Hydrogel Droplet Microarray for Genotyping Antimicrobial Resistance Determinants in Neisseria gonorrhoeae Isolates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection and Characterization of N. gonorrhoeae Clinical Isolates
2.2. Design of Oligonucleotide Probes for Microarray Immobilization and Primers for PCR Amplification
- -
- replacements Ala311→Val; Ile312→Met; Val316→Thr, Pro; Thr483→Ser; Ala501→Val, Thr, Pro; Asn512→Tyr; Gly542→Ser; Gly545→Ser and Pro551→Leu, Ser in mosaic and non-mosaic alleles of the penA gene encoding penicillin-binding protein 2 (PBP2)—resistance to cephalosporins (ceftriaxone);
- -
- insertion of an aspartic acid codon at position 345 of the PBP2 protein (insAsp345) (penA gene)—decreased susceptibility to penicillins;
- -
- substitution Leu421→Pro in penicillin-binding protein 1 (PBP1) (ponA gene)—resistance to penicillins;
- -
- substitutions Gly120→Lys, Arg, Asp, Asn, Thr and Ala121→Asp, Asn, Gly, Val, Ser in the porin protein PorB (porB gene)—decrease in cell membrane permeability and resistance to penicillins, tetracyclines, and cephalosporins;
- -
- plasmid β-lactamases and mutations Met182→Thr and Gly238→Ser—resistance to penicillins and likely emergence of resistance to cephalosporins;
- -
- substitutions Ser91→Phe, Thr and Asp95→Asn, Gly, His, Tyr, Ala in DNA gyrase (gyrA gene)—resistance to fluoroquinolones;
- -
- mutations in the promoter region of the mtrR gene (-35delA, -10insT, -10insTT)—overexpression of the MtrCDE efflux pump, resistance to penicillins, tetracyclines, macrolides, and cephalosporins;
- -
- tetM plasmid—resistance to tetracyclines;
- -
- substitution Val57→Met in ribosomal protein S10 (rpsj gene)—resistance to tetracyclines;
- -
- replacements Ser87→Asn, Arg, Ile and Glu91→Gln, Gly, Lys, Ala in topoisomerase IV (parC gene)—resistance to fluoroquinolones;
- -
- nucleotide substitutions A2058→G, C and A2059→G, C in 23S rRNA—resistance to azithromycin.
2.3. Synthesis of Oligonucleotide Probes and Primers for PCR
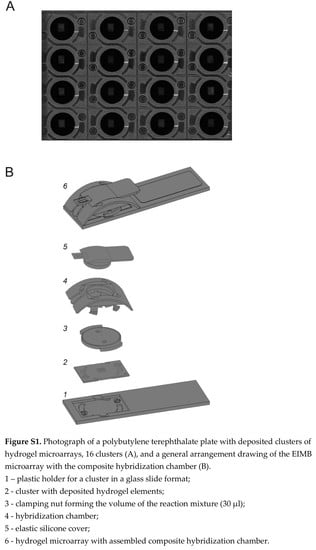
2.4. Microarray Manufacturing
2.5. Multiplex PCR
2.6. Microarray Hybridization
2.7. Detection and Interpretation of Fluorescence Signals
3. Results
3.1. Selection of Optimal Conditions for DNA Hybridization on the Hydrogel Microarray
3.1.1. Selection of the Gel Composition
3.1.2. Oligonucleotide Probes and Hybridization Conditions
3.1.3. Discrimination Ratio for Groups of Immobilized Oligonucleotides
3.2. Detection of Mutations by Microarray Analysis
- Multiplex amplification of fragments of the N. gonorrhoeae genome using specific primers with simultaneous fluorescent labeling;
- Hybridization of fluorescently labeled PCR products to the microarray (Figure 1), with the formation of hybridization complexes in the microarray elements;
- Detection of signals from the hybridization complexes and interpretation of the hybridization results.
