Dependence on Film Thickness of Guest-Induced c Perpendicular Orientation in PPO Films
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
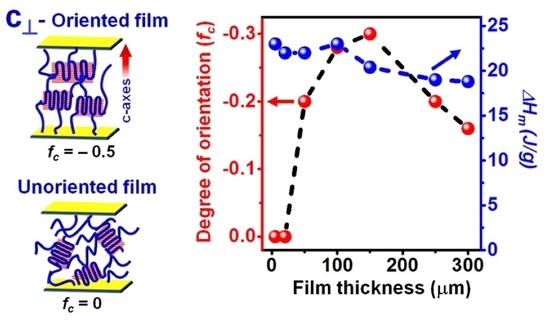
3.1. Dependence of Guest-Induced c⊥ Orientation on Film Thickness
3.2. Kinetics of Be Guest Sorption from Amorphous Films of Different Thickness
3.3. Kinetics of Guest-Induced Film Thickening and Polymer Crystallization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wegner, G. Ultrathin films of polymers: Architecture, characterization and properties. Thin Solid Film. 1992, 216, 105–116. [Google Scholar] [CrossRef]
- Schonherr, H.; Frank, C.W. Ultrathin films of poly(ethylene oxides) on oxidized silicon. 1. Spectroscopic characterization of film structure and crystallization kinetics. Macromolecules 2003, 36, 1188–1198. [Google Scholar] [CrossRef]
- Wang, Y.; Chan, C.M.; Ng, K.M.; Li, L. What Controls the Lamellar Orientation at the Surface of Polymer Films during Crystallization? Macromolecules 2008, 41, 2548–2553. [Google Scholar] [CrossRef]
- Yang, P.; Han, Y. Crystal Growth Transition from Flat-On to Edge-On Induced by Solvent Evaporation in Ultrathin Films of Polystyrene-b-Poly(ethylene oxide). Langmuir 2009, 25, 9960–9968. [Google Scholar] [CrossRef]
- Rueda, D.R.; Hernández, J.J.; García-Gutiérrez, M.C.; Ezquerra, T.A.; Soccio, M.; Lotti, N.; Munari, A.; Perlich, J.; Serna, R. Flat-On Lamellae in Spin-Coated, Stable Films of Poly(propylene azelate). Langmuir 2010, 26, 17540–17545. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.S.; Niskala, J.R.; Unruh, D.A.; Chu, C.K.; Lee, O.P.; Frechet, J.M.J. Control of Polymer-Packing Orientation in Thin Films through Synthetic Tailoring of Backbone Coplanarity. Chem. Mater. 2013, 25, 4088–4096. [Google Scholar] [CrossRef]
- Hu, R.; Pi, Y.; Wang, N.; Zhang, Q.; Feng, J.; Xu, W.; Dong, X.; Wang, D.; Yang, H. The formation of the S-shaped edge-on lamellae on the thin porous polylactic acid membrane via phase separation induced by water microdroplets. J. Appl. Polym. Sci. 2016, 133, 43355. [Google Scholar] [CrossRef]
- Heffelfinger, C.J.; Burton, R.L. X-ray determination of the crystallite orientation distributions of poly(ethylene terephthalate) films. J. Polym. Sci. 1960, 47, 289–306. [Google Scholar] [CrossRef]
- Pazur, R.J.; Purd’homme, R.E. X-ray Pole Figure and Small Angle Scattering Measurements on Tubular Blown Low-Density Poly(ethylene) Films. Macromolecules 1996, 29, 119–128. [Google Scholar] [CrossRef]
- Shibaev, V.; Bobrovsky, A.; Boiko, N. Photoactive liquid crystalline polymer systems with light-controllable structure and optical properties. Progr. Polym. Sci. 2003, 28, 729–836. [Google Scholar] [CrossRef]
- Rao, Y.Q.; Greener, J.; Avila-Orta, C.A.; Hsiao, B.S.; Blanton, T.N. The relationship between microstructure and toughness of biaxially oriented semicrystalline polyester films. Polymer 2008, 49, 2507–2514. [Google Scholar] [CrossRef]
- Jariyasakoolroj, P.; Tashiro, K.; Hai, W.; Yamamoto, H.; Chinsirikul, W.; Kerddonfag, N.; Chirachanchai, S. Isotropically small crystalline lamellae induced by high biaxial-stretching rate as a key microstructure for super-tough polylactide film. Polymer 2015, 68, 234–245. [Google Scholar] [CrossRef]
