Suppression of Smoldering of Calcium Alginate Flame-Retardant Paper by Flame-Retardant Polyamide-66
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of FR-PA/Ca-Alg Papers
2.3. Characterization
2.3.1. Physical Properties of the Prepared Paper
2.3.2. Limiting Oxygen Index (LOI) Test
2.3.3. Vertical Flammability (VF) Test
2.3.4. Scanning Electron Microscopy (SEM) Analysis
2.3.5. Raman Spectroscopy Analysis
2.3.6. Cone Calorimetry (CONE) Analysis
2.3.7. Thermogravimetric (TG) Analysis
2.3.8. Thermogravimetric Analysis Coupled with Fourier Transformed Infrared Analysis (TG-FTIR) in N2
3. Results and Discussion
3.1. Physical Properties of the Prepared Paper
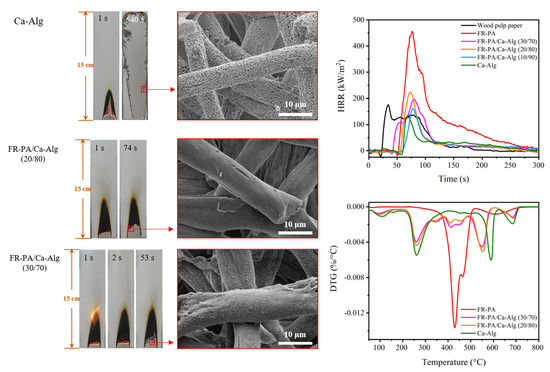
3.2. Vertical Flammability (VF) and Limiting Oxygen Index (LOI) Analysis
3.3. SEM Analysis
3.4. CONE Analysis
3.5. TG Analysis in N2 and Air
3.6. Infrared Spectrum Analysis of Gaseous Volatiles Acquired from TG Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, F.; Zhong, L.; Xu, Y.; Feng, S.; Zhang, C.; Zhang, F.; Zhang, G. Highly efficient flame-retardant kraft paper. J. Mater. Sci. 2018, 54, 1884–1897. [Google Scholar] [CrossRef]
- Choi, Y.; Joe, I.; Lee, S.E.; Oh, K.H. An experimental study on the ignition and emissions characteristics of wallpapers. J. Mech. Sci. Technol. 2009, 23, 2839–2847. [Google Scholar] [CrossRef]
- Li, W.; Zhao, L.; Zhang, Q.; Zhao, G. Flame retardance and antibacterial performance of wooden wallpaper treated with compasite modifying agent. Wood Res. 2016, 61, 423–432. [Google Scholar]
- Zhang, T.; Wu, M.; Kuga, S.; Ewulonu, C.M.; Huang, Y. Cellulose Nanofibril-Based Flame Retardant and Its Application to Paper. ACS Sustain. Chem. Eng. 2020, 8, 10222–10229. [Google Scholar] [CrossRef]
- Wang, N.; Liu, Y.; Liu, Y.; Wang, Q. Properties and mechanisms of different guanidine flame retardant wood pulp paper. J. Anal. Appl. Pyrolysis 2017, 128, 224–231. [Google Scholar] [CrossRef]
- Si, Y.; Guo, Z. Eco-friendly functionalized superhydrophobic recycled paper with enhanced flame-retardancy. J. Colloid Interface Sci. 2016, 477, 74–82. [Google Scholar] [CrossRef]
- Sha, L.; Chen, K. Surface modification of ammonium polyphosphate-diatomaceous earth composite filler and its application in flame-retardant paper. J. Therm. Anal. Calorim. 2015, 123, 339–347. [Google Scholar] [CrossRef]
- Wang, S.; Huang, J.; Chen, F. Study on Mg–Al hydrotalcites in flame-retardant paper preparation. BioResources 2012, 7, 997–1007. [Google Scholar]
- Zhou, H.; Liu, Z.; Wei, Y. Preparation of fire-retardant paper using magnesium hydroxide as flame retardant. China Pulp Pap. 2009, 28, 13–16. [Google Scholar]
- Cui, J.; Guo, H.; Yang, B.; Zhou, Y. Investigation and preparation of sizing agent with waterborne epoxy bromine carbon resin for flame retardant paper. J. Lanzhou Univ. Technol. 2010, 36, 62–65. [Google Scholar]
- Qiu, X.; Li, Z.; Li, X.; Zhang, Z. Flame retardant coatings prepared using layer by layer assembly: A review. Chem. Eng. J. 2018, 334, 108–122. [Google Scholar] [CrossRef]
