Polymers as Encapsulating Agents and Delivery Vehicles of Enzymes
Abstract
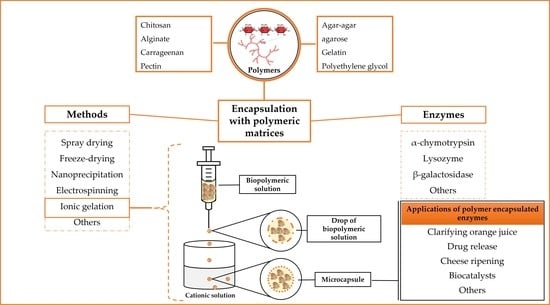
:1. Introduction
2. Polymeric Materials
3. Most Used Polymers for Enzyme Encapsulation
3.1. Chitosan
3.2. Alginate
3.3. Carrageenan
3.4. Pectin
3.5. Agar–Agar and Agarose
3.6. Gelatin
3.7. Polyethylene Glycol (PEG)
4. Enzyme Encapsulation Methods
4.1. Ionic Gelation
4.2. Spray Drying
4.3. Freeze-Drying
4.4. Nanoprecipitation
4.5. Electrospinning
5. Release Mechanisms for Encapsulated Enzymes
6. Characterization of Encapsulation Systems
7. Factors Influencing Active Molecules Encapsulation Efficiency
8. Applications of Polymer Encapsulated Enzymes
9. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kirk, O.; Borchert, T.V.; Fuglsang, C.C. Industrial Enzyme Applications. Curr. Opin. Biotechnol. 2002, 13, 345–351. [Google Scholar] [CrossRef]
- Fraga, J.L.; Souza, C.P.L.; da S. Pereira, A.; Aguieiras, E.C.G.; de Silva, L.O.; Torres, A.G.; Freire, D.G.; Amaral, P.F.F. Palm Oil Wastes as Feedstock for Lipase Production by Yarrowia lipolytica and Biocatalyst Application/Reuse. 3 Biotech 2021, 11, 191. [Google Scholar] [CrossRef]
- Nunes, P.M.B.; Fraga, J.L.; Ratier, R.B.; Rocha-Leão, M.H.M.; Brígida, A.I.S.; Fickers, P.; Amaral, P.F.F. Waste Soybean Frying Oil for the Production, Extraction, and Characterization of Cell-Wall-Associated Lipases from Yarrowia lipolytica. Bioprocess Biosyst. Eng. 2021, 44, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Da Pereira, S.A.; Fontes-Sant’Ana, G.C.; Amaral, P.F.F. Mango Agro-Industrial Wastes for Lipase Production from Yarrowia Lipolytica and the Potential of the Fermented Solid as a Biocatalyst. Food Bioprod. Process. 2019, 115, 68–77. [Google Scholar] [CrossRef]
- Bornscheuer, U.T.; Huisman, G.W.; Kazlauskas, R.J.; Lutz, S.; Moore, J.C.; Robins, K. Engineering the Third Wave of Biocatalysis. Nature 2012, 485, 185–194. [Google Scholar] [CrossRef]
- Bornscheuer, U.T.; Pohl, M. Improved Biocatalysts by Directed Evolution and Rational Protein Design. Curr. Opin. Chem. Biol. 2001, 5, 137–143. [Google Scholar] [CrossRef]
- Basso, A.; Serban, S. Industrial Applications of Immobilized Enzymes—A Review. Mol. Catal. 2019, 479, 6437–6474. [Google Scholar] [CrossRef]
- McClements, D.J. Nanoparticle- and Microparticle-Based Delivery Systems: Encapsulation, 1st ed.; CRC Press: Boca Ratón, FL, USA, 2015. [Google Scholar]
- Boostani, S.; Jafari, S.M. Controlled release of nanoencapsulated food ingredients. In Release and Bioavailability of Nanoencapsulated Food Ingredients; Elsevier: Amsterdam, The Netherlands, 2020; pp. 27–78. [Google Scholar]
- Sheldon, R.A.; van Pelt, S. Enzyme Immobilisation in Biocatalysis: Why, What and How. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamad, N.R.; Marzuki, N.H.C.; Buang, N.A.; Huyop, F.; Wahab, R.A. An Overview of Technologies for Immobilization of Enzymes and Surface Analysis Techniques for Immobilized Enzymes. Biotechnol. Biotechnol. Equip. 2015, 29, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.N.; Balkus, K.J., Jr. Perspective of Recent Progress in Immobilization of Enzymes. ACS Catal. 2011, 1, 956–968. [Google Scholar] [CrossRef]
- da S. Pereira, A.; Diniz, M.M.; de Jong, G.; Gama Filho, H.S.; dos Anjos, M.J.; Finotelli, P.V.; Fontes-Sant’Ana, G.C.; Amaral, P.F.F. Chitosan-Alginate Beads as Encapsulating Agents for Yarrowia Lipolytica Lipase: Morphological, Physico-Chemical and Kinetic Characteristics. Int. J. Biol. Macromol. 2019, 139, 621–630. [Google Scholar] [CrossRef]
- Anjani, K.; Kailasapathy, K.; Phillips, M. Microencapsulation of Enzymes for Potential Application in Acceleration of Cheese Ripening. Int. Dairy J. 2007, 17, 79–86. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Kim, M. An Overview of Techniques in Enzyme Immobilization. Appl. Sci. Converg. Technol. 2017, 26, 157–163. [Google Scholar] [CrossRef]
- Osman, R.; al Jamal, K.T.; Kan, P.L.; Awad, G.; Mortada, N.; EL-Shamy, A.E.; Alpar, O. Inhalable DNase I Microparticles Engineered with Biologically Active Excipients. Pulm. Pharmacol. Ther. 2013, 26, 700–709. [Google Scholar] [CrossRef]
- Chivers, P.R.A.; Kelly, J.A.; Hill, M.J.S.; Smith, D.K. First-Generation Shaped Gel Reactors Based on Photo-Patterned Hybrid Hydrogels. React. Chem. Eng. 2020, 5, 1112–1117. [Google Scholar] [CrossRef]
- Markwalter, C.E.; Pagels, R.F.; Hejazi, A.N.; Gordon, A.G.R.; Thompson, A.L.; Prud’homme, R.K. Polymeric Nanocarrier Formulations of Biologics Using Inverse Flash NanoPrecipitation. AAPS J. 2020, 22, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Edmans, J.G.; Murdoch, C.; Santocildes-Romero, M.E.; Hatton, P.V.; Colley, H.E.; Spain, S.G. Incorporation of Lysozyme into a Mucoadhesive Electrospun Patch for Rapid Protein Delivery to the Oral Mucosa. Mater. Sci. Eng. C 2020, 112, 110917. [Google Scholar] [CrossRef] [PubMed]
- Boudrant, J.; Woodley, J.M.; Fernandez-Lafuente, R. Parameters Necessary to Define an Immobilized Enzyme Preparation. Process. Biochem. 2020, 90, 66–80. [Google Scholar] [CrossRef]
- Jenkins, A.D.; Kratochvíl, P.; Stepto, F.T.; Suter, U.W. Glossary of Basic Terms in Polymer Science (IUPAC Recommendations 1996). Pure Appl. Chem. 1996, 68, 2287–2311. [Google Scholar] [CrossRef]
- Yadav, V.; Sharma, A.; Singh, S.K. Microencapsulation Techniques Applicable To Food Flavours Research And Development: A Comprehensive Review. Int. J. Food Nutr. Sci. 2015, 4, 119–124. [Google Scholar]
- Abbasian, M.; Massoumi, B.; Mohammad-Rezaei, R.; Samadian, H.; Jaymand, M. Scaffolding Polymeric Biomaterials: Are Naturally Occurring Biological Macromolecules More Appropriate for Tissue Engineering? Int. J. Biol. Macromol. 2019, 134, 673–694. [Google Scholar] [CrossRef] [PubMed]
- Ogueri, K.S.; Jafari, T.; Escobar Ivirico, J.L.; Laurencin, C.T. Polymeric Biomaterials For Scaffold-Based Bone Regenerative Engineering. Regen. Eng. Transl. Med. 2019, 5, 128–154. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A Comparative Review of Natural and Synthetic Biopolymer Composite Scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef] [PubMed]
- Rabanel, J.M.; Banquy, X.; Zouaoui, H.; Mokhtar, M.; Hildgen, P. Progress Technology in Microencapsulation Methods for Cell Therapy. Biotechnol. Prog. 2009, 25, 946–963. [Google Scholar] [CrossRef]
- Anderson, D.G.; Langer, R.S.; Dang, T.T. Hydrogel Encapsulated Cells And Anti-Inflammatory Drugs. U.S. Patent 9,867,781, 16 January 2018. [Google Scholar]
- Cascone, M.G.; Barbani, N.; Cristallini, C.; Giusti, P.; Ciardelli, G.; Lazzeri, L. Bioartificial Polymeric Materials Based on Polysaccharides. J. Biomater. Sci. Polym. Ed. 2001, 12, 267–281. [Google Scholar] [CrossRef]