- Tatsumi, M.; Teramoto, Y.; Nishio, Y. Different orientation patterns of cellulose nanocrystal films prepared from aqueous suspensions by shearing under evaporation. Cellulose 2015, 22, 2983–2992. [Google Scholar] [CrossRef]
- Mueller, C.J.; Gann, E.; Singh, C.R.; Thelakkat, M.; McNeill, C.R. Control of Molecular Orientation in Polydiketopyrrolopyrrole Copolymers via Diffusive Noncovalent Interactions. Chem. Mater. 2016, 28, 7088–7097. [Google Scholar] [CrossRef]
- Rizzo, P.; Lamberti, M.; Albunia, A.R.; Ruiz de Ballesteros, O.; Guerra, G. Crystalline orientation in syndiotactic polystyrene cast films. Macromolecules 2002, 35, 5854–5860. [Google Scholar] [CrossRef]
- Rizzo, P.; Spatola, A.; De Girolamo Del Mauro, A.; Guerra, G. Polymeric Films with Three Different Uniplanar Crystalline Phase Orientations. Macromolecules 2005, 38, 10089–10094. [Google Scholar] [CrossRef]
- Albunia, A.R.; Rizzo, P.; Guerra, G. Polymeric Films with Three Different Orientations of Crystalline-Phase Empty Channels. Chem. Mater. 2009, 21, 3370–3375. [Google Scholar] [CrossRef]
- Rizzo, P.; Ianniello, G.; Venditto, V.; Tarallo, O.; Guerra, G. Poly(L-lactic acid): Uniplanar Orientation in Cocrystalline Films and Structure of the Cocrystalline Form with Cyclopentanone. Macromolecules 2015, 48, 7513–7520. [Google Scholar] [CrossRef]
- Nagendra, B.; Rizzo, P.; Daniel, C.; Baldino, L.; Guerra, G. c-Perpendicular Orientation of Poly(L-lactide) Films. Polymers 2021, 13, 1572. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, P.; Ianniello, G.; Longo, S.; Guerra, G. Uniplanar Orientations and Guest Exchange in PPO Cocrystalline Films. Macromocules 2013, 46, 3995–4001. [Google Scholar] [CrossRef]
- Rizzo, P.; Gallo, C.; Vitale, V.; Tarallo, O.; Guerra, G. Nanoporous-crystalline films of PPO with parallel and perpendicular polymer chain orientations. Polymer 2019, 167, 193–201. [Google Scholar] [CrossRef]
- Venditto, V.; Milano, G.; De Girolamo Del Mauro, A.; Guerra, G.; Mochizuki, J.; Itagaki, H. Orientation and Microenvironment of Naphthalene Guest in the Host Nanoporous Phase of Syndiotactic Polystyrene. Macromolecules 2005, 38, 3696–3702. [Google Scholar] [CrossRef]
- Uda, Y.; Kaneko, F.; Tanigaki, N.; Kawaguchi, T. The First Example of a Polymer-Crystal–Organic-Dye Composite Material: The Clathrate Phase of Syndiotactic Polystyrene with Azulene. Adv. Mater. 2005, 17, 1846–1850. [Google Scholar] [CrossRef]
- Itagaki, H.; Sago, T.; Uematsu, M.; Yoshioka, G.; Correa, A.; Venditto, V.; Guerra, G. Guest Orientation in Uniplanar-Axial Polymer Host Films and in Co-Crystal Unit-Cell, Determined by Angular Distributions of Polarized Guest Fluorescence. Macromolecules 2008, 41, 9156–9164. [Google Scholar] [CrossRef]
- Sano, T.; Uchiyama, A.; Sago, T.; Itagaki, H. Fluorescence behavior of syndiotactic polystyrene and its derivative: Formation of a ground-state dimer in the solid state. Eur. Polym. 2017, 90, 114–121. [Google Scholar] [CrossRef]
- Kaneko, F.; Uda, Y.; Kajiwara, A.; Tanigaki, N. Molecular-Complex Formation of Syndiotactic Polystyrene with Stable Radical Molecules. Macromol. Rapid Commun. 2006, 27, 1643–1647. [Google Scholar] [CrossRef]
- Albunia, A.R.; D’Aniello, C.; Guerra, G.; Gatteschi, D.; Mannini, M.; Sorace, L. Ordering Magnetic Molecules within Nanoporous Crystalline Polymers. Chem. Mater. 2009, 21, 4750–4752. [Google Scholar] [CrossRef]
