Enzymatic Functionalization of Wood as an Antifouling Strategy against the Marine Bacterium Cobetia marina
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Enzymatic Hydrophobization of Wood Veneers
2.3. Water Contact Angle Measurements
2.4. X-ray Photoelectron Spectroscopy (XPS)
2.5. Fourier Transform Infrared Spectroscopy (FTIR)
2.6. Stock Bacteria and Culture Conditions
2.7. Biofilm Assay
2.8. Bacterial Surface Hydrophobicity
2.9. Protein Measurement
2.10. SEM Analysis
3. Results and Discussion
3.1. Enzymatic Hydrophobization
3.2. XPS Study
3.3. FT-IR Analysis
3.4. Biofilm Assay
3.5. Protein Measurement
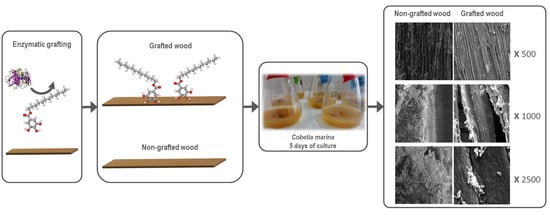
3.6. SEM Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crossman, M.; Simm, J. Sustainable Coastal Defences—The Use of Timber and Other Materials. Proc. Inst. Civ. Eng.-Munic. Eng. 2002, 151, 207–211. [Google Scholar] [CrossRef]
- Nilsson, T.; Rowell, R. Historical Wood—Structure and Properties. J. Cult. Herit. 2012, 13 (Suppl. 3), 5–9. [Google Scholar] [CrossRef]
- Jain, A.; Bhosle, N.B. Biochemical Composition of the Marine Conditioning Film: Implications for Bacterial Adhesion. Biofouling 2009, 25, 13–19. [Google Scholar] [CrossRef]
- Cooksey, K.E.; Wigglesworth-Cooksey, B. Adhesion of Bacteria and Diatoms to Surfaces in the Sea: A Review. Aquat. Microb. Ecol. 1995, 9, 87–96. [Google Scholar] [CrossRef]
- More, T.T.; Yadav, J.S.S.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y. Extracellular Polymeric Substances of Bacteria and Their Potential Environmental Applications. J. Environ. Manag. 2014, 144, 1–25. [Google Scholar] [CrossRef]
- Mieszkin, S.; Callow, M.E.; Callow, J.A. Interactions between Microbial Biofilms and Marine Fouling Algae: A Mini Review. Biofouling 2013, 29, 1097–1113. [Google Scholar] [CrossRef]
- Gittens, J.E.; Smith, T.J.; Suleiman, R.; Akid, R. Current and Emerging Environmentally-Friendly Systems for Fouling Control in the Marine Environment. Biotechnol. Adv. 2013, 31, 1738–1753. [Google Scholar] [CrossRef]
- Callow, M.E.; Callow, J.A. Marine Biofouling: A Sticky Problem. Biologist 2002, 49, 10–14. [Google Scholar]
- Krsmanovic, M.; Biswas, D.; Ali, H.; Kumar, A.; Ghosh, R.; Dickerson, A.K. Hydrodynamics and Surface Properties Influence Biofilm Proliferation. Adv. Colloid Interface Sci. 2021, 288, 102336. [Google Scholar] [CrossRef]
- Callow, M.E.; Callow, J.A. Trends in the Development of Environmentally Friendly Fouling-Resistant Marine Coatings. Nat. Commun. 2011, 2, 210–244. [Google Scholar] [CrossRef]
- Baier, R.E. Surface Behaviour of Biomaterials: The Theta Surface for Biocompatibility. J. Mater. Sci. Mater. Med. 2006, 17, 1057–1062. [Google Scholar] [CrossRef]
- Brady, R.F. Properties Which Influence Marine Fouling Resistance in Polymers Containing Silicon and Fluorine. Prog. Org. Coat. 1999, 35, 31–35. [Google Scholar] [CrossRef]
- Riva, S. Laccases: Blue Enzymes for Green Chemistry. Trends Biotechnol. 2006, 24, 219–226. [Google Scholar] [CrossRef]
- Kudanga, T.; Nyanhongo, G.S.; Guebitz, G.M.; Burton, S. Potential Applications of Laccase-Mediated Coupling and Grafting Reactions: A Review. Enzym. Microb. Technol. 2011, 48, 195–208. [Google Scholar] [CrossRef]
- Kudanga, T.; Prasetyo, E.N.; Sipilä, J.; Guebitz, G.M.; Nyanhongo, G.S. Reactivity of Long Chain Alkylamines to Lignin Moieties: Implications on Hydrophobicity of Lignocellulose Materials. J. Biotechnol. 2010, 149, 81–87. [Google Scholar] [CrossRef]