- Zaharaki, D.; Komnitsas, K.; Perdikatsis, V. Use of Analytical Techniques for Identification of Inorganic Polymer Gel Composition. J. Mater. Sci. 2010, 45, 2715–2724. [Google Scholar] [CrossRef]
- Wang, C.; Gu, P.; Hu, B.; Zhang, Q. Recent Progress in Organic Resistance Memory with Small Molecules and Inorganic–Organic Hybrid Polymers as Active Elements. J. Mater. Chem. C 2015, 3, 10055–10065. [Google Scholar] [CrossRef]
- Mahajan, A.; Aggarwal, G. Smart Polymers: Innovations In Novel Drug Delivery. Int. J. Drug Dev. Res. 2011, 3, 16–30. [Google Scholar]
- Ma, Q.; Gao, X.; Bi, X.; Han, Q.; Tu, L.; Yang, Y.; Shen, Y.; Wang, M. Dissolution and Deacetylation of Chitin in Ionic Liquid Tetrabutylammonium Hydroxide and Its Cascade Reaction in Enzyme Treatment for Chitin Recycling. Carbohydr. Polym. 2020, 230, 115605. [Google Scholar] [CrossRef] [PubMed]
- Raza, Z.A.; Khalil, S.; Ayub, A.; Banat, I.M. Recent Developments in Chitosan Encapsulation of Various Active Ingredients for Multifunctional Applications. Carbohydr. Res. 2020, 492, 108004. [Google Scholar] [CrossRef] [PubMed]
- Freitas, A.K.C.; da Silva, L.B.; da Costa, A.C.S.; Peres, A.P.S.; Pergher, S.B.C.; Acchar, W. Tricalcium Phosphate Sheets with Chitosan Obtained via Aqueous Tape Casting. Cerâmica 2020, 66, 421–425. [Google Scholar] [CrossRef]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications. Mar. Drugs 2015, 13, 5156. [Google Scholar] [CrossRef] [PubMed]
- Brumano, L.P.; da Silva, F.V.S.; Costa-Silva, T.A.; Apolinário, A.C.; Santos, J.H.P.M.; Kleingesinds, E.K.; Monteiro, G.; Rangel-Yagui, C.O.; Benyahia, B.; Junior, A.P. Development of L-Asparaginase Biobetters: Current Research Status and Review of the Desirable Quality Profiles. Front. Bioeng. Biotechnol. 2019, 6, 212. [Google Scholar] [CrossRef] [Green Version]
- Racine, E.; Sattler, S.; Escande, A. Free Will and the Brain Disease Model of Addiction: The Not So Seductive Allure of Neuroscience and Its Modest Impact on the Attribution of Free Will to People with an Addiction. Front. Psychol. 2017, 8, 1850. [Google Scholar] [CrossRef] [Green Version]
- Sacco, P.; Furlani, F.; de Marzo, G.; Marsich, E.; Paoletti, S.; Donati, I. Concepts for Developing Physical Gels of Chitosan and of Chitosan Derivatives. Gels 2018, 4, 67. [Google Scholar] [CrossRef] [Green Version]
- Bezerra, C.S.; de Lemos, F.C.M.G.; de Sousa, M.; Gonçalves, L.R.B. Enzyme Immobilization onto Renewable Polymeric Matrixes: Past, Present, and Future Trends. J. Appl. Polym. Sci. 2015, 132, 42125. [Google Scholar] [CrossRef]
- Cardoso, F.D.S.N.; Koblitz, M.G.B.; Ortiz, G.M.D.; Carvalho, J.L.V.D.; Carvalho, L.M.J.d. Study of the Parameters Used in the Encapsulation of Commercial Pectinase in Calcium Alginate and Its Effect on Its Catalytic Activity. Food Sci. Technol. 2019, 39, 247–252. [Google Scholar] [CrossRef] [Green Version]
- Miao, T.; Wang, J.; Zeng, Y.; Liu, G.; Chen, X. Polysaccharide-Based Controlled Release Systems for Therapeutics Delivery and Tissue Engineering: From Bench to Bedside. Adv. Sci. 2018, 5, 1700513. [Google Scholar] [CrossRef]
- Debele, T.A.; Mekuria, S.L.; Tsai, H.C. Polysaccharide based nanogels in the drug delivery system: Application as the carrier of pharmaceutical agents. Mater. Sci. Eng. C 2016, 68, 964–981. [Google Scholar] [CrossRef]
- Guo, X.; Wang, Y.; Qin, Y.; Shen, P.; Peng, Q. Structures, Properties and Application of Alginic Acid: A Review. Int. J. Biol. Macromol. 2020, 162, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Grace, K.; Arjun, N.; Shu-Meng, K.; Rahul, K.; So-Ra, L.; Michael, A.; Paul, D.V.; Elliot, B.; Jonathan, L. Alginate Composition, Temperature, and Presence of Islet Tissue Influence Microcapsule Permeability. Front. Bioeng. Biotechnol. 2016, 4. [Google Scholar] [CrossRef]
- Tønnesen, H.H.; Karlsen, J. Alginate in Drug Delivery Systems. Drug Dev. Ind. Pharm. 2002, 28, 837–854. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [Green Version]
- Moriyama, J.; Yoshimoto, M. Efficient Entrapment of Carbonic Anhydrase in Alginate Hydrogels Using Liposomes for Continuous-Flow Catalytic Reactions. ACS Omega 2021, 6, 6368–6378. [Google Scholar] [CrossRef]
- Shahid, F.; Aman, A.; Ul Qader, S.A. Immobilization of Dextranase Using Anionic Natural Polymer Alginate as a Matrix for the Degradation of a Long-Chain Biopolymer (Dextran). Int. J. Polym. Sci. 2019, 2019, 1354872. [Google Scholar] [CrossRef] [Green Version]
- Sankalia, M.G.; Mashru, R.C.; Sankalia, J.M.; Sutariya, V.B. Stability Improvement of Alpha-Amylase Entrapped in Kappa-Carrageenan Beads: Physicochemical Characterization and Optimization Using Composite Index. Int. J. Pharm. 2006, 312, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sankalia, M.G.; Mashru, R.C.; Sankalia, J.M.; Sutariya, V.B. Physicochemical Characterization of Papain Entrapped in Ionotropically Cross-Linked Kappa-Carrageenan Gel Beads for Stability Improvement Using Doehlert Shell Design. J. Pharm. Sci. 2006, 95, 1994–2013. [Google Scholar] [CrossRef] [PubMed]
- Dey, G.; Bhupinder, S.; Banerjee, R. Immobilization of Alpha-Amylase Produced by Bacillus Circulans GRS 313. Braz. Arch. Biol. Technol. 2003, 46, 167–176. [Google Scholar] [CrossRef]
- Won, K.; Kim, S.; Kim, K.J.; Park, H.W.; Moon, S.J. Optimization of Lipase Entrapment in Ca-Alginate Gel Beads. Process Biochem. 2005, 40, 2149–2154. [Google Scholar] [CrossRef]
- Konsoula, Z.; Liakopoulou-Kyriakides, M. Thermostable α-Amylase Production by Bacillus Subtilis Entrapped in Calcium Alginate Gel Capsules. Enzym. Microb. Technol. 2006, 39, 690–696. [Google Scholar] [CrossRef]
- Konsoula, Z.; Liakopoulou-Kyriakides, M. Starch Hydrolysis by the Action of an Entrapped in Alginate Capsules α-Amylase from Bacillus Subtilis. Process. Biochem. 2006, 41, 343–349. [Google Scholar] [CrossRef]
- Demirkan, E.; Dincbas, S.; Sevinc, N.; Ertan, F. Immobilization of B. Amyloliquefaciens α-Amylase and Comparison of Some of Its Enzymatic Properties with the Free Form. Rom. Biotechnol. Lett. 2011, 16, 6690–6701. [Google Scholar]
- Peña-Montes, C.; Mondragón-Tintor, M.E.; Castro-Rodríguez, J.A.; Bustos-Jaimes, I.; Navarro-Ocaña, A.; Farrés, A. Immobilization and biochemical properties of the enantioselective recombinant NStcI esterase of Aspergillus nidulans. Enzym. Res. 2013, 2013, 928913. [Google Scholar] [CrossRef] [Green Version]
- Pereira, A.d.S.; Fraga, J.L.; Diniz, M.M.; Fontes-Sant’ana, G.C.; Amaral, P.F.F. High Catalytic Activity of Lipase from Yarrowia Lipolytica Immobilized by Microencapsulation. Int. J. Mol. Sci. 2018, 19, 3393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zusfahair, D.R.N.; Kartika, D.; Kurniasih, M.; Nofiani, R.; Fatoni, A. Improved Reuse and Affinity of Enzyme Using Immobilized Amylase on Alginate Matrix. J. Phys. Conf. Ser. 2020, 1494, 012028. [Google Scholar] [CrossRef]
