Inner Surface Hydrophilic Modification of PVDF Membrane with Tea Polyphenols/Silica Composite Coating
Abstract
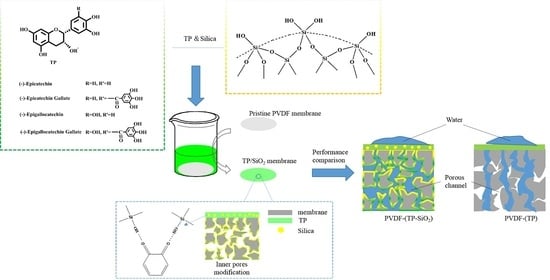
:1. Introduction
2. Experimental
3. Results and Discussion
3.1. Analysis of XPS Spectrum and Morphology
3.2. Hydrophilicity and Separation Performance of Modified PVDF Membrane
3.3. Antifouling and Stability Performance of Modified PVDF Membrane
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bengani-Lutz, P.; Zaf, R.D.; Culfaz-Emecen, P.Z.; Asatekin, A. Extremely fouling resistant zwitterionic copolymer membranes with ~1 nm pore size for treating municipal, oily and textile wastewater streams. J. Membr. Sci. 2017, 543, 184–194. [Google Scholar] [CrossRef]
- Painmanakul, P.; Sastaravet, P.; Lersjintanakarn, S.; Khaodhiar, S. Effect of bubble hydrodynamic and chemical dosage on treatment of oily wastewater by Induced Air Flotation (IAF) process. Chem. Eng. Res. Des. 2010, 88, 693–702. [Google Scholar] [CrossRef]
- Xu, Q.; Huang, Q.-S.; Luo, T.-Y.; Wu, R.-L.; Wei, W.; Ni, B.-J. Coagulation removal and photocatalytic degradation of microplastics in urban waters. Chem. Eng. J. 2021, 416, 129123. [Google Scholar] [CrossRef]
- Wu, L.; Ge, G.; Wan, J. Biodegradation of oil wastewater by free and immobilized Yarrowia lipolytica W29. J. Environ. Sci. 2009, 21, 237–242. [Google Scholar] [CrossRef]
- Yang, T.; Ma, Z.-F.; Yang, Q.-Y. Formation and performance of Kaolin/MnO2 bi-layer composite dynamic membrane for oily wastewater treatment: Effect of solution conditions. Desalination 2011, 270, 50–56. [Google Scholar] [CrossRef]
- Cheryan, M.; Rajagopalan, N. Membrane processing of oily streams: Wastewater treatment and waste reduction. J. Membr. Sci. 1998, 151, 13–28. [Google Scholar] [CrossRef]
- Judd, S.J. The status of industrial and municipal effluent treatment with membrane bioreactor technology. Chem. Eng. J. 2016, 305, 37–45. [Google Scholar] [CrossRef] [Green Version]
- Zuo, J.; Cheng, P.; Chen, X.; Yan, X.; Guo, Y.; Lang, W. Ultrahigh flux of polydopamine-coated PVDF membranes quenched in air via thermally induced phase separation for oil/water emulsion separation. Sep. Purif. Technol. 2018, 192, 348–359. [Google Scholar] [CrossRef]
- Takht Ravanchi, M.; Kaghazchi, T.; Kargari, A. Application of membrane separation processes in petrochemical industry: A review. Desalination 2009, 235, 199–244. [Google Scholar] [CrossRef]
- Peng, Y.; Guo, F.; Wen, Q.; Yang, F.; Guo, Z. A novel polyacrylonitrile membrane with a high flux for emulsified oil/water separation. Sep. Purif. Technol. 2017, 184, 72–78. [Google Scholar] [CrossRef]
- Wan Ikhsan, S.N.; Yusof, N.; Aziz, F.; Misdan, N.; Ismail, A.F.; Lau, W.-J.; Jaafar, J.; Wan Salleh, W.N.; Hayati Hairom, N.H. Efficient separation of oily wastewater using polyethersulfone mixed matrix membrane incorporated with halloysite nanotube-hydrous ferric oxide nanoparticle. Sep. Purif. Technol. 2018, 199, 161–169. [Google Scholar] [CrossRef]
- Ross, A.R.S. Identification of histidine phosphorylations in proteins using mass spectrometry and affinity-based techniques. Methods Enzymol. 2007, 423, 549–572. [Google Scholar]
- Lai, H.; Yu, X.; Liu, M.; Cheng, Z. One-step solution immersion process for the fabrication of low adhesive underwater superoleophobic copper mesh film toward high-flux oil/water separation. Appl. Surf. Sci. 2018, 448, 241–247. [Google Scholar] [CrossRef]
- Anis, S.F.; Hashaikeh, R.; Hilal, N. Microfiltration membrane processes: A review of research trends over the past decade. J. Water Process. Eng. 2019, 32, 100941. [Google Scholar] [CrossRef]