- Yasin, S.; Curti, M.; Behary, N.; Perwuelz, A.; Giraud, S.; Rovero, G.; Guan, J.; Chen, G. Process Optimization of Eco-Friendly Flame Retardant Finish for Cotton Fabric: A Response Surface Methodology Approach. Surf. Rev. Lett. 2017, 24, 1750114. [Google Scholar] [CrossRef]
- Mao, H.; Wu, X.; Qian, X.; An, X. Conductivity and flame retardancy of polyaniline-deposited functional cellulosic paper doped with organic sulfonic acids. Cellulose 2013, 21, 697–704. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, L.; Zhao, H. Recyclable flame retardant paper made from layer-by-layer assembly of zinc coordinated multi-layered coatings. Cellulose 2018, 25, 5309–5321. [Google Scholar] [CrossRef]
- Yasin, S.; Behary, N.; Curti, M.; Rovero, G. Global Consumption of Flame Retardants and Related Environmental Concerns: A Study on Possible Mechanical Recycling of Flame Retardant Textiles. Fibers 2016, 4, 16. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Ji, Q.; Shen, X.; Xia, Y.; Tan, L.; Kong, Q. Pyrolysis products and thermal degradation mechanism of intrinsically flame-retardant calcium alginate fibre. Polym. Degrad. Stabil. 2011, 96, 936–942. [Google Scholar] [CrossRef]
- Zhang, J.; Ji, Q.; Wang, F.; Tan, L.; Xia, Y. Effects of divalent metal ions on the flame retardancy and pyrolysis products of alginate fibres. Polym. Degrad. Stabil. 2012, 97, 1034–1040. [Google Scholar] [CrossRef]
- Shi, R.; Tan, L.; Zong, L.; Ji, Q.; Li, X.; Zhang, K.; Cheng, L.; Xia, Y. Influence of Na(+) and Ca(2+) on flame retardancy, thermal degradation, and pyrolysis behavior of cellulose fibers. Carbohydr. Polym. 2017, 157, 1594–1603. [Google Scholar] [CrossRef]
- Kong, Q.; Wang, B.; Ji, Q.; Xia, Y.; Guo, Z.; Yu, J. Thermal degradation and flame retardancy of calcuim alginate fibers. Chin. J. Polym. Sci. 2009, 6, 807–812. [Google Scholar] [CrossRef]
- Zhang, X.; Xia, Y.; Shi, M.; Yan, X. The flame retardancy of alginate/flame retardant viscose fibers investigated by vertical burning test and cone calorimeter. Chin. Chem. Lett. 2018, 29, 489–492. [Google Scholar] [CrossRef]
- Stockmann, P.N.; van Opdenbosch, D.; Poethig, A.; Pastoetter, D.L.; Hoehenberger, M.; Lessig, S.; Raab, J.; Woelbing, M.; Falcke, C.; Winnacker, M.; et al. Biobased chiral semi-crystalline or amorphous high-performance polyamides and their scalable stereoselective synthesis. Nat. Commun. 2020, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, G.; Liu, W.; Li, J.; Zhang, S. The anti-dripping intumescent flame retardant finishing for nylon-6,6 fabric. Polym. Degrad. Stabil. 2009, 94, 996–1000. [Google Scholar] [CrossRef]
- Weil, E.D.; Levchik, S. Current Practice and Recent Commercial Developments in Flame Retardancy of Polyamides. J. Fire Sci. 2004, 22, 251–264. [Google Scholar] [CrossRef]
- Horrocks, A.R.; Kandola, B.K.; Davies, P.J.; Zhang, S.; Padbury, S.A. Developments in flame retardant textiles—A review. Polym. Degrad. Stabil. 2005, 88, 3–12. [Google Scholar] [CrossRef]