- DeGroot, A.R.; Neufeld, R.J. Encapsulation of Urease in Alginate Beads and Protection from α-Chymotrypsin with Chitosan Membranes. Enzym. Microb. Technol. 2001, 29, 321–327. [Google Scholar] [CrossRef]
- Hosseini, S.; Varidi, M. Optimization of Microbial Rennet Encapsulation in Alginate—Chitosan Nanoparticles. Food Chem. 2021, 352, 129325. [Google Scholar] [CrossRef]
- Gür, S.; İdil, N.; Aksöz, N. Optimization of Enzyme Co-Immobilization with Sodium Alginate and Glutaraldehyde-Activated Chitosan Beads. Appl. Biochem. Biotechnol. 2018, 184, 538–552. [Google Scholar] [CrossRef]
- Rezakhani, N.; Molaei rad, A.; Parivar, K.; Khayat, M.; Etemadzade, S. Immobilization of Protease in Biopolymers (Mixture of Alginate-Chitosan). J. Paramed. Sci. 2014, 5, 108–113. [Google Scholar]
- Raghu, S.; Biol, T.J.; Pennathur, G. Enhancing the Stability of a Carboxylesterase by Entrapment in Chitosan Coated Alginate Beads. Turk. J. Biol. 2018, 42, 307–318. [Google Scholar] [CrossRef]
- Adzmi, F.; Meon, S.; Musa, M.H.; Yusuf, N.A. Preparation, characterisation and viability of encapsulated Trichoderma harzianum UPM40 in alginate-montmorillonite clay. J. Microencapsul. 2012, 29, 205–210. [Google Scholar] [CrossRef]
- Rahim, S.N.A.; Sulaiman, A.; Hamzah, F.; Hamid, K.H.K.; Rodhi, M.N.M.; Musa, M.; Edama, N.A. Enzymes Encapsulation within Calcium Alginate-Clay Beads: Characterization and Application for Cassava Slurry Saccharification. Procedia Eng. 2013, 68, 411–417. [Google Scholar] [CrossRef] [Green Version]
- Freitas, F.F.; Marquez, L.D.S.; Ribeiro, G.P.; Brandão, G.C.; Cardoso, V.L.; Ribeiro, E.J. Optimization of the Immobilization Process of β-Galatosidade by Combined Entrapment-Cross-Linking and the Kinetics of Lactose Hydrolysis. Braz. J. Chem. Eng. 2012, 29, 15–24. [Google Scholar] [CrossRef] [Green Version]
- Labus, K.; Wolanin, K.; Radosiński, Ł. Comparative Study on Enzyme Immobilization Using Natural Hydrogel Matrices—Experimental Studies Supported by Molecular Models Analysis. Catalysts 2020, 10, 489. [Google Scholar] [CrossRef]
- Kendall, W.F.; Opara, E.C. Polymeric Materials for Perm-Selective Coating of Alginate Microbeads. Methods Mol. Biol. 2017, 1479, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Bakry, A.M.; Huang, J.; Zhai, Y.; Huang, Q. Myofibrillar Protein with κ- or λ-Carrageenans as Novel Shell Materials for Microencapsulation of Tuna Oil through Complex Coacervation. Food Hydrocoll. 2019, 96, 43–53. [Google Scholar] [CrossRef]
- Khotimchenko, M.; Tiasto, V.; Kalitnik, A.; Begun, M.; Khotimchenko, R.; Leonteva, E.; Bryukhovetskiy, I.; Khotimchenko, Y. Antitumor Potential of Carrageenans from Marine Red Algae. Carbohydr. Polym. 2020, 246, 116568. [Google Scholar] [CrossRef]
- David, S.; Shani Levi, C.; Fahoum, L.; Ungar, Y.; Meyron-Holtz, E.G.; Shpigelman, A. Revisiting the Carrageenan Controversy: Do We Really Understand the Digestive Fate and Safety of Carrageenan in Our Foods? Food Funct. 2018, 9, 1344–1352. [Google Scholar] [CrossRef] [PubMed]
- Fauzi, M.A.R.D.; Pudjiastuti, P.; Wibowo, A.C.; Hendradi, E. Preparation, Properties and Potential of Carrageenan-Based Hard Capsules for Replacing Gelatine: A Review. Polymers 2021, 13, 2666. [Google Scholar] [CrossRef] [PubMed]
- Geonzon, L.C.; Bacabac, R.G.; Matsukawa, S. Network Structure and Gelation Mechanism of Kappa and Iota Carrageenan Elucidated by Multiple Particle Tracking. Food Hydrocoll. 2019, 92, 173–180. [Google Scholar] [CrossRef]
- Zheng, H.; Mao, L.; Cui, M.; Liu, J.; Gao, Y. Development of Food-Grade Bigels Based on κ-Carrageenan Hydrogel and Monoglyceride Oleogels as Carriers for β-Carotene: Roles of Oleogel Fraction. Food Hydrocoll. 2020, 105, 105855. [Google Scholar] [CrossRef]
- Silva, R.C.; Trevisan, M.G.; Garcia, J.S. β-Galactosidase Encapsulated in Carrageenan, Pectin and Carrageenan/Pectin: Comparative Study, Stability and Controlled Release. An. Acad. Bras. Ciências 2020, 92, 20180609. [Google Scholar] [CrossRef]
- Malhotra, I.; Basir, S.F. Immobilization of Invertase in Calcium Alginate and Calcium Alginate-Kappa-Carrageenan Beads and Its Application in Bioethanol Production. Prep. Biochem. Biotechnol. 2020, 50, 494–503. [Google Scholar] [CrossRef]
- Yamazaki, S.; Mori, T.; Ogino, I.; Mukai, S.R. Flexible Film-Type Catalysts Encapsulating Urease within κ-Carrageenan Hydrogel Network. Chem. Eng. J. 2015, 278, 122–128. [Google Scholar] [CrossRef]
- Olímpio, F.M.P.; Mendes, A.A.; Trevisan, M.G.; Garcia, J.S. Preparation and Delayed Release Study on Pancreatin Encapsulated into Alginate, Carrageenan and Pectin Hydrogels. J. Braz. Chem. Soc. 2020, 31, 320–330. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, R.; McClements, D.J. Lactase (β-Galactosidase) Encapsulation in Hydrogel Beads with Controlled Internal PH Microenvironments: Impact of Bead Characteristics on Enzyme Activity. Food Hydrocoll. 2017, 67, 85–93. [Google Scholar] [CrossRef] [Green Version]
- Desai, P.D.; Dave, A.M.; Devi, S. Entrapment of Lipase into K-Carrageenan Beads and Its Use in Hydrolysis of Olive Oil in Biphasic System. J. Mol. Catalysis. B Enzym. 2004, 31, 143–150. [Google Scholar] [CrossRef]
- Wardoyo, F.A.; Hidayah, F.F. Thermal and Reused Stability of Immobilized Lipase in Carrageenan. IOP Conf. Ser. Earth Environ. Sci. 2019, 292, 012028. [Google Scholar] [CrossRef]
- Hassan, M.E.; Yang, Q.; Xiao, Z. Covalent Immobilization of Glucoamylase Enzyme onto Chemically Activated Surface of κ-Carrageenan. Bull. Natl. Res. Cent. 2019, 43, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Caroço, R.F.; Kim, B.; Santacoloma, P.A.; Abildskov, J.; Lee, J.H.; Huusom, J.K. Analysis and Model-Based Optimization of a Pectin Extraction Process. J. Food Eng. 2019, 244, 159–169. [Google Scholar] [CrossRef] [Green Version]
- Chan, S.Y.; Choo, W.S.; Young, D.J.; Loh, X.J. Pectin as a Rheology Modifier: Origin, Structure, Commercial Production and Rheology. Carbohydr. Polym. 2017, 161, 118–139. [Google Scholar] [CrossRef]
- Cao, L.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Egg-Box Model-Based Gelation of Alginate and Pectin: A Review. Carbohydr. Polym. 2020, 242, 116389. [Google Scholar] [CrossRef] [PubMed]
- Picot-Allain, M.C.N.; Ramasawmy, B.; Emmambux, M.N. Extraction, Characterisation, and Application of Pectin from Tropical and Sub-Tropical Fruits: A Review. Food Rev. Int. 2020. [Google Scholar] [CrossRef]
