D.; Visvanathan, K.; Millerb, K.M.; Long, T.E. Michael addition reactions in macromolecular design for emerging technologies. Prog. Polym. Sci. 2006, 31, 487–531. [Google Scholar] [CrossRef]
- Magnaghi, V.; Conte, V.; Procacci, P.; Pivato, G.; Cortese, P.; Cavalli, E.; Pajardi, G.; Ranucci, E.; Fenili, F.; Manfredi, A.; et al. Biological performance of a novel biodegradable polyamidoamine hydrogel as guide for peripheral nerve regeneration. J. Biomed. Mater. Res.-Part A 2011, 98, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Maggioni, D.; Fenili, F.; D’Alfonso, L.; Donghi, D.; Panigati, M.; Zanoni, I.; Marzi, R.; Manfredi, A.; Ferruti, P.; D’Alfonso, G.; et al. Luminescent Rhenium and Ruthenium Complexes of an Amphoteric Poly(amidoamine) Functionalized with 1,10-Phenanthroline. Inorg. Chem. 2012, 51, 12776–12788. [Google Scholar] [CrossRef]
- Ferruti, P.; Mauro, N.; Falciola, L.; Pifferi, V.; Bartoli, C.; Gazzarri, M.; Chiellini, F.; Ranucci, E. Amphoteric, Prevailingly Cationic L-Arginine Polymers of Poly(amidoamino acid) Structure: Synthesis, Acid/Base Properties and Preliminary Cytocompatibility and Cell-Permeating Characterizations. Macromol. Biosci. 2014, 14, 390–400. [Google Scholar] [CrossRef]
- Ferruti, F.; Alongi, J.; Manfredi, A.; Ranucci, E.; Ferruti, P. Controlled Synthesis of Linear Polyamidoamino Acids. Polymers 2019, 11, 1324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manfredi, A.; Carosio, F.; Ferruti, P.; Ranucci, E.; Alongi, J. Linear polyamidoamines as novel biocompatible phosphorus-free sur-face-confined intumescent flame retardants for cotton fabrics. Polym. Degrad. Stabil. 2018, 151, 52–64. [Google Scholar] [CrossRef]
- Vandersall, H.J. Intumescent coating system, their development and chemistry. J. Fire Flam. 1971, 2, 97–140. [Google Scholar]
- Manfredi, A.; Carosio, F.; Ferruti, P.; Alongi, J.; Ranucci, E. Disulfide-containing polyamidoamines with remarkable flame retardant activity for cotton fabrics. Polym. Degrad. Stabil. 2018, 156, 1–13. [Google Scholar] [CrossRef]
- Alongi, J.; Ferruti, P.; Manfredi, A.; Carosio, F.; Feng, Z.; Hakkarainen, M.; Ranucci, E. Superior flame retardancy of cotton by synergetic effect of cellulose-derived nano-graphene oxide carbon dots and disulphide-containing polyamidoamines. Polym. Degrad. Stabil. 2019, 169, 108993. [Google Scholar] [CrossRef]
- Beduini, A.; Carosio, F.; Ferruti, P.; Ranucci, E.; Alongi, J. Sulfur-Based Copolymeric Polyamidoamines as Efficient Flame-Retardants for Cotton. Polymers 2019, 11, 1904. [Google Scholar] [CrossRef] [Green Version]
- Costes, L.; Laoutid, F.; Brohez, S.; Dubois, P. Bio-based flame retardants: When nature meets fire protection. Mater. Sci. Eng. R 2017, 117, 1–25. [Google Scholar] [CrossRef]
- Papaspyrides, C.D.; Kiliaris, P. (Eds.) Polymer Green Flame Retardants; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Basak, S.; Ali, W. Sustainable fire retardancy of textiles using bio-macromolecules. Polym. Degrad. Stabil. 2016, 133, 47–64. [Google Scholar] [CrossRef]
- Tata, J.; Alongi, J.; Carosio, J.; Frache, A. Optimization of the procedure to burn textile fabrics by cone calorimeter: Part I. Combustion behavior of polyester. Fire Mater. 2011, 35, 397–409. [Google Scholar] [CrossRef]
- ISO 5660. In Fire Test, Reaction to Fire, Rate of Heat Release (Cone Calorimeter Method); International Organization for Standardization: Geneva, Switzerland, 2002.