- -
- the mosaic penA gene encoding PBP2 with the Ile312→Met, Val316→Thr, Asn512→Tyr and Gly545→Ser substitutions (maximal signals in elements D-1 (group 1–8), K-1 (group 8–16), G-2 (group 17–25), D-3 (group 26–32), K-3 (group 33–46), D-5 (group 47–52), I-5 (group 53–60), C-6 (group 61–69) and C-8 (group 83–88));
- -
- Leu421→Pro substitution in the ponA gene (maximal signal in element F-8 (group 89–90));
- -
- Gly120→Lys and Ala121→Asn substitutions in the porB gene (maximal signal in element J-8 (group 91–123));
- -
- Ser91→Phe substitution in the gyrA gene (maximal signal in element K-11 (group 128–141, subgroup for position 91));
- -
- Asp95→Ala substitution in the gyrA gene (maximal signal in element H-12 (group 128–141, subgroup for position 95));
- -
- -35delA deletion in the promoter region of the mtrR gene (maximal signal in element K-12 (group 142–146));
- -
- Val57→Met substitution in the rpsJ gene (maximal signal in element E-13 (group 148–149));
- -
- Ser87→Arg substitution in the parC gene (maximal signal in element H-13 (group 150–166, subgroup for position 87)).
3.3. Analysis of Genetic Determinants of Antimicrobial Drug Resistance of N. gonorrhoeae in the Russian Population and Concordance of the Microarray Results with Phenotypic Data
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
On the Calculation of Background and Positive Signals of Microarray Elements
References
- ECDC. Molecular Typing of Neisseria gonorrhoeae—A Study of 2013 Isolates. 2018. Available online: https://www.ecdc.europa.eu/en/publications-data/molecular-typing-neisseria-gonorrhoeae-study-2013-isolates (accessed on 15 January 2021).
- Whiley, D.M.; Jennison, A.; Pearson, J.; Lahra, M.M. Genetic characterisation of Neisseria gonorrhoeae resistant to both ceftriaxone and azithromycin. Lancet Infect. Dis. 2018, 18, 717–718. [Google Scholar] [CrossRef]
- Eyre, D.W.; Sanderson, N.D.; Lord, E.; Regisford-Reimmer, N.; Chau, K.; Barker, L.; Morgan, M.; Newnham, R.; Golparian, D.; Unemo, M.; et al. Gonorrhoea treatment failure caused by a Neisseria gonorrhoeae strain with combined ceftriaxone and high-level azithromycin resistance, England, February 2018. Eurosurveillance 2018, 23, 1800323. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Jennison, A.V.; Whiley, D.; Lahra, M.M.; Graham, R.M.; Cole, M.J.; Hughes, G.; Fifer, H.; Andersson, M.; Edwards, A.; Eyre, D. Genetic relatedness of ceftriaxone-resistant and high-level azithromycin resistant Neisseria gonorrhoeae cases, United Kingdom and Australia, February to April 2018. Eurosurveillance 2019, 24, 1900118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unemo, M.; Shafer, W.M. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: Past, evolution, and future. Clin. Microbiol. Rev. 2014, 27, 587–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unemo, M.; Jensen, J.S. Antimicrobial-resistant sexually transmitted infections: Gonorrhoea and Mycoplasma genitalium. Nat. Rev. Urol. 2017, 14, 139–152. [Google Scholar] [CrossRef]
- Unemo, M.; Seifert, H.S.; Hook, E.W., III; Hawkes, S.; Ndowa, F.; Dillon, J.R. Gonorrhoea. Nat. Rev. Dis. Primers 2019, 5, 79. [Google Scholar] [CrossRef]
