Microstructure and Lamellae Phase of Raw Natural Rubber via Spontaneous Coagulation Assisted by Sugars
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagent
2.2. Samples Preparation
2.3. Characterization
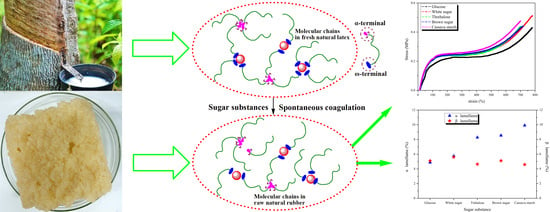
3. Results and Discussion
3.1. Influence of Different Sugars on Terminal Components
3.2. Branching Degree of Natural Rubber Molecules
3.3. Mooney Viscosity
3.4. Rheological Behavior
3.5. Green Strength
3.6. Crystallization Behavior
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salomez, M.; Subileau, M.; Intapun, J.; Bonfils, F.; Sainte-Beuve, J.; Vaysse, L.; Dubreucq, E. Micro-organisms in latex and natural rubber coagula of Hevea brasiliensis and their impact on rubber composition, structure and properties. J. Appl. Microbiol. 2014, 117, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Fatt, M.S.H.; Chen, L.; Al-Quraishi, A.A. Fracture Parameters for Natural Rubber Under Dynamic Loading. Strain 2011, 47, E505–E518. [Google Scholar] [CrossRef]
- Nimpaiboon, A.; Sakdapipanich, J. A model study on effect of glucose on the basic characteristics and physical properties of natural rubber. Polym. Test. 2013, 32, 1408–1416. [Google Scholar] [CrossRef]
- Xu, L.L.; Huang, C.; Luo, M.C.; Qu, W.; Liu, H.; Gu, Z.W.; Jing, L.M.; Huang, G.S.; Zheng, J. A rheological study on non-rubber component networks in natural rubber. RSC Adv. 2015, 5, 91742–91750. [Google Scholar] [CrossRef]
- Zhou, Y.B.; Kosugi, K.; Yamamoto, Y.; Kawahara, S. Effect of non-rubber components on the mechanical properties of natural rubber. Polym. Adv. Technol. 2017, 28, 159–165. [Google Scholar] [CrossRef]
- Luo, Y.Y.; Wang, Y.Q.; Zhong, J.P.; He, C.Z.; Li, Y.Z.; Peng, Z. Interaction Between Fumed-Silica and Epoxidized Natural Rubber. J. Inorg. Organomet. Polym. Mater. 2011, 21, 777–783. [Google Scholar] [CrossRef]
- Amnuaypornsri, S.; Sakdapipanich, J.; Tanaka, Y. Highly purified natural rubber by saponificaion of latex: Analysis of green and cured properties. J. Appl. Polym. Sci. 2010, 118, 3524–3531. [Google Scholar] [CrossRef]
- Jayaraj, S.; Egodage, S.M.; Walpalage, S. New approach for preparation of dry natural rubber nanocomposites through acid-free co-coagulation: Effect of organoclay content. J. Appl. Polym. Sci. 2018, 135. [Google Scholar] [CrossRef]
- Jayachandran, K.; Suresh, P.V.; Chandrasekaran, M. A novel Acinetobacter sp. for treating highly acidic rubber latex centrifugation effluent. Biotechnol. Lett. 1994, 16, 649–654. [Google Scholar] [CrossRef]
- Jayachandran, K.; Chandrasekaran, M. Biological coagulation of skim latex using Acinetobacter sp. isolated from natural rubber latex centrifugation effluent. Biotechnol. Lett. 1998, 20, 161–164. [Google Scholar] [CrossRef]
- Ehabe, E.; Le Roux, Y.; Ngolemasango, F.; Bonfils, F.; Nkeng, G.; Nkouonkam, B.; Sainte-Beuve, J.; Gobina, M.S. Effect of maturation on the bulk viscosity and molecular chain length of cuplump natural rubber. J. Appl. Polym. Sci. 2002, 86, 703–708. [Google Scholar] [CrossRef]
- Kurian, T.; Mathew, N.M. Natural Rubber: Production, Properties and Applications. In Biopolymers; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 403–436. [Google Scholar]
- Salomez, M.; Subileau, M.; Vallaeys, T.; Santoni, S.; Bonfils, F.; Sainte-Beuve, J.; Intapun, J.; Granet, F.; Vaysse, L.; Dubreucq, E. Microbial communities in natural rubber coagula during maturation: Impacts on technological properties of dry natural rubber. J. Appl. Microbiol. 2018, 124, 444–456. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Huang, M.F.; Zeng, Z.Q. Molecular Structures and Mechanical Properties of Microbe Rapid Coagulation Natural Rubber. Chin. J. Struct. Chem. 2011, 30, 1810–1819. [Google Scholar]
