Changes in Crystal Structure and Accelerated Hydrolytic Degradation of Polylactic Acid in High Humidity
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Crystal Structure of Annealed PLA
3.2. Influence of Accelerated Environment on Crystal Structure
3.3. Changes in Tensile Fracture after the Accelerated Test
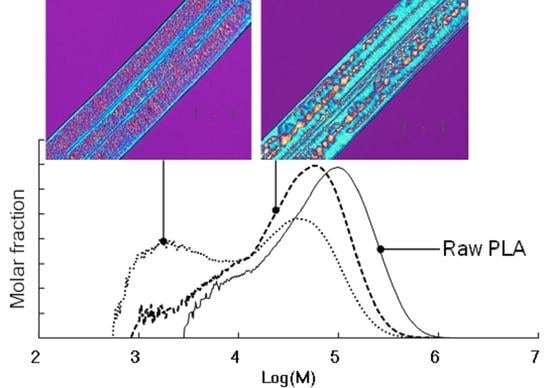
3.4. Changes in Molecular Weight Distribution after Accelerated Test
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garlotta, D. A literature review of poly(lactic acid). J. Polym. Environ. 2001, 9, 63–84. [Google Scholar] [CrossRef]
- Pasquini, N. Polypropylene Handbook, 2nd ed.; Hanser Gardner: Cincinnati, OH, USA, 2005; ISBN 9781569903858. [Google Scholar]
- European Bioplastics BIOPLASTICS MARKET DEVELOPMENT UPDATE 2020. Available online: https://www.european-bioplastics.org/market-update-2020-bioplastics-continue-to-become-mainstream-as-the-global-bioplastics-market-is-set-to-grow-by-36-percent-over-the-next-5-years/ (accessed on 18 October 2021).
- Galiè, F. Global Polypropylene Outlook. Available online: https://www.icis.com/explore/resources/icis-global-petrochemicals-outlook-seminar/ (accessed on 18 October 2021).
- Matsutani, K.; Kimura, Y. Present Situation and Future Perspectives of Poly(lactic acid). In Synthesis, Structure and Properties of Poly(lactic acid); Di Lorenzo, M.L., Androsch, R., Eds.; Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar]
- Choe, S.; Kim, Y.; Won, Y.; Myung, J. Bridging Three Gaps in Biodegradable Plastics: Misconceptions and Truths about Biodegradation. Front. Chem. 2021, 9, 671750. [Google Scholar] [CrossRef]
- Lončarski, M.; Gvoić, V.; Prica, M.; Cveticanin, L.; Agbaba, J.; Tubić, A. Sorption behavior of polycyclic aromatic hydrocarbons on biodegradable polylactic acid and various nondegradable microplastics: Model fitting and mechanism analysis. Sci. Total Environ. 2021, 785, 147289. [Google Scholar] [CrossRef]
- Manfra, L.; Marengo, V.; Libralato, G.; Costantini, M.; De Falco, F.; Cocca, M. Biodegradable polymers: A real opportunity to solve marine plastic pollution? J. Hazard. Mater. 2021, 416, 125763. [Google Scholar] [CrossRef]
- Min, K.; Cuiffi, J.D.; Mathers, R.T. Ranking environmental degradation trends of plastic marine debris based on physical properties and molecular structure. Nat. Commun. 2020, 11, 727. [Google Scholar] [CrossRef]
- MacDonald, R.T.; McCarthy, S.P.; Gross, R.A. Enzymatic degradability of poly(lactide): Effects of chain stereochemistry and material crystallinity. Macromolecules 1996, 29, 7356–7361. [Google Scholar] [CrossRef]
- Tsuji, H.; Ikada, Y. Properties and morphology of poly(L-lactide). II. Hydrolysis in alkaline solution. J. Polym. Sci. Part A Polym. Chem. 1998, 36, 59–66. [Google Scholar] [CrossRef]
- Tsuji, H.; Tsuruno, T. Water vapor permeability of poly(L-lactide)/poly(D-lactide) stereocomplexes. Macromol. Mater. Eng. 2010, 295, 709–715. [Google Scholar] [CrossRef]
- Tsuji, H.; Ikada, Y. Properties and morphology of poly(L-lactide) 4. Effects of structural parameters on long-term hydrolysis of poly(L-lactide) in phosphate-buffered solution. Polym. Degrad. Stab. 2000, 67, 179–189. [Google Scholar] [CrossRef]
- Tsuji, H.; Mizuno, A.; Ikada, Y. Properties and morphology of poly(L-lactide). III. Effects of initial crystallinity on long-term in vitro hydrolysis of high molecular weight poly(L-lactide) film in phosphate-buffered solution. J. Appl. Polym. Sci. 2000, 77, 1452–1464. [Google Scholar] [CrossRef]