- Daniel, C.; Rufolo, C.; Bobba, F.; Scarfato, A.; Cucolo, A.; Guerra, G. Ferroelectric co-crystalline polymers. J. Mater. Chem. 2011, 21, 19074–19079. [Google Scholar] [CrossRef]
- Stegmaier, P.; De Girolamo Del Mauro, A.; Venditto, V.; Guerra, G. Optical recording materials based on photoisomerization of guest molecules of a polymeric crystalline host phase. Adv. Mater. 2005, 17, 1166–1168. [Google Scholar] [CrossRef]
- Rizzo, P.; Abbate, S.; Longhi, G.; Guerra, G. Circularly polarized luminescence of syndiotactic polystyrene. Opt. Mater. 2017, 73, 595–601. [Google Scholar] [CrossRef]
- De Rosa, C.; Guerra, G.; Petraccone, V.; Pirozzi, B. Crystal structure of the emptied clathrate form (*e form) of syndiotactic polystyrene. Macromolecules 1997, 30, 4147–4152. [Google Scholar] [CrossRef]
- Bhoje Gowd, E.; Shibayama, N.; Tashiro, K. Structural Changes in Thermally Induced Phase Transitions of Uniaxially Oriented δe Form of Syndiotactic Polystyrene Investigated by Temperature-Dependent Measurements of X-ray Fiber Diagrams and Polarized Infrared Spectra. Macromolecules 2006, 39, 8412–8418. [Google Scholar] [CrossRef]
- Petraccone, V.; Ruiz de Ballesteros, O.; Tarallo, O.; Rizzo, P.; Guerra, G. Nanoporous Polymer Crystals with Cavities and Channels. Chem. Mater. 2008, 20, 3663–3668. [Google Scholar] [CrossRef]
- Itagaki, H.; Sano, T.; Okabe, T.; Sano, S.; Ebihara, H.; Tomono, F.; Dohra, H. Polymerization of Aniline in Tubular Cavities of the Crystalline Phase of Syndiotactic Polystyrene: Proposal of a Preparation Method of Sophisticated Polymer Composites. ACS Macro Lett. 2017, 6, 1099–1103. [Google Scholar] [CrossRef]
- Daniel, C.; Longo, S.; Fasano, G.; Vitillo, J.G.; Guerra, G. Nanoporous Crystalline Phases of Poly(2,6-Dimethyl-1,4-phenylene)oxide. Chem. Mater. 2011, 23, 3195–3200. [Google Scholar] [CrossRef]
- Nagendra, B.; Cozzolino, A.; Daniel, C.; Rizzo, P.; Guerra, G.; Auriemma, F.; De Rosa, C.; D’Alterio, M.C.; Tarallo, O.; Nuzzo, A. Two nanoporous-crystalline forms of PPO and related co-crystalline forms. Macromolecules 2019, 52, 9646–9656. [Google Scholar] [CrossRef]
- Yu, A.; Alentiev, I.S.; Levin, M.I.; Buzin, N.A.; Belov, R.Y.; Nikiforov, S.V.; Chirkov, I.V.; Blagodatskikh, A.S.; Kechekyan, P.A.; Kechekyan, V.G.; et al. Gas transport parameters, density and free volume of nanocrystalline poly-2,6-dimethylphenylene oxide. Polymer 2021, 226, 123804. [Google Scholar] [CrossRef]
- Venditto, V.; De Girolamo Del Mauro, A.; Mensitieri, G.; Milano, G.; Musto, P.; Rizzo, P.; Guerra, G. Anisotropic Guest Diffusion in the δ Crystalline Host Phase of Syndiotactic Polystyrene: Transport Kinetics in Films with Three Different Uniplanar Orientations of the Host Phase. Chem. Mater. 2006, 18, 2205–2210. [Google Scholar] [CrossRef]
- Albunia, A.R.; Rizzo, P.; Guerra, G. Control of guest transport in polymer films by structure and orientation of nanoporous-crystalline phases. Polymers 2013, 54, 1671–1678. [Google Scholar] [CrossRef]
- Daniel, C.; Rizzo, P.; Nagendra, B.; Cozzolino, A.; Guerra, G. High guest diffusivity of PPO films with perpendicular orientation of chain axes of nanoporous-crystalline phases. Polymer 2021, 229, 124005. [Google Scholar] [CrossRef]
- Cozzolino, A.; Nagendra, B.; Rizzo, P.; Daniel, C.; Guerra, G. Fast uptake of organic pollutants from dilute aqueous solutions by nanoporous-crystalline PPO films with c-perpendicular orientation. J. Eur. Polym. 2021, 161, 110864. [Google Scholar] [CrossRef]
