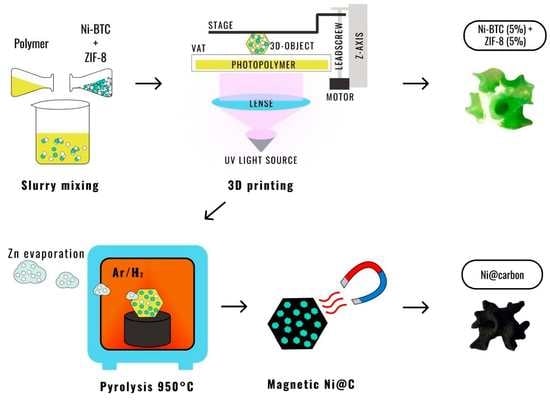
3D-Printed Porous Magnetic Carbon Materials Derived from Metal–Organic Frameworks
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, J.Y.; An, J. Fundamentals and applications of 3D printing for novel materials. Appl. Mater. Today 2017, 7, 120–133. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, C.J. Three-dimensional printing for catalytic applications: Current status and perspectives. Adv. Funct. Mater. 2017, 27, 1701134. [Google Scholar] [CrossRef]
- Awad, A.; Trenfield, S.J. 3D printed medicines: A new branch of digital healthcare. Int. J. Pharm. 2018, 548, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lim, G.J. Binder-free 3D printing of covalent organic framework (COF) monoliths for CO2 adsorption. Chem. Eng. J. 2021, 403, 126333. [Google Scholar] [CrossRef]
- Pandis, P.K.; Papaioannou, S. Differential scanning calorimetry based evaluation of 3D printed PLA for phase change materials encapsulation or as container material of heat storage tanks. Energy Procedia 2019, 161, 429–437. [Google Scholar] [CrossRef]
- Belka, M.; Bączek, T. Additive manufacturing and related technologies–the source of chemically active materials in separation science. Trends Analyt. Chem. 2021, 142, 116322. [Google Scholar] [CrossRef]
- Femmer, T.; Flack, I. Additive manufacturing in fluid process engineering. Chem. Ing. Tech. 2016, 88, 535–552. [Google Scholar] [CrossRef]
- Kantaros, A.; Diegel, O. 3D printing: Making an innovative technology widely accessible through makerspaces and outsourced services. Mater. Today 2021, in press. [Google Scholar]
- Lokesh, K.; Jain, P.K. Selection of rapid prototyping technology. Adv. Prod. Eng. Manag. 2010, 5, 75–84. [Google Scholar]
- Talley, S.J.; Branch, B. Impact of filler composition on mechanical and dynamic response of 3-D printed silicone-based nanocomposite elastomers. Compos. Sci. Technol. 2020, 198, 108258. [Google Scholar] [CrossRef]
- Scott, P.J.; Meenakshisundaram, V. 3D printing latex: A route to complex geometries of high molecular weight polymers. ACS Appl. Mater. Interfaces 2020, 12, 10918–10928. [Google Scholar] [CrossRef]
- Bekas, D.G.; Hou, Y. 3D printing to enable multifunctionality in polymer-based composites: A review. Compos. Part B Eng. 2019, 179, 107540. [Google Scholar] [CrossRef]
- Liu, D.; Jiang, P. 3D printing of metal-organic frameworks decorated hierarchical porous ceramics for high-efficiency catalytic degradation. Chem. Eng. J. 2020, 397, 125392. [Google Scholar] [CrossRef]
- Pei, R.; Fan, L. 3D-Printed metal-organic frameworks within biocompatible polymers as excellent adsorbents for organic dyes removal. J. Hazard. Mater. 2020, 384, 121418. [Google Scholar] [CrossRef]
- Wei, X.; Li, D. 3D printable graphene composite. Sci. Rep. 2015, 5, 11181. [Google Scholar] [CrossRef] [Green Version]
- Lawson, S.; Al-Naddaf, Q. UTSA-16 growth within 3D-printed Co-Kaolin monoliths with high selectivity for CO2/CH4, CO2/N2, and CO2/H2 separation. ACS Appl. Mater. Interfaces 2018, 10, 19076–19086. [Google Scholar] [CrossRef] [PubMed]
- Figuerola, A.; Medina, D.A. Metal–organic framework mixed-matrix coatings on 3D printed devices. Appl. Mater. Today 2019, 16, 21–27. [Google Scholar] [CrossRef]
- Azuaje, J.; Rama, A. Catalytic performance of a metal-free graphene oxide-Al2O3 composite assembled by 3D printing. J. Eur. Ceram. Soc. 2021, 41, 1399–1406. [Google Scholar] [CrossRef]
- Cherevko, A.I.; Denisov, G.L. Composite Materials Manufactured by Photopolymer 3D Printing with Metal-Organic Frameworks. Russ. J. Coord. Chem. 2021, 47, 319–325. [Google Scholar] [CrossRef]
- Yaghi, O.M.; Li, G. Selective binding and removal of guests in a microporous metal–organic framework. Nature 1995, 378, 703–706. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, L.; Olson, D.H. Separation of hydrocarbons with a microporous metal–organic framework. Angew. Chem. Int. Ed. 2006, 45, 616–619. [Google Scholar] [CrossRef]