- Harrison, O.B.; Clemence, M.; Dillard, J.P.; Tang, C.M.; Trees, D.; Grad, Y.H.; Maiden, M.C. Genomic analyses of Neisseria gonorrhoeae reveal an association of the gonococcal genetic island with antimicrobial resistance. J. Infect. 2016, 73, 578–587. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Busó, L.; Golparian, D.; Corander, J.; Grad, Y.H.; Ohnishi, M.; Flemming, R.; Parkhill, J.; Bentley, S.D.; Unemo, M.; Harris, S.R. The impact of antimicrobials on gonococcal evolution. Nat. Microbiol. 2019, 4, 1941–1950. [Google Scholar] [CrossRef] [PubMed]
- Yin, G.; Bie, S.; Gu, H.; Shu, X.; Zheng, W.; Peng, K.; Zhao, H.; Li, F.; Chen, B.; Botchway, B.O.A.; et al. Application of gene chip technology in the diagnostic and drug resistance detection of Helicobacter pylori in children. J. Gastroenterol. Hepatol. 2020, 35, 1331–1339. [Google Scholar] [CrossRef]
- Damin, F.; Galbiati, S.; Gagliardi, S.; Cereda, C.; Dragoni, F.; Fenizia, C.; Savasi, V.; Sola, L.; Chiari, M. CovidArray: A microarray-based assay with high sensitivity for the detection of Sars-Cov-2 in nasopharyngeal swabs. Sensors 2021, 21, 2490. [Google Scholar] [CrossRef]
- Shaskolskiy, B.; Kandinov, I.; Kravtsov, D.; Filippova, M.; Chestkov, A.; Solomka, V.; Kubanov, A.; Deryabin, D.; Dementieva, E.; Gryadunov, D. Prediction of ceftriaxone MIC in Neisseria gonorrhoeae using DNA microarray technology and regression analysis. J. Antimicrob. Chemother. 2021, 76, 3151–3158. [Google Scholar] [CrossRef]
- Khodakov, D.; Li, J.; Zhang, J.X.; Zhang, D.Y. Highly multiplexed rapid DNA detection with single-nucleotide specificity via convective PCR in a portable device. Nat. Biomed. Eng. 2021, 5, 702–712. [Google Scholar] [CrossRef]
- Sola, L.; Damin, F.; Chiari, M. Array of multifunctional polymers for localized immobilization of biomolecules on microarray substrates. Anal. Chim. Acta 2019, 1047, 188–196. [Google Scholar] [CrossRef]
- Chiodi, E.; Damin, F.; Sola, L.; Ferraro, L.; Brambilla, D.; Ünlü, M.S.; Chiari, M. A reliable, label free quality control method for the production of DNA microarrays with clinical applications. Polymers 2021, 13, 340. [Google Scholar] [CrossRef] [PubMed]
- Sinitsyna, E.S.; Walter, J.G.; Vlakh, E.G.; Stahl, F.; Kasper, C.; Tennikova, T.B. Macroporous methacrylate-based monoliths as platforms for DNA microarrays. Talanta 2012, 93, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Glotov, A.S.; Sinitsyna, E.S.; Danilova, M.M.; Vashukova, E.S.; Walter, J.G.; Stahl, F.; Baranov, V.S.; Vlakh, E.G.; Tennikova, T.B. Detection of human genome mutations associated with pregnancy complications using 3-D microarray based on macroporous polymer monoliths. Talanta 2016, 147, 537–546. [Google Scholar] [CrossRef]
- Korzhikova-Vlakh, E.; Antipchik, M.; Tennikova, T. Macroporous polymer monoliths in thin layer format. Polymers 2021, 13, 1059. [Google Scholar] [CrossRef] [PubMed]
- Le Goff, G.C.; Srinivas, R.L.W.; Hill, A.; Doyle, P.S. Hydrogel microparticles for biosensing. Eur. Polym. J. 2015, 72, 386–412. [Google Scholar] [CrossRef] [Green Version]