- Alentiev, A.; Drioli, E.; Gokzhaev, M.; Golemme, G.; Ilinich, O.; Lapkin, A.; Volkov, V.; Yampolskii, Y. Gas permeation properties of phenylene oxide polymers. J. Membr. Sci. 1998, 138, 99–107. [Google Scholar] [CrossRef]
- Chowdhury, G.; Vujosevic, R.; Matsuura, T.; Laverty, B. Effects of polymer molecular weight and chemical modification on the gas transport properties of poly(2,6-dimethyl-1,4-phenylene oxide). J. Appl. Polym. Sci. 2000, 77, 1137–1143. [Google Scholar] [CrossRef]
- Tsujita, Y. Gas sorption and permeation of glassy polymers with microvoids. Prog. Polym. Sci. 2003, 28, 1377–1401. [Google Scholar] [CrossRef]
- Huang, Y.; Paul, D.R. Physical aging of thin glassy polymer films monitored by gas permeability. Polymer 2004, 45, 8377–8393. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, X.; Paul, D.R. Physical aging of thin glassy polymer films: Free volume interpretation. J. Membr. Sci. 2006, 277, 219–229. [Google Scholar] [CrossRef]
- Horn, N.R.; Paul, D.R. Carbon Dioxide Sorption and Plasticization of Thin Glassy Polymer Films Tracked by Optical Methods. Macromolecules 2012, 45, 2820–2834. [Google Scholar] [CrossRef]
- Minelli, M.; De Angelis, M.G.; Sarti, G.C. Predictive calculations of gas solubility and permeability in glassy polymeric membranes: An overview. Front. Chem. Sci. Eng. 2017, 11, 405–413. [Google Scholar] [CrossRef]
- Soniat, M.; Tesfaye, M.; Mafi, A.; Brooks, D.J.; Humphrey, N.D.; Weng, L.-C.; Merinov, B.; Goddard, W.A., III; Weber, A.Z.; Houle, F.A. Permeation of CO2 and N2 through glassy poly(dimethyl phenylene) oxide under steady- and presteady-state conditions. J. Polym. Sci. 2020, 58, 1207–1228. [Google Scholar] [CrossRef] [Green Version]
- Nagendra, B.; Rizzo, P.; Daniel, C.; Guerra, G. Planar Orientation and Transparency of Nanoporous-Crystalline Polymer Films. Macromolecules 2021, 54, 6605–6611. [Google Scholar] [CrossRef]
- Nagendra, B.; Golla, M.; Gallo, C.; Daniel, C.; Rizzo, P.; Guerra, G.; Baldino, L.; Reverchon, E. Mechanisms Determining Different Planar Orientations in PPO Films Crystallized by Guest Sorption. Polymer 2021, 235, 124242. [Google Scholar] [CrossRef]
- Musto, P.; Loianno, V.; Scherillo, G.; La Manna, P.; Galizia, M.; Guerra, G.; Mensitieri, G. Benzene-Induced Crystallization of PPO: A Combined Thermodynamic and Vibrational Spectroscopy Study. Ind. Eng. Chem. Res. 2020, 59, 5402–5411. [Google Scholar] [CrossRef]
- Daniel, C.; Zhovner, D.; Guerra, G. Thermal Stability of Nanoporous Crystalline and Amorphous Phases of Poly(2,6-dimethyl-1,4-phenylene) Oxide. Macromolecules 2013, 46, 449–454. [Google Scholar] [CrossRef]
- Nagendra, B.; Golla, M.; Daniel, C.; Rizzo, P.; Guerra, G. Melting of nanoporous-crystalline and co-crystalline solution cast films of poly(2,6-dimethyl-1,4-phenylene) oxide. Polymer 2021, 228, 123935. [Google Scholar] [CrossRef]
- Karasz, F.E.; Bair, H.E.; O’Reilly, J.M. Thermodynamic properties of poly(2,6-dimethyl-1,4-phenylene ether). J. Polym. Sci. Polym. Phys. Ed. 1968, 6, 1141–1148. [Google Scholar] [CrossRef]
- Turska, E.; Benecki, W. Studies of liquid-induced crystallization of bisphenol A polycarbonate. J. Appl. Polym. Sci. 1979, 23, 3489–3500. [Google Scholar] [CrossRef]