- Kudanga, T.; Prasetyo, E.N.; Widsten, P.; Kandelbauer, A.; Jury, S.; Heathcote, C.; Sipilä, J.; Weber, H.; Nyanhongo, G.S.; Guebitz, G.M. Laccase Catalyzed Covalent Coupling of Fluorophenols Increases Lignocellulose Surface Hydrophobicity. Bioresour. Technol. 2010, 101, 2793–2799. [Google Scholar] [CrossRef]
- Filgueira, D.; Holmen, S.; Melbø, J.K.; Moldes, D.; Echtermeyer, A.; Chinga-Carrasco, G. Enzymatic-Assisted Modification of Thermomechanical Pulp Fibres To Improve the Interfacial Adhesion with Poly (Lactic acid) for 3D Printing. ACS Sustain. Chem. Eng. 2017, 5, 9338–9346. [Google Scholar] [CrossRef]
- Garcia-Ubasart, J.; Vidal, T.; Torres, A.L.; Rojas, O.J. Laccase-Mediated Coupling of Nonpolar Chains for the Hydrophobization of Lignocellulose. Biomacromolecules 2013, 14, 1637–1644. [Google Scholar] [CrossRef]
- Hossain, K.M.G.; González, M.D.; Lozano, G.R.; Tzanov, T. Multifunctional Modification of Wool Using an Enzymatic Process in Aqueous-Organic Media. J. Biotechnol. 2009, 141, 58–63. [Google Scholar] [CrossRef]
- Warne Zoueki, C.; Ghoshal, S.; Tufenkji, N. Bacterial Adhesion to Hydrocarbons: Role of Asphaltenes and Resins. Colloids Surf. B Biointerfaces 2010, 79, 219–226. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, M.; Gutnick, D.; Rosenberg, E. Adherence of Bacteria to Hydrocarbons: A Simple Method for Measuring Cell-Surface Hydrophobicity. FEMS Microbiol. Lett. 1980, 9, 29–33. [Google Scholar] [CrossRef]
- Saastamoinen, P.; Mattinen, M.L.; Hippi, U.; Nousiainen, P.; Sipilä, J.; Lille, M.; Suurnäkki, A.; Pere, J. Laccase Aided Modification of Nanofibrillated Cellulose with Dodecyl Gallate. BioResources 2012, 7, 5749–5770. [Google Scholar] [CrossRef] [Green Version]
- Itoh, N.; Katsube, Y.; Yamamoto, K.; Nakajima, N.; Yoshida, K. Laccase-Catalyzed Conversion of Green Tea Catechins in the Presence of Gallic Acid to Epitheaflagallin and Epitheaflagallin 3-O-Gallate. Tetrahedron 2007, 63, 9488–9492. [Google Scholar] [CrossRef]
- Areskogh, D.; Li, J.; Nousiainen, P.; Gellerstedt, G.; Sipilä, J.; Henriksson, G. Oxidative Polymerisation of Models for Phenolic Lignin End-Groups by Laccase. Holzforschung 2010, 64, 21–34. [Google Scholar] [CrossRef]
- Filgueira, D.; Moldes, D.; Fuentealba, C.; García, D.E. Condensed Tannins from Pine Bark: A Novel Wood Surface Modifier Assisted by Laccase. Ind. Crops Prod. 2017, 103, 185–194. [Google Scholar] [CrossRef]
- Grönqvist, S.; Viikari, L.; Niku-Paavola, M.L.; Orlandi, M.; Canevali, C.; Buchert, J. Oxidation of Milled Wood Lignin with Laccase, Tyrosinase and Horseradish Peroxidase. Appl. Microbiol. Biotechnol. 2005, 67, 489–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto, P.C.; Evtuguin, D.V.; Neto, C.P. Effect of Structural Features of Wood Biopolymers on Hardwood Pulping and Bleaching Performance. Ind. Eng. Chem. Res. 2005, 44, 9777–9784. [Google Scholar] [CrossRef]
- Choi, J.W.; Faix, O.; Meier, D. Characterization of Residual Lignins from Chemical Pulps of Spruce (Picea abies L.) and Beech (Fagus sylvatica L.) by Analytical Pyrolysis-Gas Chromatography/Mass Spectrometry. Holzforschung 2001, 55, 185–192. [Google Scholar] [CrossRef]
- Saito, K.; Kato, T.; Tsuji, Y.; Fukushima, K. Identifying the Characteristic Secondary Ions of Lignin Polymer Using ToF-SIMS. Biomacromolecules 2005, 6, 678–683. [Google Scholar] [CrossRef]
- Alves, A.; Schwanninger, M.; Pereira, H.; Rodrigues, J. Calibration of NIR to Assess Lignin Composition (H/G Ratio) in Maritime Pine Wood Using Analytical Pyrolysis as the Reference Method. Holzforschung 2006, 60, 29–31. [Google Scholar] [CrossRef]