- Schartel, B.; Bartholomai, M.; Knoll, U. Some comments on the main fire retardancy mechanisms in polymer nanocomposites. Polym. Adv. Technol. 2006, 17, 772–777. [Google Scholar] [CrossRef]
- Smith, M.W.; Pecha, B.; Helms, G.; Scudiero, L.; Garcia-Perez, M. Chemical and morphological evaluation of chars produced from primary biomass constituents: Cellulose, xylan, and lignin. Biomass Bioenergy 2017, 104, 17–35. [Google Scholar] [CrossRef]
- Morgan, D.J. Comments on the XPS Analysis of Carbon Materials. Carbon 2021, 7, 51. [Google Scholar] [CrossRef]
- Davies, P.J.; Horrocks, A.R.; Alderson, A. The sensitisation of thermal decomposition of ammonium polyphosphate by selected metal ions and their potential for improved cotton fabric flame retardancy. Polym. Degrad. Stabil. 2005, 88, 114–122. [Google Scholar] [CrossRef]
- Schartel, B.; Hull, R. Development of fire-retardant materials. Interpretation of cone calorimeter data. Fire Mater. 2007, 31, 327–354. [Google Scholar] [CrossRef]











| PAA 2 | [α-Amino Acid] 3 (g) | LiOH∙H2O (g, mol) | H2O (mL) |
|---|---|---|---|
| M-GLY | 7.5 | 4.2, 0.1 | 35 |
| M-ALA | 8.9 | 4.2, 0.1 | 40 |
| M-VAL | 11.7 | 4.2, 0.1 | 42 |
| M-LEU | 13.1 | 4.2, 0.1 | 45 |
| M-HIS | 15.5 | 4.2, 0.1 | 53 |
| M-SER | 10.5 | 4.2, 0.1 | 45 |
| M-ASN | 13.2 | 4.2, 0.1 | 50 |
| M-GLN | 14.6 | 4.2, 0.1 | 60 |
| M-ASP | 13.3 | 8.4, 0.2 | 55 |
| M-GLU | 14.6 | 8.4, 0.2 | 60 |
| PAA | Structure of the Repeat Unit | PAA | Structure of the Repeat Unit |
|---|---|---|---|
| M-GLY |  | M-ALA |  |
| M-VAL |  | M-LEU |  |
| M-HIS |  | M-SER |  |
| M-ASN |  | M-GLN |  |
| M-ASP |  | M-GLU |  |
| Sample | Tonset10% 1 (°C) | Tmax1 2 (°C) | Tmax2 3 (°C) | RMF800 4 (%) |
|---|---|---|---|---|
| Nitrogen | ||||
| M-GLY | 152 | 270 | - | 12 |
| M-ALA | 160 | 302 | - | 16 |
| M-VAL | 223 | 275 | - | 14 |
| M-LEU | 116 | 270 | - | 9 |
| M-HIS | 135 | 260 | - | 20 |
| M-SER | 129 | 245 | - | 18 |
| M-ASN | 126 | 308 | - | 26 |
| M-GLN | 145 | 315 | - | 21 |
| M-ASP | 157 | 230 | - | 26 |
| M-GLU | 172 | 340 | - | 26 |
| Air | ||||
| M-GLY | 205 | 260 | 610 | 3 |
| M-ALA | 150 | 290 | 550 | 7 |
| M-VAL | 229 | 274 | 540 | 9 |
| M-LEU | 102 | 256 | 510 | 6 |
| M-HIS | 123 | 255 | 560 | 1 |
| M-SER | 126 | 236 | 541 | 3 |
| M-ASN | 149 | 254 | 560 | 5 |
| M-GLN | 159 | 270 | 500 | 1 |
| M-ASP | 155 | 225 | 616 | 4 |
| M-GLU | 177 | 320 | 530 | 4 |
| Sample | RMF (%) 1 | Sample | RMF (%) 1 |
|---|---|---|---|
| M-GLY | 98.0 | M-SER | 98.9 |
| M-ALA | 98.6 | M-ASN | 98.9 |
| M-VAL | 99.5 | M-GLN | 99.5 |
| M-LEU | 90.0 | M-ASP | 98.6 |
| M-HIS | 98.8 | M-GLU | 99.7 |
| Sample | Tonset10% 1 (°C) | Tmax1 2 (°C) | Tmax2 3 (°C) | RMF800 4 (%) |
|---|---|---|---|---|
| Nitrogen | ||||
| COT | 329 | 355 | - | 5.5 |
| COT/M-ALA | 274 | 332 | - | 19.0 |
| COT/M-VAL | 265 | 340 | - | 15.5 |
| COT/M-LEU | 262 | 321 | - | 17.0 |
| COT/M-HIS | 264 | 347 | - | 20.0 |
| COT/M-SER | 277 | 343 | - | 16.0 |
| COT/M-ASN | 278 | 342 | - | 20.0 |
| COT/M-GLN | 266 | 341 | - | 17.5 |
| COT/M-ASP | 290 | 354 | - | 15.0 |