- Beyer, A.; Pollok, S.; Berg, A.; Weber, K.; Popp, J. Easy daylight fabricated hydrogel array for colorimetric DNA analysis. Macromol. Biosci. 2014, 14, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Gryadunov, D.A.; Shaskolskiy, B.L.; Nasedkina, T.V.; Rubina, A.Y.; Zasedatelev, A.S. The EIMB hydrogel microarray technology: Thirty years later. Acta Nat. 2018, 10, 4–18. [Google Scholar] [CrossRef]
- Leinsoo, A.T.; Shaskol’skii, B.L.; Dement’eva, E.I.; Gryadunov, D.A.; Kubanov, A.A.; Chestkov, A.V.; Obraztsova, O.A.; Shpilevaya, M.V.; Deryabin, D.G. Oligonucleotide microchip for the identification of infectious agents of reproductive system with simultaneous analysis of determinants of resistance to antimicrobial substances. Bull. Exp. Biol. Med. 2017, 164, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Kubanov, A.; Solomka, V.; Plakhova, X.; Chestkov, A.; Petrova, N.; Shaskolskiy, B.; Dementieva, E.; Leinsoo, A.; Gryadunov, D.; Deryabin, D. Summary and trends of the Russian Gonococcal Antimicrobial Surveillance Programme, 2005 to 2016. J. Clin. Microbiol. 2019, 57, e02024-18. [Google Scholar] [CrossRef] [Green Version]
- Shaskolskiy, B.; Dementieva, E.; Kandinov, I.; Filippova, M.; Petrova, N.; Plakhova, X.; Chestkov, A.; Kubanov, A.; Deryabin, D.; Gryadunov, D. Resistance of Neisseria gonorrhoeae isolates to beta-lactams (benzylpenicillin and ceftriaxone) in Russia, 2015–2017. PLoS ONE 2019, 14, e0220339. [Google Scholar] [CrossRef] [Green Version]
- EUCAST. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 11.0. 2021. Available online: http://www.eucast.org (accessed on 15 June 2021).
- Ohnishi, M.; Ono, E.; Shimuta, K.; Watanabe, H.; Okamura, N. Identification of TEM-135 beta-lactamase in penicillinase-producing Neisseria gonorrhoeae isolates in Japan. Antimicrob. Agents Chemother. 2010, 54, 3021–3023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohnishi, M.; Golparian, D.; Shimuta, K.; Saika, T.; Hoshina, S.; Iwasaku, K.; Nakayama, S.; Kitawaki, J.; Unemo, M. Is Neisseria gonorrhoeae initiating a future era of untreatable gonorrhea? Detailed characterization of the first strain with high-level resistance to ceftriaxone. Antimicrob. Agents Chemother. 2011, 55, 3538–3545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, F.; Starosta, A.L.; Arenz, S.; Sohmen, D.; Dönhöfer, A.; Wilson, D.N. Tetracycline antibiotics and resistance mechanisms. Biol. Chem. 2014, 395, 559–575. [Google Scholar] [CrossRef]
- Zapun, A.; Morlot, C.; Taha, M. Resistance to beta-lactams in Neisseria ssp. due to chromosomally encoded penicillin-binding proteins. Antibiotics 2016, 5, 35. [Google Scholar] [CrossRef]
- Unemo, M.; Golparian, D.; Nicholas, R.; Ohnishi, M.; Gallay, A.; Sednaoui, P. High-level cefixime- and ceftriaxone-resistant Neisseria gonorrhoeae in France: Novel penA mosaic allele in a successful international clone causes treatment failure. Antimicrob. Agents Chemother. 2012, 56, 1273–1280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lysov, Y.; Barsky, V.; Urasov, D.; Urasov, R.; Cherepanov, A.; Mamaev, D.; Yegorov, Y.; Chudinov, A.; Surzhikov, S.; Rubina, A.; et al. Microarray analyzer based on wide field fluorescent microscopy with laser illumination and a device for speckle suppression. Biomed. Opt. Express. 2017, 8, 4798–4810. [Google Scholar] [CrossRef] [Green Version]