- Bilal, M.; Adeel, M.; Rasheed, T.; Zhao, Y.; Iqbal, H.M.N. Emerging Contaminants of High Concern and Their Enzyme-Assisted Biodegradation–A Review. Environ. Int. 2019, 124, 336–353. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Chen, J.; Zhang, H.; Wu, D.; Ye, X.; Linardt, R.J.; Chen, S. Gelling Mechanism of RG-I Enriched Citrus Pectin: Role of Arabinose Side-Chains in Cation- and Acid-Induced Gelation. Food Hydrocoll. 2020, 101, 105536. [Google Scholar] [CrossRef]
- Costas, L.; Bosio, V.E.; Pandey, A.; Castro, G.R. Effects of Organic Solvents on Immobilized Lipase in Pectin Microspheres. Appl. Biochem. Biotechnol. 2008, 151, 578–586. [Google Scholar] [CrossRef]
- Jadhav, S.B.; Singhal, R.S. Co-Conjugation Vis-à-Vis Individual Conjugation of α-Amylase and Glucoamylase for Hydrolysis of Starch. Carbohydr. Polym. 2013, 98, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, S.; Gernet, M.; Pradeau, D.; Andremont, A.; Fattal, E. Evaluation of Critical Formulation Parameters Influencing the Bioactivity of β-Lactamases Entrapped in Pectin Beads. Int. J. Pharm. 2006, 324, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Muanruksa, P.; Dujjanutat, P.; Kaewkannetra, P. Entrapping Immobilisation of Lipase on Biocomposite Hydrogels toward for Biodiesel Production from Waste Frying Acid Oil. Catalysts 2020, 10, 834. [Google Scholar] [CrossRef]
- Sharma, M.; Sangwan, R.S.; Khatkar, B.S.; Singh, S.P. Alginate-Pectin Co-Encapsulation of Dextransucrase and Dextranase for Oligosaccharide Production from Sucrose Feedstocks. Bioprocess. Biosyst. Eng. 2019, 42, 1681–1693. [Google Scholar] [CrossRef]
- Satar, R.; Matto, M.; Husain, Q. Studies on Calcium Alginate-Pectin Gel Entrapped Concanavalin A-Bitter Gourd (Momordica Charantia) Peroxidase Complex. J. Sci. Ind. Res. 2008, 67, 609–615. [Google Scholar]
- Cruz, M.; Fernandes, K.; Cysneiros, C.; Nassar, R.; Caramori, S. Improvement of Starch Digestion Using α-Amylase Entrapped in Pectin-Polyvinyl Alcohol Blend. BioMed Res. Int. 2015, 2015, 145903. [Google Scholar] [CrossRef]
- Gómez, L.; Ramírez, H.L.; Neira-Carrillo, A.; Villalonga, R. Polyelectrolyte Complex Formation Mediated Immobilization of Chitosan-Invertase Neoglycoconjugate on Pectin-Coated Chitin. Bioprocess. Biosyst. Eng. 2006, 28, 387–395. [Google Scholar] [CrossRef]
- Cargnin, M.A.; Gasparin, B.C.; dos Santos Rosa, D.; Paulino, A.T. Performance of Lactase Encapsulated in Pectin-Based Hydrogels during Lactose Hydrolysis Reactions. LWT 2021, 150, 111863. [Google Scholar] [CrossRef]
- Chen, X.; Fu, X.; Huang, L.; Xu, J.; Gao, X. Agar Oligosaccharides: A Review of Preparation, Structures, Bioactivities and Application. Carbohydr. Polym. 2021, 265, 118076. [Google Scholar] [CrossRef]
- Imam, H.T.; Marr, P.C.; Marr, A.C. Enzyme Entrapment, Biocatalyst Immobilization without Covalent Attachment. Green Chemistry 2021, 23, 4980–5005. [Google Scholar] [CrossRef]
- Nishinari, K.; Fang, Y. Relation between Structure and Rheological/Thermal Properties of Agar. A Mini-Review on the Effect of Alkali Treatment and the Role of Agaropectin. Food Struct. 2017, 13, 24–34. [Google Scholar] [CrossRef]
- Sassolas, A.; Hayat, A.; Marty, J.-L. Enzyme Immobilization by Entrapment Within a Gel Network. Methods Mol. Biol. 2013, 1051, 229–239. [Google Scholar] [CrossRef]
- Huo, J.; Aguilera-Sigalat, J.; El-Hankari, S.; Bradshaw, D. Magnetic MOF Microreactors for Recyclable Size-Selective Biocatalysis. Chem. Sci. 2015, 6, 1938–1943. [Google Scholar] [CrossRef] [Green Version]
- Mahajan, R.; Gupta, V.K.; Sharma, J. Comparison and Suitability of Gel Matrix for Entrapping Higher Content of Enzymes for Commercial Applications. Indian, J. Pharm. Sci. 2010, 72, 223–228. [Google Scholar] [CrossRef] [Green Version]
- Prakash, O.; Jaiswal, N. Immobilization of a Thermostable-Amylase on Agarose and Agar Matrices and Its Application in Starch Stain Removal. World Appl. Sci. J. 2011, 13, 572–577. [Google Scholar]
- Vieira, M.F.; Vieira, A.M.S.; Zanin, G.M.; Tardioli, P.W.; Mateo, C.; Guisán, J.M. β-Glucosidase Immobilized and Stabilized on Agarose Matrix Functionalized with Distinct Reactive Groups. J. Mol. Catal. B Enzym. 2011, 69, 47–53. [Google Scholar] [CrossRef] [Green Version]
- Zucca, P.; Fernandez-Lafuente, R.; Sanjust, E. Agarose and Its Derivatives as Supports for Enzyme Immobilization. Molecules 2016, 21, 1577. [Google Scholar] [CrossRef]
- Ghebremedhin, M.; Seiffert, S.; Vilgis, T.A. Physics of Agarose Fluid Gels: Rheological Properties and Microstructure. Curr. Res. Food Sci. 2021, 4, 436–448. [Google Scholar] [CrossRef]
- Russ, N.; Zielbauer, B.I.; Koynov, K.; Vilgis, T.A. Influence of Nongelling Hydrocolloids on the Gelation of Agarose. Biomacromolecules 2013, 14, 4116–4124. [Google Scholar] [CrossRef]
- Nordqvist, D.; Vilgis, T.A. Rheological Study of the Gelation Process of Agarose-Based Solutions. Food Biophys. 2011, 6, 450–460. [Google Scholar] [CrossRef]
- Vilgis, T.A. Gels: Model Systems for Soft Matter Food Physics. Curr. Opin. Food Sci. 2015, 3, 71–84. [Google Scholar] [CrossRef]
- Pedroche, J.; del Mar Yust, M.; Mateo, C.; Fernández-Lafuente, R.; Girón-Calle, J.; Alaiz, M.; Vioque, J.; Guisán, J.M.; Millán, F. Effect of the Support and Experimental Conditions in the Intensity of the Multipoint Covalent Attachment of Proteins on Glyoxyl-Agarose Supports: Correlation between Enzyme–Support Linkages and Thermal Stability. Enzym. Microb. Technol. 2007, 40, 1160–1166. [Google Scholar] [CrossRef]
- Kunkel, J.; Asuri, P. Function, Structure, and Stability of Enzymes Confined in Agarose Gels. PLoS ONE 2014, 9, e86785. [Google Scholar] [CrossRef]
- Karim, A.; Bibi, Z.; Rehman, H.U.; Aman, A.; Qader, S.A.U.; Rashid, M.H. Single Step Immobilization of CMCase within Agarose Gel Matrix: Kinetics and Thermodynamic Studies. Colloids Surf. B Biointerfaces 2021, 200, 111583. [Google Scholar] [CrossRef]
- Silva-Salinas, A.; Rodríguez-Delgado, M.; Gómez-Treviño, J.; López-Chuken, U.; Olvera-Carranza, C.; Blanco-Gámez, E.A. Novel Thermotolerant Amylase from Bacillus Licheniformis Strain LB04: Purification, Characterization and Agar-Agarose. Microorganisms 2021, 9, 1857. [Google Scholar] [CrossRef]
- Sattar, H.; Aman, A.; Qader, S.A.U. Agar-Agar Immobilization: An Alternative Approach for the Entrapment of Protease to Improve the Catalytic Efficiency, Thermal Stability and Recycling Efficiency. Int. J. Biol. Macromol. 2018, 111, 917–922. [Google Scholar] [CrossRef]
- Mariod, A.A.; Adam, H.F. Review: Gelatin, Source, Extraction and Industrial Applications. Acta Svi. Pol. Technol. Aliment. 2013, 12, 135–147. [Google Scholar]