- Mu, Y.; Feng, H.; Wang, S.; Zhang, S.; Luan, J.; Zhang, M.; Yu, Z.; Wang, G. Combined strategy of blending and surface modification as an effective route to prepare antifouling ultrafiltration membranes. J. Colloid Interface Sci. 2021, 589, 1–12. [Google Scholar] [CrossRef]
- Wang, J.; Qiu, M.; He, C. A zwitterionic polymer/PES membrane for enhanced antifouling performance and promoting hemocompatibility. J. Membr. Sci. 2020, 606, 118119. [Google Scholar] [CrossRef]
- Lin, Y.; Wu, H.C.; Yasui, T.; Yoshioka, T.; Matsuyama, H. Development of an HKUST-1 nanofiller-templated poly(ether sulfone) mixed matrix membrane for a highly efficient ultrafiltration process. ACS Appl. Mater. Interfaces 2019, 11, 18782–18796. [Google Scholar] [CrossRef]
- Zhang, G.; Zhou, M.; Xu, Z.; Jiang, C.; Shen, C.; Meng, Q. Guanidyl-functionalized graphene/polysulfone mixed matrix ultrafiltration membrane with superior permselective, antifouling and antibacterial properties for water treatment. J. Colloid Interface Sci. 2019, 540, 295–305. [Google Scholar] [CrossRef]
- Nayak, K.; Tripathi, B.P. Molecularly grafted PVDF membranes with in-air superamphiphilicity and underwater superoleophobicity for oil/water separation. Sep. Purif. Technol. 2021, 259, 118068. [Google Scholar] [CrossRef]
- Hou, S.; Zheng, J.; Zhang, S.; Li, S. Novel amphiphilic PEO-grafted cardo poly(aryl ether sulfone) copolymer: Synthesis, characterization and antifouling performance. Polymer 2015, 77, 48–54. [Google Scholar] [CrossRef]
- Li, S.L.; Shan, X.; Zhao, Y.; Hu, Y. Fabrication of a novel nanofiltration membrane with enhanced performance via interfacial polymerization through the incorporation of a new zwitterionic diamine monomer. ACS Appl. Mater. Interfaces 2019, 11, 42846–42855. [Google Scholar] [CrossRef]
- Zhou, C.; Shi, Y.; Sun, C.; Yu, S.; Liu, M.; Gao, C. Thin-film composite membranes formed by interfacial polymerization with natural material sericin and trimesoyl chloride for nanofiltration. J. Membr. Sci. 2014, 471, 381–391. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Z.; Dai, L.; Zhang, S. Preparation of a highly permeable nanofiltration membrane using a novel acyl chloride monomer with -PO(Cl)2 group. Desalination 2018, 431, 56–65. [Google Scholar] [CrossRef]
- Yuan, B.; Jiang, C.; Li, P.; Sun, H.; Li, P.; Yuan, T.; Sun, H.; Niu, Q.J. Ultrathin polyamide membrane with decreased porosity designed for outstanding water-softening performance and superior antifouling properties. ACS Appl. Mater. Interfaces 2018, 10, 43057–43067. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, J.; Li, C. Low pressure-driven thin film composite membranes for Cr (VI) removal based on nanofibrous mats supported layer-by-layer assembly coatings. Polym. Eng. Sci. 2015, 55, 421–428. [Google Scholar] [CrossRef]
- Arslan, M.; Dönmez, G.; Ergün, A.; Okutan, M.; Albayrak Arı, G.; Deligöz, H. Preparation, characterization, and separation performances of novel surface modified LbL composite membranes from polyelectrolyte blends and MWCNT. Polym. Eng. Sci. 2019, 60, 341–351. [Google Scholar] [CrossRef]
- Fan, W.; Zhang, X.; Li, C. Functional fibrous compositions: Applications and perspectives. Compos. Commun. 2019, 15, 68–75. [Google Scholar] [CrossRef]