- Cañas, A.I.; Camarero, S. Laccases and Their Natural Mediators: Biotechnological Tools for Sustainable Eco-Friendly Processes. Biotechnol. Adv. 2010, 28, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, S.; Fernández-Costas, C.; Sanromán, M.A.; Moldes, D. Polymerisation of Kraft Lignin from Black Liquors by Laccase from Myceliophthora Thermophila: Effect of Operational Conditions and Black Liquor Origin. Bioresour. Technol. 2013, 131, 288–294. [Google Scholar] [CrossRef]
- Fernández-Fernández, M.; Sanromán, M.A.; Moldes, D. Potential of Laccase for Modification of Eucalyptus Globulus Wood: A XPS Study. Wood Sci. Technol. 2014, 48, 151–160. [Google Scholar] [CrossRef]
- Vázquez, G.; Ríos, R.; Freire, M.S.; Antorrena, G.; González-Álvarez, J. Surface Characterization of Eucalyptus and Ash Wood Veneers by XPS, TOF-SIMS, Optic Profilometry and Contact Angle Measurements. WIT Trans. Eng. Sci. 2011, 72, 187–198. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.; Tang, Z.; Clinton, R.M.; Hess, D.W.; Breedveld, V. Fabrication of Highly Amphiphobic Paper Using Pulp Debonder. Cellulose 2016, 23, 3885–3899. [Google Scholar] [CrossRef]
- George, M.; Mussone, P.G.; Bressler, D.C. Surface and Bulk Transformation of Thermomechanical Pulp Using Fatty Acyl Chlorides: Influence of Reaction Parameters on Surface, Morphological, and Thermal Properties. J. Wood Chem. Technol. 2016, 36, 114–128. [Google Scholar] [CrossRef]
- Vanhatalo, K.; Maximova, N.; Perander, A.; Johansson, L.; Haimi, E.; Dahl, O. Comparison of Conventional and Lignin-Rich. BioResources 2016, 11, 4037–4054. [Google Scholar] [CrossRef] [Green Version]
- Javanbakht, T.; Raphael, W.; Tavares, J.R. Physicochemical Properties of Cellulose Nanocrystals Treated by Photo-Initiated Chemical Vapour Deposition (PICVD). Can. J. Chem. Eng. 2016, 94, 1135–1139. [Google Scholar] [CrossRef]
- Granda, L.A.; Espinach, F.X.; Tarrés, Q.; Méndez, J.A.; Delgado-Aguilar, M.; Mutjé, P. Towards a Good Interphase between Bleached Kraft Softwood Fibers and Poly(Lactic) Acid. Compos. Part B Eng. 2016, 99, 514–520. [Google Scholar] [CrossRef]
- González, D.; Santos, V.; Parajó, J.C. Silane-Treated Lignocellulosic Fibers as Reinforcement Material in Polylactic Acid Biocomposites. J. Thermoplast. Compos. Mater. 2012, 25, 1005–1022. [Google Scholar] [CrossRef]
- Nguila Inari, G.; Pétrissans, M.; Dumarcay, S.; Lambert, J.; Ehrhardt, J.J.; Šernek, M.; Gérardin, P. Limitation of XPS for Analysis of Wood Species Containing High Amounts of Lipophilic Extractives. Wood Sci. Technol. 2011, 45, 369–382. [Google Scholar] [CrossRef]
- Sernek, M.; Kamke, F.A.; Glasser, W.G. Comparative Analysis of Inactivated Wood Surfaces Comparative Analysis of Inactivated Wood Surfaces. Holzforschung 2004, 58, 22–31. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Fernández, M.; Sanromán, M.Á.; Moldes, D. Wood Hydrophobization by Laccase-Assisted Grafting of Lauryl Gallate. J. Wood Chem. Technol. 2015, 35, 156–165. [Google Scholar] [CrossRef]
- Etoh, H.; Ban, N.; Fujiyoshi, J.; Murayama, N.; Sugiyama, K.; Watanabe, N.; Sakata, K.; Ina, K.; Miyoshi, H.; Iwamura, H. Quantitative Analysis of the Antimicrobial Activity and Membrane-Perturbation Potency of Antifouling Para-Substituted Alkylphenols. Biosci. Biotechnol. Biochem. 1994, 58, 467–469. [Google Scholar] [CrossRef]
- Kubo, I.; Xiao, P.; Fujita, K. Anti-MRSA Activity of Alkyl Gallates. Bioorg. Med. Chem. Lett. 2002, 12, 113–116. [Google Scholar] [CrossRef]
- Baier, R.E.; Shafrin, E.G.; Zisman, W.A. Adhesion: Mechanisms That Assist or Impede It. Science 1968, 162, 1360–1368. [Google Scholar] [CrossRef]