- Intapun, J.; Sainte-Beuve, J.; Bonfils, F.; Tanrattanakul, V.; Dubreucq, E.; Vaysse, L. Effect of Microorganisms During the Initial Coagulum Maturation of Hevea Natural Rubber. J. Appl. Polym. Sci. 2010, 118, 1341–1348. [Google Scholar] [CrossRef]
- Nimpaiboon, A.; Sriring, M.; Kumarn, S.; Sakdapipanich, J. Reducing and stabilizing the viscosity of natural rubber by using sugars: Interference of the Maillard reaction between proteins and sugars. J. Appl. Polym. Sci. 2020, 137, 49389. [Google Scholar] [CrossRef]
- Singh, M.; Easa, A.M.; Azahari, B. Effect of Maillard reaction in ammonia preserved natural rubber latex using reducing sugars. J. Rubb. Res. 2020, 23, 365–374. [Google Scholar] [CrossRef]
- Kanmani, P.; Yuvapriya, S. Exopolysaccharide from Bacillus sp. YP03: Its properties and application as a flocculating agent in wastewater treatment. Int. J. Environ. Sci. Technol. 2018, 15, 2551–2560. [Google Scholar] [CrossRef]
- Bellacicco, S.; Prades, A.; Char, C.; Vaysse, L.; Granet, F.; Lacote, R.; Gohet, E.; Flori, A.; Sainte-Beuve, J.; Bonfils, F. The Sugar and Polyol Composition of Hevea Brasiliensis Latex Depends on the Clonal Origin of the Tree. J. Rubber Res. 2018, 21, 224–235. [Google Scholar] [CrossRef]
- Tupý, J.; Resing, W.L. Anaerobic respiration in latex of Hevea brasiliensis substrate and limiting factors. Biol. Plant. 1968, 10, 72. [Google Scholar] [CrossRef]
- Guan, J.; Zhao, F.; Gu, T.; Liu, H.; Luo, M.-C.; Liao, S. Role of endogenous glucose on natural rubber molecular chains and natural network architecture based on biological action and chelation. Polymer 2020, 202, 122752. [Google Scholar] [CrossRef]
- Xie, Z.J.; Sebald, G.; Guyomar, D. Temperature dependence Of the elastocaloric effect in natural rubber. Phys. Lett. A 2017, 381, 2112–2116. [Google Scholar] [CrossRef] [Green Version]
- Kohjiya, S.; Junkong, P.; Ikeda, Y. Crystallization of Natural Rubber: Its unique Feature. KGK Kautsch. Gummi Kunstst. 2017, 70, 38–48. [Google Scholar]
- Kawahara, S.; Takano, K.; Yunyongwattanakorn, J.; Isono, Y.; Hikosaka, M.; Sakdapipanich, J.T.; Tanaka, Y. Crystal Nucleation and Growth of Natural Rubber Purified by Deproteinization and Trans-esterification. Polym. J. 2004, 36, 361–367. [Google Scholar] [CrossRef] [Green Version]
- Chenal, J.M.; Chazeau, L.; Bomal, Y.; Gauthier, C. New insights into the cold crystallization of filled natural rubber. J. Polym. Sci. Part B Polym. Phys. 2007, 45, 955–962. [Google Scholar] [CrossRef]
- Zhang, J.C.; Zhang, T.X.; Dong, M.J.; Liu, G.S.; Dong, Y.Y. Study on degrees of mesomorphic zone of polymer.I.Determination of degrees of crystallinity of Eucommia ulmoides gum and natural rubber by dynamic mechanical thermal analysis. Polym. Test. 2017, 63, 511–520. [Google Scholar] [CrossRef]
- Burfield, D.R.; Tanaka, Y. Cold crystallization of natural rubber and its synthetic analogues: The influence of chain microstructure. Polymer 1987, 28, 907–910. [Google Scholar] [CrossRef]
- Kawahara, S.; Kakubo, T.; Nishiyama, N.; Tanaka, Y.; Isono, Y.; Sakdapipanich, J.T. Crystallization behavior and strength of natural rubber: Skim rubber, deproteinized natural rubber, and pale crepe. J. Appl. Polym. Sci. 2015, 78, 1510–1516. [Google Scholar] [CrossRef]
- Yunyongwattanakorn, J.; Sakdapipanich, J.T.; Kawahara, S.; Hikosaka, M.; Tanaka, Y. Effect of gel on crystallization behavior of natural rubber after accelerated storage hardening test. J. Appl. Polym. Sci. 2007, 106, 455–461. [Google Scholar] [CrossRef]
- Kawahara, S.; Kakubo, T.; Sakdapipanich, J.T.; Isono, Y.; Tanaka, Y. Characterization of fatty acids linked to natural rubber—role of linked fatty acids on crystallization of the rubber. Polymer 2000, 41, 7483–7488. [Google Scholar] [CrossRef]
- Natta, G. Properties of isotactic, atactic, and stereoblock homopolymers, random and block copolymers of α-olefins. J. Polym. Sci. 1959, 34, 531–549. [Google Scholar] [CrossRef]