- Sultana, S.; Ali, M.E.; Ahamad, M.N.U. Gelatine, Collagen, and Single Cell Proteins as a Natural and Newly Emerging Food Ingredients. In Preparation and Processing of Religious and Cultural Foods, 1st ed.; Ali, M.E., Nizar, N.N.A., Eds.; Woodhead Publishing: Duxford, UK, 2018; pp. 215–239. [Google Scholar] [CrossRef]
- Alipal, J.; Mohd Pu’ad, N.A.S.; Lee, T.C.; Nayan, N.H.M.; Sahari, N.; Basri, H.; Idris, M.I.; Abdullah, H.Z. A Review of Gelatin: Properties, Sources, Process, Applications, and Commercialisation. Mater. Today Proc. 2021, 42, 240–250. [Google Scholar] [CrossRef]
- Djabourov, M. Gels. In NMR and MRI of Gels; De Deene, Y., Ed.; The Royal Society of Chemistry: Croydon, UK, 2020; pp. 1–44. [Google Scholar]
- Long, H.; Ma, K.; Xiao, Z.; Ren, X.; Yang, G. Preparation and Characteristics of Gelatin Sponges Crosslinked by Microbial Transglutaminase. PeerJ 2017, 5, e3665. [Google Scholar] [CrossRef] [Green Version]
- Savoca, M.P.; Tonoli, E.; Atobatele, A.G.; Verderio, E.A.M. Biocatalysis by Transglutaminases: A Review of Biotechnological Applications. Micromachines 2018, 9, 562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labus, K.; Drozd, A.; Trusek-Holownia, A. Preparation and Characterisation of Gelatine Hydrogels Predisposed to Use as Matrices for Effective Immobilisation of Biocatalystst. Chem. Pap. 2016, 70, 523–530. [Google Scholar] [CrossRef]
- Salleh, H.M.; Mel, M.; Jami, M.S.; Amid, A.; Bala, M. Optimization of Spray Drying Process Conditions for Recombinant Stem Bromelain. Adv. Environ. Biol. 2014, 8, 696–703. [Google Scholar]
- Li, J.; Ma, J.; Jiang, Y.; Jiang, T.; Wang, Y.; Chen, Y.; Liu, S. Immobilizing Enzymes in Regular-Sized Gelatin Microspheres through a Membrane Emulsification Method. J. Mater. Sci. 2016, 51, 6357–6369. [Google Scholar] [CrossRef]
- Al-Khafaji, M.A.J.; Ewadh, M.J. Immobilization of Urease in Gelatin Beads for Urea Estimation. Natl. J. Chem. 2009, 33, 131–137. [Google Scholar]
- Gan, Z.; Zhang, T.; Liu, Y.; Wu, D. Temperature-Triggered Enzyme Immobilization and Release Based on Cross-Linked Gelatin Nanoparticles. PLoS ONE 2012, 7, e47154. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Singh, S.P. Enzyme entrapment approaches and their applications. In Biomass, Biofuels, Biochemicals; Singh, S.P., Pandey, A., Sighania, R.R., Larroche, C., Li, Z., Eds.; Elsevier: Oxford, UK, 2020; pp. 191–216. [Google Scholar]
- Mogharabi, M.; Nassiri-Koopaei, N.; Bozorgi-Koushalshahi, M.; Nafissi-Varcheh, N.; Bagherzadeh, G.; Faramarzi, M.A. Immobilization of Laccase in Alginate-Gelatin Mixed Gel and Decolorization of Synthetic Dyes. Bioinorg. Chem. Appl. 2012, 2012, 823830. [Google Scholar] [CrossRef] [PubMed]
- Nagatomo, H.; Matsushita, Y.; Sugamoto, K.; Matsui, T. Preparation and Properties of Gelatin-Immobilized β-Glucosidase from Pyrococcus Furiosus. Biosci. Biotechnol. Biochem. 2005, 69, 128–136. [Google Scholar] [CrossRef] [Green Version]
- Jaiswal, N.; Prakash, O.; Talat, M.; Hasan, S.H.; Pandey, R.K. A-Amylase Immobilization on Gelatin: Optimization of Process Variables. J. Genet. Eng. Biotechnol. 2012, 10, 161–167. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.W.; Wang, N.; Zhou, Y.J.; He, T.; Yu, X.Q. Enhancement of Activity and Stability of Lipase by Microemulsion-Based Organogels (MBGs) Immobilization and Application for Synthesis of Arylethyl Acetate. J. Mol. Catal. B Enzym. 2012, 78, 65–71. [Google Scholar] [CrossRef]
- Patel, A.R.; Remijn, C.; Cabero, A.I.M.; Heussen, P.C.M.; ten Hoorn, J.W.M.S.; Velikov, K.P. Novel All-Natural Microcapsules from Gelatin and Shellac for Biorelated Applications. Adv. Funct. Mater. 2013, 23, 4710–4718. [Google Scholar] [CrossRef]
- D’Souza, A.; Shegokar, R. Polymer: Lipid Hybrid Nanostructures in Cancer Drug Delivery: Successes and Limitations. In Nanoarchitectonics for Smart Delivery and Drug Targeting; Holban, A.M., Grumezescu, A.M., Eds.; William Andrew Publishing: New York, NY, USA, 2016; pp. 431–463. ISBN 978-0-323-47347-7. [Google Scholar]
- Sikka, M.P.; Midha, V.K. The role of biopolymers and biodegradable polymeric dressings in managing chronic wounds. In Advanced Textiles for Wound Care; Rajendran, S., Ed.; Woodhead Publishing: Sawston, UK, 2019; pp. 463–488. [Google Scholar]
- Wang, Y.; Feng, C.; Guo, R.; Ma, Y.; Yuan, Y.; Liu, Y. Cellulase Immobilized by Sodium Alginate-Polyethylene Glycol-Chitosan for Hydrolysis Enhancement of Microcrystalline Cellulose. Process. Biochem. 2021, 107, 38–47. [Google Scholar] [CrossRef]
- Liao, R.; Pon, J.; Chungyoun, M.; Nance, E. Enzymatic Protection and Biocompatibility Screening of Enzyme-Loaded Polymeric Nanoparticles for Neurotherapeutic Applications. Biomaterials 2020, 257, 120238. [Google Scholar] [CrossRef]
- Piao, M.; Zou, D.; Yang, Y.; Ren, X.; Qin, C.; Piao, Y. Multi-Functional Laccase Immobilized Hydrogel Microparticles for Efficient Removal of Bisphenol, A. Materials 2019, 12, 704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ataide, J.A.; Geraldes, D.C.; Gérios, E.F.; Bissaco, F.M.; Cefali, L.C.; Oliveira-Nascimento, L.; Mazzola, P.G. Freeze-Dried Chitosan Nanoparticles to Stabilize and Deliver Bromelain. J. Drug Deliv. Sci. Technol. 2021, 61, 102225. [Google Scholar] [CrossRef]
- Liu, H.; Nakagawa, K.; Kato, D.I.; Chaudhary, D.; Tadé, M.O. Enzyme Encapsulation in Freeze-Dried Bionanocomposites Prepared from Chitosan and Xanthan Gum Blend. Mater. Chem. Phys. 2011, 129, 488–494. [Google Scholar] [CrossRef]
- Steinhilber, D.; Witting, M.; Zhang, X.; Staegemann, M.; Paulus, F.; Friess, W.; Küchler, S.; Haag, R. Surfactant Free Preparation of Biodegradable Dendritic Polyglycerol Nanogels by Inverse Nanoprecipitation for Encapsulation and Release of Pharmaceutical Biomacromolecules. J. Control. Release 2013, 169, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Gabrielczyk, J.; Duensing, T.; Buchholz, S.; Schwinges, A.; Jördening, H.J. A Comparative Study on Immobilization of Fructosyltransferase in Biodegradable Polymers by Electrospinning. Appl. Biochem. Biotechnol. 2018, 185, 847–862. [Google Scholar] [CrossRef]
- de Souza Simões, L.; Madalena, D.A.; Pinheiro, A.C.; Teixeira, J.A.; Vicente, A.A.; Ramos, Ó.L. Micro- and Nano Bio-Based Delivery Systems for Food Applications: In Vitro Behavior. Adv. Colloid Interface Sci. 2017, 243, 23–45. [Google Scholar] [CrossRef] [Green Version]
- Aguiar, J.; Estevinho, B.N.; Santos, L. Microencapsulation of Natural Antioxidants for Food Application—The Specific Case of Coffee Antioxidants—A Review. Trends Food Sci. Technol. 2016, 58, 21–39. [Google Scholar] [CrossRef]