| COT/M-GLU | 256 | 333 | - | 21.5 |
| Air | ||||
| COT | 329 | 352 | 480 | - |
| COT/M-ALA | 270 | 315 | 440 | - |
| COT/M-VAL | 272 | 324 | 429 | - |
| COT/M-LEU | 271 | 309 | 426 | - |
| COT/M-HIS | 265 | 327 | 441 | - |
| COT/M-SER | 276 | 321 | 435 | - |
| COT/M-ASN | 287 | 336 | 445 | - |
| COT/M-GLN | 273 | 327 | 442 | - |
| COT/M-ASP | 291 | 340 | 457 | - |
| COT/M-GLU | 256 | 323 | 431 | - |
| Sample | Combustion Time 1 (s) | Extinguishment | RMF 2 (%) |
|---|---|---|---|
| COT | 53 | NO | <1 |
| COT/M-GLY | 80 | YES | 81 |
| COT/M-ALA | 94 | YES | 69 |
| COT/M-VAL | 70 | YES | 64 |
| COT/M-LEU | 82 | YES | 80 |
| COT/M-SER | 65 | YES | 79 |
| COT/M-ASN | 80 | YES | 77 |
| COT/M-GLN | 51 | YES | 87 |
| COT/M-HIS | 65 | YES | 84 |
| COT/M-ASP | 50 | YES | 81 |
| COT/M-GLU | 54 | YES | 90 |
| Sample | Combustion Time 1 (s) | Extinguishment | RMF 2 (%) |
|---|---|---|---|
| COT | 53 | NO | <1 |
| COT/M-GLY | 120 | NO | 25 |
| COT/M-ALA | 93 | NO | 23 |
| COT/M-VAL | 92 | NO | 18 |
| COT/M-LEU | 111 | NO | 17 |
| COT/M-SER | 102 | NO | 25 |
| COT/M-ASN | 81 | NO | 32 |
| COT/M-GLN | 66 | YES | 74 |
| COT/M-HIS | 96 | NO | 17 |
| COT/M-ASP | 84 | NO | 17 |
| COT/M-GLU | 60 | YES | 84 |
| Sample | TTI (s) 1 | pKHRR 2 (kW m−2) (Reduction %) | FPI3 (sm2kW−1) | THR 4 (MJ m−2) | RMF 5 (%) |
|---|---|---|---|---|---|
| COT | 28 ± 1 | 131 ± 5 | 0.21 ± 0.01 | 3.5 ± 0.2 | 0 |
| COT/M-ALA | 25 ± 1 | 114 ± 5 (−13) | 0.22 ± 0.01 | 3.3 ± 0.2 | 4.0 ± 0.2 |
| COT/M-VAL | 25 ± 1 | 122 ± 0 (−7) | 0.20 ± 0.01 | 4.1 ± 0.2 | 2.5 ± 0.7 |
| COT/M-LEU | 24 ± 3 | 121 ± 3 (−8) | 0.20 ± 0.01 | 3.8 ± 0.2 | 4.0 ± 0.4 |
| COT/M-SER | 23 ± 4 | 110 ± 9 (−16) | 0.21 ± 0.01 | 3.4 ± 0.2 | 4.0 ± 0.4 |
| COT/M-ASN | 24 ± 3 | 101 ± 7 (−23) | 0.24 ± 0.01 | 3.4 ± 0.2 | 4.5 ± 0.7 |
| COT/M-GLN | 25 ± 1 | 96 ± 1 (−27) | 0.26 ± 0.01 | 2.9 ± 0.1 | 5.5 ± 0.7 |
| COT/M-HIS | 23 ± 1 | 88 ± 13 (−33) | 0.26 ± 0.01 | 3.3 ± 0.2 | 3.5 ± 0.7 |
| COT/M-ASP | 26 ± 1 | 117 ± 6 (−10) | 0.22 ± 0.01 | 4.2 ± 0.2 | 3.0 ± 0.4 |
| COT/M-GLU | 25 ± 1 | 119 ± 22 (−9) | 0.21 ± 0.01 | 4.0 ± 0.2 | 3.5 ± 0.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beduini, A.; Carosio, F.; Ferruti, P.; Ranucci, E.; Alongi, J. Polyamidoamines Derived from Natural α-Amino Acids as Effective Flame Retardants for Cotton. Polymers 2021, 13, 3714. https://doi.org/10.3390/polym13213714
Beduini A, Carosio F, Ferruti P, Ranucci E, Alongi J. Polyamidoamines Derived from Natural α-Amino Acids as Effective Flame Retardants for Cotton. Polymers. 2021; 13(21):3714. https://doi.org/10.3390/polym13213714
Chicago/Turabian StyleBeduini, Alessandro, Federico Carosio, Paolo Ferruti, Elisabetta Ranucci, and Jenny Alongi. 2021. "Polyamidoamines Derived from Natural α-Amino Acids as Effective Flame Retardants for Cotton" Polymers 13, no. 21: 3714. https://doi.org/10.3390/polym13213714
APA StyleBeduini, A., Carosio, F., Ferruti, P., Ranucci, E., & Alongi, J. (2021). Polyamidoamines Derived from Natural α-Amino Acids as Effective Flame Retardants for Cotton. Polymers, 13(21), 3714. https://doi.org/10.3390/polym13213714