- Horrocks, A.R.; Zhang, S. Char Formation in Polyamides (Nylons 6 and 6.6) and Wool Keratin Phosphorylated by Polyol Phosphoryl Chlorides. Text. Res. J. 2004, 74, 433–441. [Google Scholar] [CrossRef]
- Stoddard, J.W.; Pickett, O.A.; Cicero, C.J. Flame-retarded nylon carpets. Text. Res. J. 1975, 45, 474–483. [Google Scholar] [CrossRef]
- Parker, E.M.; Gielen, A.C.; McDonald, E.M.; Shields, W.C.; Trump, A.R.; Koon, K.M.; Jones, V. Fire and scald burn risks in urban communities: Who is at risk and what do they believe about home safety? Health Educ. Res. 2013, 28, 599–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Tao, Y.; Wang, B.; Li, P.; Xu, Y.; Jiang, Z.; Dong, C.; Zhu, P. Fully bio-based fire-safety viscose/alginate blended nonwoven fabrics: Thermal degradation behavior, flammability, and smoke suppression. Cellulose 2020, 27, 6037–6053. [Google Scholar] [CrossRef]
- Zhang, X.; Xia, Y.; Yan, X.; Shi, M. Efficient suppression of flammability in flame retardant viscose fiber through incorporating with alginate fiber. Mater. Lett. 2018, 215, 106–109. [Google Scholar] [CrossRef]
- Wang, B.; Li, P.; Xu, Y.; Jiang, Z.; Dong, C.; Liu, Y.; Zhu, P. Bio-based, nontoxic and flame-retardant cotton/alginate blended fibres as filling materials: Thermal degradation properties, flammability and flame-retardant mechanism. Compos. Part B Eng. 2020, 194, 108038. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, M. Flame retardant vinylon/poly(m-phenylene isophthalamide) blended fibers with synergistic flame retardancy for advanced fireproof textiles. J. Hazard. Mater. 2019, 365, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, B.; Xu, Y.; Li, P.; Liu, Y.; Zhu, P. Convenient blending of alginate fibers with polyamide fibers for flame-retardant non-woven fabrics. Cellulose 2020, 27, 8341–8349. [Google Scholar] [CrossRef]
- Fukatsu, K. Thermal degradation behaviour of aromatic polyamide fiber blended with cotton fiber. Polym. Degrad. Stabil. 2002, 75, 479–484. [Google Scholar] [CrossRef]
- Sehaqui, H.; Liu, A.; Zhou, Q.; Berglund, L.A. Fast Preparation Procedure for Large, Flat Cellulose and Cellulose/Inorganic Nanopaper Structures. Biomacromolecules 2010, 11, 2195–2198. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Li, Z.; Shi, R.; Quan, F.; Wang, B.; Ma, X.; Ji, Q.; Tian, X.; Xia, Y. Preparation and Properties of an Alginate-Based Fiber Separator for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 38175–38182. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Liu, C.; Duan, Z.; Yu, B.; Liu, M.; Song, P. Interface engineering of MXene towards super-tough and strong polymer nanocomposites with high ductility and excellent fire safety. Chem. Eng. J. 2020, 399, 125829. [Google Scholar] [CrossRef]
- Dufresne, W.J.B.; Rufledt, C.J.; Marshall, C.P. Raman spectroscopy of the eight natural carbonate minerals of calcite structure. J. Raman Spectrosc. 2018, 49, 1999–2007. [Google Scholar] [CrossRef]