- Bobone, S.; Miele, E.; Cerroni, B.; Roversi, D.; Bocedi, A.; Nicolai, E.; di Venere, A.; Placidi, E.; Ricci, G.; Rosato, N.; et al. Liposome-Templated Hydrogel Nanoparticles as Vehicles for Enzyme-Based Therapies. Langmuir 2015, 31, 7572–7580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tawfeek, H.M.; Khidr, S.H.; Samy, E.M.; Ahmed, S.M.; Gaskell, E.E.; Hutcheon, G.A. Evaluation of Biodegradable Polyester-Co-Lactone Microparticles for Protein Delivery. Drug Dev. Ind. Pharm. 2014, 40, 1213–1222. [Google Scholar] [CrossRef] [Green Version]
- Galliani, M.; Santi, M.; del Grosso, A.; Cecchettini, A.; Santorelli, F.M.; Hofmann, S.L.; Lu, J.Y.; Angella, L.; Cecchini, M.; Signore, G. Cross-Linked Enzyme Aggregates as Versatile Tool for Enzyme Delivery: Application to Polymeric Nanoparticles. Bioconjugate Chem. 2018, 29, 2225–2231. [Google Scholar] [CrossRef]
- Flores-Fernández, G.M.; Griebenow, K. Glycosylation Improves α-Chymotrypsin Stability upon Encapsulation in Poly(Lactic-Co-Glycolic)Acid Microspheres. Results Pharma Sci. 2012, 2, 46–51. [Google Scholar] [CrossRef] [Green Version]
- Portilla-Arias, J.A.; Camargo, B.; García-Alvarez, M.; de Ilarduya, A.M.; Muñoz-Guerra, S. Nanoparticles Made of Microbial Poly(γ-Glutamate)s for Encapsulation and Delivery of Drugs and Proteins. J. Biomater. Sci. Polym. Ed. 2009, 20, 1065–1079. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.L.; Musin, E.V.; Dubrovskii, A.V.; Tikhonenko, S.A. Effect of Pollyallylamine on Alcoholdehydrogenase Structure and Activity. Polymers 2020, 12, 832. [Google Scholar] [CrossRef] [Green Version]
- Dhanjai; Lu, X.; Wu, L.; Chen, J.; Lu, Y. Robust Single-Molecule Enzyme Nanocapsules for Biosensing with Significantly Improved Biosensor Stability. Anal. Chem. 2020, 92, 5830–5837. [Google Scholar] [CrossRef] [PubMed]
- Housseiny, M.M.; Aboelmagd, H.I. Nano-Encapsulation of Naringinase Produced by Trichoderma Longibrachiatum ATCC18648 on Thermally Stable Biopolymers for Citrus Juice Debittering. J. Microbiol. 2019, 57, 521–531. [Google Scholar] [CrossRef]
- Wagner, I.; Nagy, Z.K.; Vass, P.; Fehér, C.; Barta, Z.; Vigh, T.; Sóti, P.L.; Harasztos, A.H.; Pataki, H.; Balogh, A.; et al. Stable Formulation of Protein-Type Drug in Electrospun Polymeric Fiber Followed by Tableting and Scaling-up Experiments. Polym. Adv. Technol. 2015, 26, 1461–1467. [Google Scholar] [CrossRef]
- Yaghoobi, N.; Majidi, R.F.; Faramarzi, M.A.; Baharifar, H.; Amani, A. Preparation, Optimization and Activity Evaluation of PLGA/Streptokinase Nanoparticles Using Electrospray. Adv. Pharm. Bull. 2017, 7, 131–139. [Google Scholar] [CrossRef] [Green Version]
- Morales-Cruz, M.; Flores-Fernández, G.M.; Morales-Cruz, M.; Orellano, E.A.; Rodriguez-Martinez, J.A.; Ruiz, M.; Griebenow, K. Two-Step Nanoprecipitation for the Production of Protein-Loaded PLGA Nanospheres. Results Pharma Sci. 2012, 2, 79–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindhoud, S.; de Vries, R.; Norde, W.; Stuart, M.A.C. Structure and Stability of Complex Coacervate Core Micelles with Lysozyme. Biomacromolecules 2007, 8, 2219–2227. [Google Scholar] [CrossRef] [PubMed]
- Babich, O.; Dyshlyuk, L.; Prosekov, A.; Noskova, S.; Ivina, O.; Pavsky, V.; Ivanova, S.; Bulgakova, O. Study of the Potential of the Capsule Shell Based on Natural Polysaccharides in Targeted Delivery of the L-Phenylalanine Ammonia-Lyase Enzyme Preparation. Pharmaceuticals 2020, 13, 63. [Google Scholar] [CrossRef] [Green Version]
- Estevinho, B.N.; Damas, A.M.; Martins, P.; Rocha, F. Study of the Inhibition Effect on the Microencapsulated Enzyme β-Galactosidase. Environ. Eng. Manag. J. 2012, 11, 1923–1930. [Google Scholar] [CrossRef]
- Chong-Cerda, R.; Levin, L.; Castro-Rios, R.; Hérnandez-Luna, C.E.; González-Horta, A.; Gutiérrez-Soto, G.; Chávez-Montes, A. Nanoencapsulated Laccases Obtained by Double-Emulsion Technique. Effects on EnzymeActivity PH-Dependence and Stability. Catalysts 2020, 10, 1085. [Google Scholar] [CrossRef]
- Tawfeek, H.M.; Evans, A.R.; Iftikhar, A.; Mohammed, A.R.; Shabir, A.; Somavarapu, S.; Hutcheon, G.A.; Saleem, I.Y. Dry Powder Inhalation of Macromolecules Using Novel PEG-Co-Polyester Microparticle Carriers. Int. J. Pharm. 2013, 441, 611–619. [Google Scholar] [CrossRef] [Green Version]
- Larrañaga, A.; Isa, I.L.M.; Patil, V.; Thamboo, S.; Lomora, M.; Fernández-Yague, M.A.; Sarasua, J.R.; Palivan, C.G.; Pandit, A. Antioxidant Functionalized Polymer Capsules to Prevent Oxidative Stress. Acta Biomater. 2018, 67, 21–31. [Google Scholar] [CrossRef]
- Karamitros, C.S.; Yashchenok, A.M.; Möhwald, H.; Skirtach, A.G.; Konrad, M. Preserving Catalytic Activity and Enhancing Biochemical Stability of the Therapeutic Enzyme Asparaginase by Biocompatible Multilayered Polyelectrolyte Microcapsules. Biomacromolecules 2013, 14, 4398–4406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lino, P.R.; Leandro, J.; Amaro, M.; Gonçalves, L.M.D.; Leandro, P.; Almeida, A.J. In Silico and in Vitro Tailoring of a Chitosan Nanoformulation of a Human Metabolic Enzyme. Pharmaceutics 2021, 13, 329. [Google Scholar] [CrossRef]
- Mahmoud, K.F.; Abo-Elmagd, H.I.; Housseiny, M.M. Micro- and Nano-Capsulated Fungal Pectinase with Outstanding Capabilities of Eliminating Turbidity in Freshly Produced Juice. Food Sci. Technol. Int. 2018, 24, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Patil, J.S.; Kamalapur, M.V.; Marapur, S.C.; Kadam, D. Ionotropic Gelation And Polyelectrolyte Complexation: The Novel Techniques To Design Hydrogel Particulate Sustained, Modulated Drug Delivery System: A Review. Dig. J. Nanomater. Biostructures 2010, 5, 241–248. [Google Scholar]
- Comunian, T.A.; Favaro-Trindade, C.S. Microencapsulation Using Biopolymers as an Alternative to Produce Food Enhanced with Phytosterols and Omega-3 Fatty Acids: A Review. Food Hydrocoll. 2016, 61, 442–457. [Google Scholar] [CrossRef]
- Bahreini, E.; Aghaiypour, K.; Abbasalipourkabir, R.; Mokarram, A.R.; Goodarzi, M.T.; Saidijam, M. Preparation and Nanoencapsulation of L-Asparaginase II in Chitosan-Tripolyphosphate Nanoparticles and in Vitro Release Study. Nanoscale Res. Lett. 2014, 9, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Vimal, A.; Kumar, A. Antimicrobial Potency Evaluation of Free and Immobilized L-Asparaginase Using Chitosan Nanoparticles. J. Drug Deliv. Sci. Technol. 2021, 61, 102231. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M.N.; Hu, H.; Wang, W.; Zhang, X. Enhanced Bio-Catalytic Performance and Dye Degradation Potential of Chitosan-Encapsulated Horseradish Peroxidase in a Packed Bed Reactor System. Sci. Total. Environ. 2017, 575, 1352–1360. [Google Scholar] [CrossRef]