- Mikhailovich, V.; Lapa, S.; Gryadunov, D.; Sobolev, A.; Strizhkov, B.; Chernyh, N.; Skotnikova, O.; Irtuganova, O.; Moroz, A.; Litvinov, V.; et al. Identification of rifampin-resistant Mycobacterium tuberculosis strains by hybridization, PCR, and ligase detection reaction on oligonucleotide microchips. J. Clin. Microbiol. 2001, 39, 2531–2540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorokin, N.V.; Chechetkin, V.R.; Pan’kov, S.V.; Somova, O.G.; Livshitsm, M.A.; Donnikov, M.Y.; Turygin, A.Y.; Barsky, V.E.; Zasedatelev, A.S. Kinetics of hybridization on surface oligonucleotide microchips: Theory, experiment, and comparison with hybridization on gel-based microchips. J. Biomol. Struct. Dyn. 2006, 24, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, N.V.; Chechetkin, V.R.; Livshits, M.A.; Pan’kov, S.V.; Donnikov, M.Y.; Gryadunov, D.A.; Lapa, S.A.; Zasedatelev, A.S. Discrimination between perfect and mismatched duplexes with oligonucleotide gel microchips: Role of thermodynamic and kinetic effects during hybridization. J. Biomol. Struct. Dyn. 2005, 22, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Kaprou, G.D.; Bergšpica, I.; Alexa, E.A.; Alvarez-Ordóñez, A.; Prieto, M. Rapid methods for antimicrobial resistance diagnostics. Antibiotics 2021, 10, 209. [Google Scholar] [CrossRef]
- Shaskolskiy, B.; Dementieva, E.; Kandinov, I.; Chestkov, A.; Kubanov, A.; Deryabin, D.; Gryadunov, D. Genetic diversity of Neisseria gonorrhoeae multi-antigen sequence types in Russia and Europe. Int. J. Infect. Dis. 2020, 93, 1–8. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaskolskiy, B.; Kandinov, I.; Kravtsov, D.; Vinokurova, A.; Gorshkova, S.; Filippova, M.; Kubanov, A.; Solomka, V.; Deryabin, D.; Dementieva, E.; et al. Hydrogel Droplet Microarray for Genotyping Antimicrobial Resistance Determinants in Neisseria gonorrhoeae Isolates. Polymers 2021, 13, 3889. https://doi.org/10.3390/polym13223889
Shaskolskiy B, Kandinov I, Kravtsov D, Vinokurova A, Gorshkova S, Filippova M, Kubanov A, Solomka V, Deryabin D, Dementieva E, et al. Hydrogel Droplet Microarray for Genotyping Antimicrobial Resistance Determinants in Neisseria gonorrhoeae Isolates. Polymers. 2021; 13(22):3889. https://doi.org/10.3390/polym13223889
Chicago/Turabian StyleShaskolskiy, Boris, Ilya Kandinov, Dmitry Kravtsov, Alexandra Vinokurova, Sofya Gorshkova, Marina Filippova, Alexey Kubanov, Victoria Solomka, Dmitry Deryabin, Ekaterina Dementieva, and et al. 2021. "Hydrogel Droplet Microarray for Genotyping Antimicrobial Resistance Determinants in Neisseria gonorrhoeae Isolates" Polymers 13, no. 22: 3889. https://doi.org/10.3390/polym13223889
APA StyleShaskolskiy, B., Kandinov, I., Kravtsov, D., Vinokurova, A., Gorshkova, S., Filippova, M., Kubanov, A., Solomka, V., Deryabin, D., Dementieva, E., & Gryadunov, D. (2021). Hydrogel Droplet Microarray for Genotyping Antimicrobial Resistance Determinants in Neisseria gonorrhoeae Isolates. Polymers, 13(22), 3889. https://doi.org/10.3390/polym13223889