- Maruyama, K.; Kagi, H.; Komatsu, K.; Yoshino, T.; Nakano, S. Pressure-induced phase transitions of vaterite, a metastable phase of CaCO3. J. Raman Spectrosc. 2017, 48, 1449–1453. [Google Scholar] [CrossRef]
- Keshavarzian, A.; Haghighi, M.N.; Taromi, F.A.; Abedini, H. Phosphorus-based flame retardant poly (butylene terephthalate): Synthesis, flame retardancy and thermal behavior. Polym. Degrad. Stabil. 2020, 180, 109310. [Google Scholar] [CrossRef]
- Luo, H.; Rao, W.; Zhao, P.; Wang, L.; Liu, Y.; Yu, C. An efficient organic/inorganic phosphorus–nitrogen–silicon flame retardant towards low-flammability epoxy resin. Polym. Degrad. Stabil. 2020, 178, 109195. [Google Scholar] [CrossRef]
- Cui, M.; Li, J.; Qin, D.; Sun, J.; Chen, Y.; Xiang, J.; Yan, J.; Fan, H. Intumescent flame retardant behavior of triazine group and ammonium polyphosphate in waterborne polyurethane. Polym. Degrad. Stabil. 2021, 183, 109439. [Google Scholar] [CrossRef]
- Yu, B.; Xing, W.; Guo, W.; Qiu, S.; Wang, X.; Lo, S.; Hu, Y. Thermal exfoliation of hexagonal boron nitride for effective enhancements on thermal stability, flame retardancy and smoke suppression of epoxy resin nanocomposites via sol–gel process. J. Mater. Chem. A 2016, 4, 7330–7340. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, T.; He, S.; Xiao, H.; Dai, H.; Yuan, B.; Chen, X.; Yang, X. Flame-retardant polyvinyl alcohol/cellulose nanofibers hybrid carbon aerogel by freeze drying with ultra-low phosphorus. Appl. Surf. Sci. 2019, 497, 143775. [Google Scholar] [CrossRef]
- Hirschler, M.M. Poly(vinyl chloride) and its fire properties. Fire Mater. 2017, 41, 993–1006. [Google Scholar] [CrossRef]
- Vahabi, H.; Kandola, B.K.; Saeb, M.R. Flame Retardancy Index for Thermoplastic Composites. Polymers 2019, 11, 407. [Google Scholar] [CrossRef] [Green Version]
- Ma, J. Analysis on the Fire Risk Existing in the Storage of Textile Materials and Textile Goods. Procedia Eng. 2014, 71, 271–275. [Google Scholar] [CrossRef]
- Maqsood, M.; Langensiepen, F.; Seide, G. The Efficiency of Biobased Carbonization Agent and Intumescent Flame Retardant on Flame Retardancy of Biopolymer Composites and Investigation of their Melt-Spinnability. Molecules 2019, 24, 1513. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Qi, S.; Liu, Q.; Gao, H.; Liang, S.; Feng, W.; Jiang, M. Thermal degradation and flame retardant mechanism of sulfonated polyoxadiazole fibers modified by metal ions. J. Polym. Res. 2020, 27, 12. [Google Scholar] [CrossRef]
- Yu, B.; Yuen, A.C.Y.; Xu, X.; Zhang, Z.C.; Yang, W.; Lu, H.; Fei, B.; Yeoh, G.H.; Song, P.; Wang, H. Engineering MXene surface with POSS for reducing fire hazards of polystyrene with enhanced thermal stability. J. Hazard. Mater. 2021, 401, 123342. [Google Scholar] [CrossRef]








| Samples | LOI (%) | After-Flame Time a (s) | Afterglow Time a (s) | Damaged Length a (mm) |
|---|---|---|---|---|
| Wood pulp paper | 18.5 (0.17) b | 7.5 ± 1 | - | All damaged |
| Ca-Alg | 27.3 (0.17) | 0 | 540 ± 10 | All damaged |