- Jaiswal, N.; Pandey, V.P.; Dwivedi, U.N. Immobilization of Papaya Laccase in Chitosan Led to Improved Multipronged Stability and Dye Discoloration. Int. J. Biol. Macromol. 2016, 86, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Gharsallaoui, A.; Roudaut, G.; Chambin, O.; Voilley, A.; Saurel, R. Applications of Spray-Drying in Microencapsulation of Food Ingredients: An Overview. Food Res. Int. 2007, 40, 1107–1121. [Google Scholar] [CrossRef]
- Arpagaus, C.; Collenberg, A.; Rütti, D.; Assadpour, E.; Jafari, S.M. Nano Spray Drying for Encapsulation of Pharmaceuticals. Int. J. Pharm. 2018, 546, 194–214. [Google Scholar] [CrossRef] [PubMed]
- Cal, K.; Sollohub, K. Spray Drying Technique. I: Hardware and Process Parameters. J. Pharm. Sci. 2010, 99, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Samborska, K.; Witrowa-Rajchert, D.; Gonçalves, A. Spray-Drying of α-Amylase—The Effect of Process Variables on the Enzyme Inactivation. Dry. Technol. 2005, 23, 941–953. [Google Scholar] [CrossRef]
- Shishir, M.R.I.; Xie, L.; Sun, C.; Zheng, X.; Chen, W. Advances in Micro and Nano-Encapsulation of Bioactive Compounds Using Biopolymer and Lipid-Based Transporters. Trends Food Sci. Technol. 2018, 78, 34–60. [Google Scholar] [CrossRef]
- Vishali, D.A.; Monisha, J.; Sivakamasundari, S.K.; Moses, J.A.; Anandharamakrishnan, C. Spray freeze drying: Emerging applications in drug delivery. J. Control. Release 2019, 300, 93–101. [Google Scholar] [CrossRef]
- Martínez Rivas, C.J.; Tarhini, M.; Badri, W.; Miladi, K.; Greige-Gerges, H.; Nazari, Q.A.; Galindo Rodríguez, S.A.; Román, R.Á.; Fessi, H.; Elaissari, A. Nanoprecipitation Process: From Encapsulation to Drug Delivery. Int. J. Pharm. 2017, 532, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Fessi, H.; Puisieux, F.; Devissaguet, J.P.; Ammoury, N.; Benita, S. Nanocapsule Formation by Interfacial Polymer Deposition Following Solvent Displacement. Int. J. Pharm. 1989, 55, R1–R4. [Google Scholar] [CrossRef]
- Echegoyen, Y.; Fabra, M.J.; Castro-Mayorga, J.L.; Cherpinski, A.; Lagaron, J.M. High Throughput Electro-Hydrodynamic Processing in Food Encapsulation and Food Packaging Applications: Viewpoint. Trends Food Sci. Technol. 2017, 60, 71–79. [Google Scholar] [CrossRef]
- Mendes, A.C.; Stephansen, K.; Chronakis, I.S. Electrospinning of Food Proteins and Polysaccharides. Food Hydrocoll. 2017, 68, 53–68. [Google Scholar] [CrossRef]
- Luraghi, A.; Peri, F.; Moroni, L. Electrospinning for Drug Delivery Applications: A Review. J. Control. Release 2021, 334, 463–484. [Google Scholar] [CrossRef]
- Fredenberg, S.; Wahlgren, M.; Reslow, M.; Axelsson, A. The Mechanisms of Drug Release in Poly(Lactic-Co-Glycolic Acid)-Based Drug Delivery Systems—A Review. Int. J. Pharm. 2011, 415, 34–52. [Google Scholar] [CrossRef] [PubMed]
- Boostani, S.; Jafari, S.M. A Comprehensive Review on the Controlled Release of Encapsulated Food Ingredients; Fundamental Concepts to Design and Applications. Trends Food Sci. Technol. 2021, 109, 303–321. [Google Scholar] [CrossRef]
- Mora-Huertas, C.E.; Fessi, H.; Elaissari, A. Polymer-Based Nanocapsules for Drug Delivery. Int. J. Pharm. 2010, 385, 113–142. [Google Scholar] [CrossRef] [PubMed]
- Miladi, K.; Sfar, S.; Fessi, H.; Elaissari, A. Nanoprecipitation Process: From Particle Preparation to In Vivo Applications. In Polymer Nanoparticles for Nanomedicines; Vauthier, C., Ponchel, G., Eds.; Springer International Publishing: Gewerbestrasse, Switzerland, 2016; pp. 17–53. [Google Scholar]
- Spink, C.H. Differential Scanning Calorimetry. Methods Cell Biol. 2008, 84, 115–141. [Google Scholar] [PubMed]
- Kita, K.; Dittrich, C. Drug Delivery Vehicles with Improved Encapsulation Efficiency: Taking Advantage of Specific Drug-Carrier Interactions. Expert Opin. Drug Deliv. 2011, 8, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Eghbal, N.; Choudhary, R. Complex Coacervation: Encapsulation and Controlled Release of Active Agents in Food Systems. LWT 2018, 90, 254–264. [Google Scholar] [CrossRef]
- Sharma, N.; Madan, P.; Lin, S. Effect of Process and Formulation Variables on the Preparation of Parenteral Paclitaxel-Loaded Biodegradable Polymeric Nanoparticles: A Co-Surfactant Study. Asian J. Pharm. Sci. 2016, 11, 404–416. [Google Scholar] [CrossRef] [Green Version]
- Jyothi, N.; Venkata, N.; Jyothi, N.; Prasanna, P.M.; Narayan Sakarkar, S.; Surya Prabha, K.; Ramaiah, P.S.; Srawan, G.Y. Microencapsulation Techniques, Factors Influencing Encapsulation Efficiency. Artic. J. Microencapsul. 2010, 27, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Srikar, G.; Rani, A.P. Study on Influence of Polymer and Surfactant on in Vitro Performance of Biodegradable Aqueous-Core Nanocapsules of Tenofovirdisoproxil Fumarate by Response Surface Methodology. Braz. J. Pharm. Sci. 2019, 55, e18736. [Google Scholar] [CrossRef] [Green Version]
- Shoba, E.; Lakra, R.; Kiran, M.S.; Korrapati, P.S. Design and Development of Papain–Urea Loaded PVA Nanofibers for Wound Debridement. RSC Adv. 2014, 4, 60209–60215. [Google Scholar] [CrossRef]
- Mak, W.C.; Cheung, K.Y.; Trau, D. Diffusion Controlled and Temperature Stable Microcapsule Reaction Compartments for High-Throughput Microcapsule-PCR. Adv. Funct. Mater. 2008, 18, 2930–2937. [Google Scholar] [CrossRef]
- Price, A.D.; Zelikin, A.N.; Wark, K.L.; Caruso, F. A Biomolecular “Ship-in-a-bottle”: Continuous RNA Synthesis Within Hollow Polymer Hydrogel Assemblies. Adv. Mater. 2010, 22, 720–723. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.M.; Yu, H.; Guigas, G.; Fery, A.; Weiss, M.; Patzel, V.; Trau, D. Engineering and Design of Polymeric Shells: Inwards Interweaving Polymers as Multilayer Nanofilm, Immobilization Matrix, or Chromatography Resins. ACS Appl. Mater. Interfaces 2017, 9, 5447–5456. [Google Scholar] [CrossRef]






| Encapsulation Method | Enzyme | Encapsulating Polymers | Main Results | Refs. |
|---|---|---|---|---|
| Liposome entrapment | Superoxide dismutase | Polyacrylamide | Good encapsulation efficiency (37%) and maintenance of enzyme activity. | [144] |
| Emulsion solvent evaporation | α-chymotrypsin and lysozyme | Poly (glycerol adipate-co-o-pentadecalactone) Poly (1,3-propanediol adipate-co-o-pentadecalactone) | Little difference in encapsulation was observed between the different polymers; Changes in polymer chemistry showed greater effects. | [145] |
| Crosslinked enzyme aggregates | Thioesterase, galactosylceramidase, α-glucosidase, and β- glucosidase | Poly (lactide-co-glycolide) | Excellent activity retention (usually around 60%); enzymatic activity is fully recovered in primary fibroblasts upon treatment. | [146] |
| Solid-in-oil-in-water | α-chymotrypsin | Poly (lactic-co-glycolic) acid | Maximum encapsulation efficiency of 61%. | [147] |