- Karunakaran, E.; Biggs, C.A. Mechanisms of Bacillus Cereus Biofilm Formation: An Investigation of the Physicochemical Characteristics of Cell Surfaces and Extracellular Proteins. Appl. Microbiol. Biotechnol. 2011, 89, 1161–1175. [Google Scholar] [CrossRef]
- Li, J.; Xie, Z.; Wang, G.; Ding, C.; Jiang, H.; Wang, P. Preparation and Evaluation of Amphiphilic Polymer as Fouling-Release Coating in Marine Environment. J. Coat. Technol. Res. 2017, 14, 1237–1245. [Google Scholar] [CrossRef]
- Van Zoelen, W.; Buss, H.G.; Ellebracht, N.C.; Lynd, N.A.; Fischer, D.A.; Finlay, J.; Hill, S.; Callow, M.E.; Callow, J.; Kramer, E.J.; et al. Sequence of Hydrophobic and Hydrophilic Residues in Amphiphilic Polymer Coatings A Ff Ects Surface Structure and Marine Antifouling/Fouling Release Properties. ACS Macro Lett. 2014, 3, 364–368. [Google Scholar] [CrossRef]
- Bauer, S.; Arpa-Sancet, M.P.; Finlay, J.A.; Callow, M.E.; Callow, J.A.; Rosenhahn, A. Adhesion of Marine Fouling Organisms on Hydrophilic and Amphiphilic Polysaccharides. Langmuir 2013, 29, 4039–4047. [Google Scholar] [CrossRef]
- Dundua, A.; Franzka, S.; Ulbricht, M. Improved Antifouling Properties of Polydimethylsiloxane Films via Formation of Polysiloxane/Polyzwitterion Interpenetrating Networks. Macromol. Rapid Commun. 2016, 37, 2030–2036. [Google Scholar] [CrossRef] [PubMed]
- Lelchat, F.; Cérantola, S.; Brandily, C.; Colliec-Jouault, S.; Baudoux, A.C.; Ojima, T.; Boisset, C. The Marine Bacteria Cobetia marina DSMZ 4741 Synthesizes an Unexpected K-Antigen-like Exopolysaccharide. Carbohydr. Polym. 2015, 124, 347–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, P.S.; Costerton, J.W. Antibiotic Resistance of Bacteria in Biofilms. Lancet 2001, 358, 135–138. [Google Scholar] [CrossRef]
- Ordax, M.; Marco-Noales, E.; López, M.M.; Biosca, E.G. Exopolysaccharides Favor the Survival of Erwinia amylovora under Copper Stress through Different Strategies. Res. Microbiol. 2010, 161, 549–555. [Google Scholar] [CrossRef]







| Sample | Elements | C1s Components | O1s Components | O/C Ratio | C1/C2 Ratio | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C1s | O1s | N1s | C1% | C2% | C3% | C4% | O1% | O2% | |||
| UW | 65.29 | 30.85 | 1.29 | 47.25 | 38.98 | 11.30 | 2.47 | 16.82 | 83.19 | 0.48 | 1.21 |
| LG | 66.60 | 29.06 | 1.72 | 53.79 | 30.63 | 11.05 | 4.54 | 17.30 | 82.71 | 0.44 | 1.76 |
| L | 65.39 | 26.75 | 6.42 | 38.70 | 42.46 | 16.60 | 2.25 | 30.40 | 69.61 | 0.41 | 0.91 |
| L + LG | 70.09 | 24.95 | 3.24 | 53.68 | 31.99 | 11.77 | 2.56 | 26.68 | 73.32 | 0.36 | 1.68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filgueira, D.; Bolaño, C.; Gouveia, S.; Moldes, D. Enzymatic Functionalization of Wood as an Antifouling Strategy against the Marine Bacterium Cobetia marina. Polymers 2021, 13, 3795. https://doi.org/10.3390/polym13213795
Filgueira D, Bolaño C, Gouveia S, Moldes D. Enzymatic Functionalization of Wood as an Antifouling Strategy against the Marine Bacterium Cobetia marina. Polymers. 2021; 13(21):3795. https://doi.org/10.3390/polym13213795
Chicago/Turabian StyleFilgueira, Daniel, Cristian Bolaño, Susana Gouveia, and Diego Moldes. 2021. "Enzymatic Functionalization of Wood as an Antifouling Strategy against the Marine Bacterium Cobetia marina" Polymers 13, no. 21: 3795. https://doi.org/10.3390/polym13213795
APA StyleFilgueira, D., Bolaño, C., Gouveia, S., & Moldes, D. (2021). Enzymatic Functionalization of Wood as an Antifouling Strategy against the Marine Bacterium Cobetia marina. Polymers, 13(21), 3795. https://doi.org/10.3390/polym13213795