- Thuong, N.T.; Yamamoto, Y.; Nghia, P.T.; Kawahara, S. Analysis of damage in commercial natural rubber through NMR spectroscopy. Polym. Degrad. Stab. 2016, 123, 155–161. [Google Scholar] [CrossRef] [Green Version]
- Nimpaiboon, A.; Sriring, M.; Sakdapipanich, J.T. Molecular structure and storage hardening of natural rubber: Insight into the reactions between hydroxylamine and phospholipids linked to natural rubber molecule. J. Appl. Polym. Sci. 2016, 133, 43753. [Google Scholar] [CrossRef]
- Chollakup, R.; Suwanruji, P.; Tantatherdtam, R.; Smitthipong, W. New approach on structure-property relationships of stabilized natural rubbers. J. Polym. Res. 2019, 26, 37. [Google Scholar] [CrossRef]
- Tao, R.; Rice, K.D.; Djakeu, A.S.; Mrozek, R.A.; Cole, S.T.; Freeney, R.M.; Forster, A.M. Rheological Characterization of Next-Generation Ballistic Witness Materials for Body Armor Testing. Polymers 2019, 11, 447. [Google Scholar] [CrossRef] [Green Version]
- Shao, H.F.; Yu, Q.H.; Xiao, P.; Yao, W.; Huang, B.C. Study on viscoelastic properties of epoxidized trans-1,4-polyisoprene by rubber process analyzer. Polym. Sci. Ser. A 2012, 54, 760–766. [Google Scholar] [CrossRef]
- Shen, H.W.; Luan, T.; Xie, B.H.; Yang, W.; Yang, M.B. Rheological Behaviors and Molecular Weight Distribution Characteristics of Bimodal High-Density Polyethylene. J. Appl. Polym. Sci. 2011, 121, 1543–1549. [Google Scholar] [CrossRef]
- Shao, H.F.; Wang, S.L.; He, A.H. The influence of molecular weight on high shear rate macroscopic rheological properties of polybutene-1 melts through rubber-processing analyzer. Polym. Bull. 2016, 73, 3209–3220. [Google Scholar] [CrossRef]
- Nun-Anan, P.; Wisunthorn, S.; Pichaiyut, S.; Vennemann, N.; Nakason, C. Novel approach to determine non-rubber content in Hevea brasiliensis: Influence of clone variation on properties of un-vulcanized natural rubber. Ind. Crop. Prod. 2018, 118, 38–47. [Google Scholar] [CrossRef]
- Phillips, P.J.; Edwards, B.C. High-pressure phases in polymers. I. Spherulitic growth kinetics in cis-polyisoprene. J. Polym. Sci. Polym. Phys. Ed. 1975, 13, 1819–1829. [Google Scholar] [CrossRef]
- Wang, C.; Chen, C.-C.; Hung, C.-H.; Lin, K.-S. Lamellar morphologies and crystal stability of syndiotactic polystyrene in α-crystalline form. Polymer 2004, 45, 6681–6689. [Google Scholar] [CrossRef]












| Sugars | Tensile Strength (MPa) | Elongation at Break (%) | 100% Modulus (MPa) | 300% Modulus (MPa) |
|---|---|---|---|---|
| Glucose | 0.43 ± 0.03 | 780.3 ± 33.0 | 0.17 ± 0.01 | 0.23 ± 0.01 |
| White sugar | 0.51 ± 0.08 | 780.6 ± 60.0 | 0.21 ± 0.03 | 0.24 ± 0.03 |
| Trehalose | 0.44 ± 0.08 | 705.0 ± 76.4 | 0.21 ± 0.01 | 0.24 ± 0.00 |
| Brown sugar | 0.44 ± 0.01 | 719.5 ± 9.3 | 0.21 ± 0.01 | 0.25 ± 0.01 |
| Cassava starch | 0.48 ± 0.02 | 699.8 ± 33.6 | 0.22 ± 0.01 | 0.26 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, W.; Guan, J.; Liu, H.; Cheng, S.; Zhao, F.; Liao, S. Microstructure and Lamellae Phase of Raw Natural Rubber via Spontaneous Coagulation Assisted by Sugars. Polymers 2021, 13, 4306. https://doi.org/10.3390/polym13244306
Bai W, Guan J, Liu H, Cheng S, Zhao F, Liao S. Microstructure and Lamellae Phase of Raw Natural Rubber via Spontaneous Coagulation Assisted by Sugars. Polymers. 2021; 13(24):4306. https://doi.org/10.3390/polym13244306
Chicago/Turabian StyleBai, Wanna, Jie Guan, Huan Liu, Shihong Cheng, Fuchun Zhao, and Shuangquan Liao. 2021. "Microstructure and Lamellae Phase of Raw Natural Rubber via Spontaneous Coagulation Assisted by Sugars" Polymers 13, no. 24: 4306. https://doi.org/10.3390/polym13244306
APA StyleBai, W., Guan, J., Liu, H., Cheng, S., Zhao, F., & Liao, S. (2021). Microstructure and Lamellae Phase of Raw Natural Rubber via Spontaneous Coagulation Assisted by Sugars. Polymers, 13(24), 4306. https://doi.org/10.3390/polym13244306