- Chen, P.C.; Xu, Z.K. Mineral-coated polymer membranes with superhydrophilicity and underwater superoleophobicity for effective oil/water separation. Sci. Rep. 2013, 3, 2776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, K.; Duan, H.; Chen, G.Y.; Liu, X.; Yang, W.; Wang, D. Cleaning of oil fouling with water enabled by zwitterionic polyelectrolyte coatings: Overcoming the imperative challenge of oil-water separation membranes. ACS Nano 2015, 9, 9188–9198. [Google Scholar] [CrossRef]
- Yang, X.; He, Y.; Zeng, G.; Chen, X.; Shi, H.; Qing, D.; Li, F.; Chen, Q. Bio-inspired method for preparation of multiwall carbon nanotubes decorated superhydrophilic poly(vinylidene fluoride) membrane for oil/water emulsion separation. Chem. Eng. J. 2017, 321, 245–256. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, C.; Chen, J.; Li, X. Biocompatible, functional spheres based on oxidative coupling assembly of green tea polyphenols. J. Am. Chem. Soc. 2013, 135, 4179–4182. [Google Scholar] [CrossRef] [PubMed]
- Reitzer, F.; Allais, M.; Ball, V.; Meyer, F. Polyphenols at interfaces. Adv. Colloid Interface Sci. 2018, 257, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Jiang, B.; Zhang, L.; Huang, Z.; Sun, Y.; Zong, Y.; Zhang, H. Low-pressure electroneutral loose nanofiltration membranes with polyphenol-inspired coatings for effective dye/divalent salt separation. Chem. Eng. J. 2019, 359, 1442–1452. [Google Scholar] [CrossRef]
- Sharma, R.K.; Sharma, S.; Dutta, S.; Zboril, R.; Gawande, M.B. Silica-nanosphere-based organic–inorganic hybrid nanomaterials: Synthesis, functionalization and applications in catalysis. Green Chem. 2015, 17, 3207–3230. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, L.; Deng, F.; Zhao, D.; Zhang, C.; Zhang, C. Hydrophilic modification of PVDF porous membrane via a simple dip-coating method in plant tannin solution. RSC Adv. 2016, 6, 71287–71294. [Google Scholar] [CrossRef]
- Liu, C.; Wu, L.; Zhang, C.; Chen, W.; Luo, S. Surface hydrophilic modification of PVDF membranes by trace amounts of tannin and polyethyleneimine. Appl. Surf. Sci. 2018, 457, 695–704. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, Z.; Weng, J.; Yu, D.; Liang, Y.; Xu, X.; Qiao, Z.; Zhang, G.; Yang, H.; Wu, X. Molecular understanding and design of porous polyurethane hydrogels with ultralow-oil-adhesion for oil-water separation. ACS Appl. Mater. Interfaces 2020, 12, 56530–56540. [Google Scholar] [CrossRef]
- Zuo, C.; Wang, L.; Tong, Y.; Shi, L.; Ding, W.; Li, W. Co-deposition of pyrogallol/polyethyleneimine on polymer membranes for highly efficient treatment of oil-in-water emulsion. Sep. Purif. Technol. 2021, 267, 118660. [Google Scholar] [CrossRef]




| UOCA/(°) | Adhesion Work/(mN/m) | |
|---|---|---|
| M0 | 111 | 18.07 |
| M1 | 153 | 3.12 |
| M2 | 158 | 2.05 |
| M3 | 160 | 1.7 |
| M4 | 159 | 1.87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Q.; Ji, X.; Tian, J.; Jin, X.; Wu, L. Inner Surface Hydrophilic Modification of PVDF Membrane with Tea Polyphenols/Silica Composite Coating. Polymers 2021, 13, 4186. https://doi.org/10.3390/polym13234186
Xu Q, Ji X, Tian J, Jin X, Wu L. Inner Surface Hydrophilic Modification of PVDF Membrane with Tea Polyphenols/Silica Composite Coating. Polymers. 2021; 13(23):4186. https://doi.org/10.3390/polym13234186
Chicago/Turabian StyleXu, Qiang, Xiaoli Ji, Jiaying Tian, Xiaogang Jin, and Lili Wu. 2021. "Inner Surface Hydrophilic Modification of PVDF Membrane with Tea Polyphenols/Silica Composite Coating" Polymers 13, no. 23: 4186. https://doi.org/10.3390/polym13234186
APA StyleXu, Q., Ji, X., Tian, J., Jin, X., & Wu, L. (2021). Inner Surface Hydrophilic Modification of PVDF Membrane with Tea Polyphenols/Silica Composite Coating. Polymers, 13(23), 4186. https://doi.org/10.3390/polym13234186