- Durning, C.J.; Russel, W.B. A mathematical model for diffusion with induced crystallization: 2. Polymer 1985, 26, 131–140. [Google Scholar] [CrossRef]
- Waywood, W.J.; Durning, C.J. Solvent-induced crystallization of a compatible polymer blend. Polym. Eng. Sci. 1987, 27, 1265–1274. [Google Scholar] [CrossRef]
- Ouyang, H.; Lee, W.-H.; Shiue, S.-T.; Lin, T.-L. Solvent-induced crystallization in poly(ethylene terephthalate) during mass transport. J. Polym. Sci. Part B Polym. Phys. 2002, 40, 1444–1453. [Google Scholar] [CrossRef]
- Tashiro, K.; Yoshioka, A. Molecular mechanism of solvent-induced crystallization of syndiotactic polystyrene glass. 2. Detection of enhanced motion of the amorphous chains in the induction period of crystallization. Macromolecules 2002, 35, 410–414. [Google Scholar] [CrossRef]
- Gupper, A.; Chan, K.L.A.; Kazarian, S.G. FT-IR imaging of solvent-indiced crystallization in polymers. Macromolecules 2004, 37, 6498–6503. [Google Scholar] [CrossRef]







| Sorption Time (min) | BE Guest Sorption (At/At∞) | Crystallinity Index WAXD (%) (±2) | Melting Enthalpy (ΔHm) in J/g (±0.5) | Film Thickness (μm) (±4) |
|---|---|---|---|---|
| 0 | 0 | 0 | 0 | 100 |
| 5 | 0.08 | 0 | 1.5 | 100 |
| 20 | 0.11 | 3.5 | 2.3 | 100 |
| 40 | 0.15 | 13 | 4.5 | 100 |
| 80 | 0.20 | 15 | 7.0 | 110 |
| 100 | 0.45 | 20 | 11.5 | 120 |
| 110 | 0.78 | 29 | 12.0 | 130 |
| 120 | 0.85 | 25 | 12.7 | 155 |
| 130 | 0.91 | 28 | 13.3 | 165 |
| 140 | 0.93 | 38 | 15.5 | 180 |
| 160 | 0.94 | 44 | 16.6 | 190 |
| 240 | 0.95 | 46.6 | 17.8 | 200 |
| 320 | 1 | 48 | 20.3 | 200 |
| 840 | 0.94 | 44 | 21.6 | 210 |
| 2800 | 0.95 | 46 | 22.0 | 210 |
| annealed at 40 °C | - | 52 | 23.4 | 210 |
| annealed at 60 °C | - | 60 | 27.1 | 210 |
| Guest Sorption Time (min) | BE Guest Sorption (At/At∞) | Crystallinity Index WAXD (%) (±2) | Melting Enthalpy (ΔHm) in J/g (±0.5) | Film Thickness (μm) (±4) |
|---|---|---|---|---|
| 0 | 0 | 0 | 0 | 150 |
| 5 | 0.01 | 0 | 0.2 | 150 |
| 20 | 0.10 | 4.2 | 1.02 | 150 |
| 40 | 0.12 | 11.6 | 3.5 | 155 |
| 80 | 0.22 | 19.4 | 6.9 | 155 |
| 100 | 0.30 | 21.9 | 9.1 | 165 |
| 140 | 0.42 | 24.3 | 11.1 | 180 |
| 160 | 0.48 | 27.4 | 12.6 | 200 |
| 200 | 0.58 | 31.1 | 12.8 | 220 |
| 220 | 0.80 | 36.9 | 14.5 | 250 |
| 240 | 0.86 | 39.6 | 15.6 | 270 |
| 300 | 0.92 | 43.5 | 16 | 300 |
| 640 | 1.0 | 49.8 | 17.3 | 315 |
| 840 | 0.83 | 51 | 18.4 | 325 |
| 1280 | 0.73 | 49.2 | 20 | 345 |
| 2800 | 0.73 | 50.3 | 20.4 | 330 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagendra, B.; Vignola, E.; Daniel, C.; Rizzo, P.; Guerra, G. Dependence on Film Thickness of Guest-Induced c Perpendicular Orientation in PPO Films. Polymers 2021, 13, 4384. https://doi.org/10.3390/polym13244384
Nagendra B, Vignola E, Daniel C, Rizzo P, Guerra G. Dependence on Film Thickness of Guest-Induced c Perpendicular Orientation in PPO Films. Polymers. 2021; 13(24):4384. https://doi.org/10.3390/polym13244384
Chicago/Turabian StyleNagendra, Baku, Emanuele Vignola, Christophe Daniel, Paola Rizzo, and Gaetano Guerra. 2021. "Dependence on Film Thickness of Guest-Induced c Perpendicular Orientation in PPO Films" Polymers 13, no. 24: 4384. https://doi.org/10.3390/polym13244384
APA StyleNagendra, B., Vignola, E., Daniel, C., Rizzo, P., & Guerra, G. (2021). Dependence on Film Thickness of Guest-Induced c Perpendicular Orientation in PPO Films. Polymers, 13(24), 4384. https://doi.org/10.3390/polym13244384