| Precipitation-dialysis | α-chymotrypsin | Poly (γ-glutamic acid) | Considerable amounts of α-chymotrypsin were encapsulated (20–25%); the encapsulation contributed to the preservation of enzyme activity over time. | [148] |
| Adsorption | Alcohol dehydrogenase | Polyallylamine Polystyrene sulfonate | The affinity of alcohol dehydrogenase to the substrate was 1.7 times lower than that of the native enzyme. | [149] |
| Polymerization | Glucose oxidase | - | Thermal stability and tolerance to organic solvents were significantly improved. | [150] |
| Homogenization | Naringinase | Sodium alginate or chitosan | The process improved the kinetics and operational stability, so it could be useful as a debittering agent for citrus juice industries. | [151] |
| Electrospinning | Lysozyme | Poly(vinylpyrrolidone) and Eudragit RS100 | High encapsulation efficiency and preservation of enzyme activity were achieved (93.4 ± 7.0% and 96.1 ± 3.3%, respectively). | [19] |
| Fructosyltransferase | Group of biodegradable polymers | Good results have been obtained; however, further research is needed to reduce the leaching of the encapsulated enzyme from electrophilized fibers. | [141] | |
| β-galactosidase | Polyvinylpyrrolidone | 97% of the original activity was maintained; there were no changes in pH and temperature profiles; high storage stability (β-galactosidase activity decreased by only 4% after one year). | [152] | |
| Electrospray | Streptokinase | Poly (lactic-co-glycolic acid) | The method proved to be an interesting approach to encapsulate enzymes; other studies are necessary to ensure the maintenance of enzyme activity after electrospray. | [153] |
| Freeze-drying | Bromelain | Chitosan | The freeze-dried method can effectively improve the stability of bromelain and nanoparticles. | [138] |
| Firefly luciferase | Chitosan and xanthan gum | Enzymatic activities of the encapsulated and the released enzyme were confirmed for over 30 days. | [139] | |
| Nanoprecipitation | Lysozyme and horseradish peroxidase | Poly (lactic acid) Poly (ethylene glycol) | Lysozyme and horseradish peroxidase were shown to retain 99% activity after processing. | [18] |
| Asparaginase | Polyglycerol | Enzymes were encapsulated with an efficacy of 100% and, after release, full enzyme activity and structural integrity were retained. | [140] | |
| Lysozyme and α-chymotrypsin | Poly (lactic-co-glycolic) acid | High encapsulation efficiencies (>70%) and residual activity (>90%). | [154] | |
| Coacervation complex | Lysozyme | Poly (acrylic acid)-block-poly(acrylamide) Poly(N,N-dimethylaminoethyl methacrylate) | The stability of the micelles containing a larger fraction of lysozyme was lower. | [155] |
| Extrusion | α-amylase | Gelatin and shellac | The enzyme showed good stability after encapsulation and can be recycled 10 times. | [132] |
| Thermal gelation | l-phenylalanine ammonia-lyase | Plant hydrocolloids | Good results were obtained; however, new studies are necessary. | [156] |
| Spray drying | β-galactosidase | Chitosan | Encapsulation increased the diffusional effect of the released enzyme and reduced the initial activity of the enzyme. | [157] |
| DNase I | Poly (lactic-co-glycolic) acid | High encapsulation efficiency (>80%); microparticles loaded with DNase I showed high inhalation rates and increased mucolytic activity. | [16] | |
| Double emulsion | Laccase | Eudragitfi L 100-55 | Increased stability of the enzyme at acidic pHs (2.0–5.0). | [158] |
| α-chymotrypsin | Poly (ethylene glycol)-co-poly (glycerol adipate-co-ɷ-pentadecalactone) | Good throughput and encapsulation efficiency; encapsulation kept the bioactivity of α-chymotrypsin and protected it from adverse preparation conditions. | [159] | |
| Layer-by-layer | Catalase | Poly (allylamine hydrochloride) dextran sulfate | Catalase remained active inside the polymer capsules; polymer capsules showed potential to prevent oxidative stress. | [160] |
| l-asparaginase | Poly dextran/poly-l-arginine | Encapsulation improved proteolytic resistance and thermal inactivation of l-asparaginase. | [161] | |
| Ionic gelation | Human phenylalanine hydroxylase | Chitosan | Effective in maintaining protein stability and enzymatic function. | [162] |
| Bromelain | Chitosan | High encapsulation efficiency (85.1%); improved the stability of bromelain. | [138] | |
| Pectinase | Sodium alginate | Pectinase can be used to hydrolyze pectic substances in orange juice; maintenance of enzyme stability activity during recycles. | [163] | |
| Lipase | Sodium alginate and Chitosan | High encapsulation yield (99.8%); improvement of enzyme activity. | [57] |
| Enzyme | Method | Application | Refs. |
|---|---|---|---|
| Lysozyme | Electrospinning | Drug delivery/delivery of biopharmaceuticals to the oral mucosa. | [19] |
| β-galactosidase | Electrospinning | Oral drug delivery. | [152] |
| Fructosyltransferase | Electrospinning | Biocatalysts. | [141] |
| Papain | Electrospinning | Wound debridement. | [190] |
| Phosphatase | Freeze-drying | Reaction engineering. | [17] |
| Bromelain | Freeze-drying/ionic gelation | Wound healing and blood circulation improvement. | [138] |
| l-asparaginase | Ionic gelation | Drug release. | [167] |
| Pectinase | Ionic gelation | Clarifying orange juice. | [163] |
| Flavourzyme | Ionic gelation | Cheese ripening. | [14] |
| Aminopeptidase | Ionic gelation | Food industry: accelerating; cheddar cheese ripening through peptide hydrolysis. | [191] |
| Lysozyme; α-chymotrypsin | Nanoprecipitation | Novel treatments in immunology, oncology, or enzyme therapies. | [154] |
| DNAse 1 | Spray-drying | Delivery of particulates carrying therapeutics to patients with cystic fibrosis. | [16] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da S. Pereira, A.; Souza, C.P.L.; Moraes, L.; Fontes-Sant’Ana, G.C.; Amaral, P.F.F. Polymers as Encapsulating Agents and Delivery Vehicles of Enzymes. Polymers 2021, 13, 4061. https://doi.org/10.3390/polym13234061
da S. Pereira A, Souza CPL, Moraes L, Fontes-Sant’Ana GC, Amaral PFF. Polymers as Encapsulating Agents and Delivery Vehicles of Enzymes. Polymers. 2021; 13(23):4061. https://doi.org/10.3390/polym13234061
Chicago/Turabian Styleda S. Pereira, Adejanildo, Camila P. L. Souza, Lidiane Moraes, Gizele C. Fontes-Sant’Ana, and Priscilla F. F. Amaral. 2021. "Polymers as Encapsulating Agents and Delivery Vehicles of Enzymes" Polymers 13, no. 23: 4061. https://doi.org/10.3390/polym13234061
APA Styleda S. Pereira, A., Souza, C. P. L., Moraes, L., Fontes-Sant’Ana, G. C., & Amaral, P. F. F. (2021). Polymers as Encapsulating Agents and Delivery Vehicles of Enzymes. Polymers, 13(23), 4061. https://doi.org/10.3390/polym13234061