- Matsuda, R.; Kitaura, R. Highly controlled acetylene accommodation in a metal–organic microporous material. Nature 2005, 436, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.Y.A.; Chang, H.A. Zeolitic Imidazole Framework-67 (ZIF-67) as a heterogeneous catalyst to activate peroxymonosulfate for degradation of Rhodamine B in water. J. Taiwan Inst. Chem. Eng. 2015, 53, 40–45. [Google Scholar] [CrossRef]
- Horcajada, P.; Chalati, T. Porous metal–organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat. Mater. 2010, 9, 172–178. [Google Scholar] [CrossRef]
- Gladysiak, A.; Nguyen, T.N. A recyclable metal-organic framework as a dual detector and adsorbent for ammonia. Chem. Eur. J. 2017, 23, 13602–13606. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.; Dong, Y. Recent advances of metal-organic frameworks in corrosion protection: From synthesis to applications. Chem. Eng. J. 2021, 132823, in press. [Google Scholar] [CrossRef]
- Yuan, S.; Feng, L. Stable metal—Organic frameworks: Design, synthesis, and applications. Adv. Mater. 2018, 30, 1704303. [Google Scholar] [CrossRef] [Green Version]
- Halevi, O.; Tan, J.M. Hydrolytically Stable MOF in 3D-Printed Structures. Adv. Sustain. Syst. 2018, 2, 1700150. [Google Scholar] [CrossRef]
- Bible, M.; Sefa, M. 3D-printed acrylonitrile butadiene styrene-metal organic framework composite materials and their gas storage properties. 3D Print. Addit. Manuf. 2018, 5, 63–72. [Google Scholar] [CrossRef]
- Thakkar, H.; Eastman, S. 3D-printed metal–organic framework monoliths for gas adsorption processes. ACS Appl. Mater. Interfaces 2017, 9, 35908–35916. [Google Scholar] [CrossRef]
- Salazar-Aguilar, A.D.; Quintanilla, A. Iron-based metal-organic frameworks integrated into 3D printed ceramic architectures. Open Ceram. 2021, 5, 100047. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, Z. Synthesis of carbon quantum dots and zinc oxide nanosheets by pyrolysis of novel metal–organic framework compounds. J. Alloys Compd. 2015, 642, 148–152. [Google Scholar] [CrossRef]
- Qiu, B.; Yang, C. Highly dispersed Co-based Fischer–Tropsch synthesis catalysts from metal–organic frameworks. J. Mater. Chem. A 2017, 5, 8081–8086. [Google Scholar] [CrossRef]
- Wezendonk, T.A.; Santos, V.P. Elucidating the nature of Fe species during pyrolysis of the Fe-BTC MOF into highly active and stable Fischer–Tropsch catalysts. ACS Catal. 2016, 6, 3236–3247. [Google Scholar] [CrossRef]
- Thangavel, B.; Berchmans, S. Ni@ Carbon Nanotubes Derived from Ni-MOF as a Superior Electrocatalyst for Hydrogen Evolution Reaction in Acidic Medium. Energy Fuels 2020, 35, 1866–1873. [Google Scholar] [CrossRef]
- Meng, J.; Niu, C. General oriented formation of carbon nanotubes from metal–organic frameworks. J. Am. Chem. Soc. 2017, 139, 8212–8221. [Google Scholar] [CrossRef]
- Chen, F.; Yu, C. A core-shell structured metal-organic frameworks-derived porous carbon nanowires as a superior anode for alkaline metal-ion batteries. Appl. Surf. Sci. 2021, 541, 148473. [Google Scholar] [CrossRef]
- Shen, K.; Chen, X. Development of MOF-derived carbon-based nanomaterials for efficient catalysis. ACS Catal. 2016, 6, 5887–5903. [Google Scholar] [CrossRef]
- Zhang, J.; An, B. Pyrolysis of metal–organic frameworks to hierarchical porous Cu/Zn-nanoparticle@ carbon materials for efficient CO 2 hydrogenation. Mater. Chem. Front. 2017, 1, 2405–2409. [Google Scholar] [CrossRef]
- Chen, X.; Yu, E. In situ pyrolysis of Ce-MOF to prepare CeO2 catalyst with obviously improved catalytic performance for toluene combustion. Chem. Eng. J. 2018, 344, 469–479. [Google Scholar] [CrossRef]