- Brown, R.P. Predictive techniques and models for durability tests. Polym. Test. 1995, 14, 403–414. [Google Scholar] [CrossRef]
- Zhang, X.; Espiritu, M.; Bilyk, A.; Kurniawan, L. Morphological behaviour of poly(lactic acid) during hydrolytic degradation. Polym. Degrad. Stab. 2008, 93, 1964–1970. [Google Scholar] [CrossRef]
- Righetti, M.C.; Gazzano, M.; Di Lorenzo, M.L.; Androsch, R. Enthalpy of melting of α′- and α-crystals of poly(L-lactic acid). Eur. Polym. J. 2015, 70, 215–220. [Google Scholar] [CrossRef]
- Henricks, J.; Boyum, M.; Zheng, W. Crystallization kinetics and structure evolution of a polylactic acid during melt and cold crystallization. J. Therm. Anal. Calorim. 2015, 120, 1765–1774. [Google Scholar] [CrossRef]
- Srithep, Y.; Pholharn, D.; Akkaprasa, T. Effect of molecular weight of poly(L-lactic acid) on the stereocomplex formation between enantiomeric poly(lactic acid)s blendings. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Beijing, China, 19–22 August 2019; IOP Publishing: Bristol, UK, 2019; Volume 526, p. 012024. [Google Scholar]
- Srithep, Y.; Nealey, P.; Turng, L.S. Effects of annealing time and temperature on the crystallinity and heat resistance behavior of injection-molded poly(lactic acid). Polym. Eng. Sci. 2013, 53, 580–588. [Google Scholar] [CrossRef]
- Androsch, R.; Schick, C.; Di Lorenzo, M.L. Melting of conformationally disordered crystals (α′ phase) of poly(l -lactic acid). Macromol. Chem. Phys. 2014, 215, 1134–1139. [Google Scholar] [CrossRef]
- Di Lorenzo, M.L.; Androsch, R. Influence of α′-/α-crystal polymorphism on properties of poly(l-lactic acid). Polym. Int. 2019, 68, 320–334. [Google Scholar] [CrossRef]
- Vyavahare, O.; Ng, D.; Hsu, S.L. Analysis of structural rearrangements of poly(lactic acid) in the presence of water. J. Phys. Chem. B 2014, 118, 4185–4193. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, W. Polymer Degradation: Principles and Practical applications; Carl Hanser Verlag: Munich, Germany, 1981; pp. 15–19. ISBN 3446132643. [Google Scholar]









| Annealing | χc * | Tensile Properties | |||
|---|---|---|---|---|---|
| Temperature | Modulus | Strength | Strain at Break | ||
| (°C) | (%) | (MPa) | (MPa) | (%) | |
| STD | - | - | 2730 | 57.9 | >50 |
| A80 | 80 | 1 | 3370 | 69.0 | >50 |
| A90 | 90 | 30 | 3270 | 71.0 | >50 |
| A100 | 100 | 32 | 3520 | 69.6 | 28 |
| A110 | 110 | 29 | 3300 | 68.0 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobayashi, Y.; Ueda, T.; Ishigami, A.; Ito, H. Changes in Crystal Structure and Accelerated Hydrolytic Degradation of Polylactic Acid in High Humidity. Polymers 2021, 13, 4324. https://doi.org/10.3390/polym13244324
Kobayashi Y, Ueda T, Ishigami A, Ito H. Changes in Crystal Structure and Accelerated Hydrolytic Degradation of Polylactic Acid in High Humidity. Polymers. 2021; 13(24):4324. https://doi.org/10.3390/polym13244324
Chicago/Turabian StyleKobayashi, Yutaka, Tsubasa Ueda, Akira Ishigami, and Hiroshi Ito. 2021. "Changes in Crystal Structure and Accelerated Hydrolytic Degradation of Polylactic Acid in High Humidity" Polymers 13, no. 24: 4324. https://doi.org/10.3390/polym13244324
APA StyleKobayashi, Y., Ueda, T., Ishigami, A., & Ito, H. (2021). Changes in Crystal Structure and Accelerated Hydrolytic Degradation of Polylactic Acid in High Humidity. Polymers, 13(24), 4324. https://doi.org/10.3390/polym13244324