| FR-PA/Ca-Alg (10/90) | 30.3 (0.17) | 0 | 125 ± 5 | 51 ± 5 |
| FR-PA/Ca-Alg (20/80) | 28.7 (0.28) | 0.4 ± 0.2 | 74 ± 6 | 65 ± 4 |
| FR-PA/Ca-Alg (30/70) | 29.0 (0.22) | 1.4 ± 0.3 | 53 ± 5 | 63 ± 4 |
| FR-PA/Ca-Alg (40/60) | 25.5 (0.35) | 14.2 ± 0.6 | - | All damaged |
| Samples | TTI (s) | pHRR (kW/m2) | t-pHRR (s) | THR (MJ/m2) | TSP (m2/m2) | av-EHC (MJ/kg) | Residue (%) |
|---|---|---|---|---|---|---|---|
| Wood pulp paper | 17 | 176.92 | 34 | 11.63 | 0.11 | 12.31 | 20.90 |
| FR-PA | 45 | 456.54 | 77 | 27.76 | 4.92 | 27.12 | 3.35 |
| FR-PA/Ca-Alg (30/70) | 37 | 197.31 | 80 | 11.27 | 0.57 | 15.06 | 13.25 |
| FR-PA/Ca-Alg (20/80) | 50 | 223.17 | 73 | 8.77 | 0.61 | 14.21 | 14.08 |
| FR-PA/Ca-Alg (10/90) | 52 | 161.63 | 79 | 7.61 | 0.32 | 12.16 | 14.98 |
| Ca-Alg | 58 | 135.83 | 70 | 7.12 | 0.05 | 16.53 | 12.15 |
| Samples | T5% (°C) | Tmax−A (°C) | Rmax−A (%/°C) | Tmax−B (°C) | Rmax−B (%/°C) | Tmax−C (°C) | Rmax−C (%/°C) | Tmax−D (°C) | Rmax−D (%/°C) | W f (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| FR-PA | 375 | - | - | - | - | 427 | −0.0039 | - | - | 3.99 |
| FR-PA/Ca-Alg (30/70) | 120 | 103 | −0.0003 | 259 | −0.0013 | 420 | −0.0028 | 720 | −0.0001 | 25.72 |
| FR-PA/Ca-Alg (20/80) | 105 | 104 | −0.0004 | 259 | −0.0014 | 419 | −0.0019 | 719 | −0.0001 | 28.20 |
| Ca-Alg | 104 | 103 | −0.0005 | 259 | −0.0018 | - | - | 712 | −0.0001 | 35.34 |
| Samples | T5% (°C) | Tmax−1 (°C) | Rmax−1 (%/°C) | Tmax−2 (°C) | Rmax−2 (%/°C) | Tmax−3 (°C) | Rmax−3 (%/°C) | Tmax−4 (°C) | Rmax−4 (%/°C) | Tmax−5 (°C) | Rmax−5 (%/°C) | W f (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FR-PA | 379 | - | - | - | - | 429 | −0.0137 | 607 | −0.0008 | - | - | 0.89 |
| FR-PA/Ca-Alg (30/70) | 141 | 94 | −0.0007 | 259 | −0.0040 | 412 | −0.0023 | 550 | −0.0045 | 687 | −0.0012 | 9.64 |
| FR-PA/Ca-Alg (20/80) | 128 | 98 | −0.0008 | 260 | −0.0044 | 412 | −0.0018 | 552 | −0.0051 | 689 | −0.0013 | 9.33 |
| Ca-Alg | 116 | 109 | −0.0011 | 260 | −0.0055 | - | - | 589 | −0.0060 | 685 | −0.0019 | 10.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, K.; Tian, X.; Cao, Y.; He, Y.; Xia, Y.; Quan, F. Suppression of Smoldering of Calcium Alginate Flame-Retardant Paper by Flame-Retardant Polyamide-66. Polymers 2021, 13, 430. https://doi.org/10.3390/polym13030430
Xu K, Tian X, Cao Y, He Y, Xia Y, Quan F. Suppression of Smoldering of Calcium Alginate Flame-Retardant Paper by Flame-Retardant Polyamide-66. Polymers. 2021; 13(3):430. https://doi.org/10.3390/polym13030430
Chicago/Turabian StyleXu, Kai, Xing Tian, Ying Cao, Yaqi He, Yanzhi Xia, and Fengyu Quan. 2021. "Suppression of Smoldering of Calcium Alginate Flame-Retardant Paper by Flame-Retardant Polyamide-66" Polymers 13, no. 3: 430. https://doi.org/10.3390/polym13030430
APA StyleXu, K., Tian, X., Cao, Y., He, Y., Xia, Y., & Quan, F. (2021). Suppression of Smoldering of Calcium Alginate Flame-Retardant Paper by Flame-Retardant Polyamide-66. Polymers, 13(3), 430. https://doi.org/10.3390/polym13030430