- Wang, C.; Kaneti, Y.V. Metal–organic framework-derived one-dimensional porous or hollow carbon-based nanofibers for energy storage and conversion. Mater. Horiz. 2018, 5, 394–407. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.J.; Fan, J.M. A novel synergistic composite with multi-functional effects for high-performance Li–S batteries. Energy Environ. Sci. 2016, 9, 1998–2004. [Google Scholar] [CrossRef]
- Gadipelli, S.; Travis, W. A thermally derived and optimized structure from ZIF-8 with giant enhancement in CO2 uptake. Energy Environ. Sci. 2014, 7, 2232–2238. [Google Scholar] [CrossRef]
- Lu, G.; Lu, G.M. Mechanical properties of porous materials. J. Porous Mater. 1999, 6, 359–368. [Google Scholar] [CrossRef]
- Yaghi, O.M.; Li, H. Construction of porous solids from hydrogen-bonded metal complexes of 1, 3, 5-benzenetricarboxylic acid. J. Am. Chem. Soc. 1996, 118, 9096–9101. [Google Scholar] [CrossRef]
- Tsai, C.W.; Langner, E.H. The effect of synthesis temperature on the particle size of nano-ZIF-8. Microporous Mesoporous Mater. 2016, 221, 8–13. [Google Scholar] [CrossRef]
- Jia, D.; Zhao, J. Highly dispersed Ni nanocatalysts supported by MOFs derived hierarchical N-doped porous carbon for hydrogenation of dicyclopentadiene. Carbon 2021, 184, 855–863. [Google Scholar] [CrossRef]
- Rouquerol, J.; Rouquerol, F. Adsorption by Powders and Porous Solids: Principles, Methodology and Applications, 2nd ed.; Academic Press: New York, NY, USA, 2013. [Google Scholar]
- Linares-Solano, A.; Stoeckli, F. Commentary on the paper “On the adsorption affinity coefficient of carbon dioxide in microporous carbons” by ES Bickford et al. (Carbon 2004; 42: 1867–71). Carbon 2005, 43, 658–660. [Google Scholar] [CrossRef]
- Rouquerol, J.; Llewellyn, P. Is the BET equation applicable to microporous adsorbents. Stud. Surf. Sci. Catal. 2007, 160, 49–56. [Google Scholar]
- Kaneti, Y.V.; Tang, J. Nanoarchitectured design of porous materials and nanocomposites from metal-organic frameworks. Adv. Mater. 2017, 29, 1604898. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Bhadra, B.N. Metal-organic framework-derived carbons: Preparation from ZIF-8 and application in the adsorptive removal of sulfamethoxazole from water. Catal. Today 2018, 301, 90–97. [Google Scholar] [CrossRef]
- Lai, W.; Ge, L. In situ Raman spectroscopic study towards the growth and excellent HER catalysis of Ni/Ni(OH)2 heterostructure. Int. J. Hydrogen Energy 2021, 46, 26861–26872. [Google Scholar] [CrossRef]
- Hu, Q.; Wang, X. Preparation of graphitic carbon nanofibres by in situ catalytic graphitisation of phenolic resins. Ceram. Int. 2013, 39, 8487–8492. [Google Scholar] [CrossRef]
- Miller, M.S.; Stageberg, F.E. Influence of rf magnetron sputtering conditions on the magnetic, crystalline, and electrical properties of thin nickel films. J. Appl. Phys. 1994, 75, 5779–5781. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cherevko, A.I.; Nikovskiy, I.A.; Nelyubina, Y.V.; Skupov, K.M.; Efimov, N.N.; Novikov, V.V. 3D-Printed Porous Magnetic Carbon Materials Derived from Metal–Organic Frameworks. Polymers 2021, 13, 3881. https://doi.org/10.3390/polym13223881
Cherevko AI, Nikovskiy IA, Nelyubina YV, Skupov KM, Efimov NN, Novikov VV. 3D-Printed Porous Magnetic Carbon Materials Derived from Metal–Organic Frameworks. Polymers. 2021; 13(22):3881. https://doi.org/10.3390/polym13223881
Chicago/Turabian StyleCherevko, Anton I., Igor A. Nikovskiy, Yulia V. Nelyubina, Kirill M. Skupov, Nikolay N. Efimov, and Valentin V. Novikov. 2021. "3D-Printed Porous Magnetic Carbon Materials Derived from Metal–Organic Frameworks" Polymers 13, no. 22: 3881. https://doi.org/10.3390/polym13223881
APA StyleCherevko, A. I., Nikovskiy, I. A., Nelyubina, Y. V., Skupov, K. M., Efimov, N. N., & Novikov, V. V. (2021). 3D-Printed Porous Magnetic Carbon Materials Derived from Metal–Organic Frameworks. Polymers, 13(22), 3881. https://doi.org/10.3390/polym13223881